Abstract
Purpose of Review
Gestational diabetes (GDM) is associated with adverse pregnancy and neonatal outcomes and increased maternal risk for subsequent type 2 diabetes. The best diagnostic strategy for GDM is debated and the role of oral antidiabetic medications (OAD) for treatment is unclear. In this paper, we review methods of GDM diagnosis, updates in GDM therapy, and interventions to reduce future type 2 diabetes in women with a history of GDM.
Recent Findings
A “one-step” screening protocol for GDM using 75-g, 2-h oral glucose tolerance testing at 24–28 weeks gestation is recommended by the International Association of the Diabetes and Pregnancy Study Groups, the American Diabetes Association, and the Endocrine Society. This strategy identifies a milder degree of hyperglycemia and thus increases GDM prevalence. Studies indicate that in these cases of mild hyperglycemia, treatment decreases pregnancy and neonatal complications. Insulin analogues including detemir, aspart, and lispro have been shown to be safe in pregnancy with a pregnancy category B classification. Growing literature suggests that sulfonylureas cross the placenta and are associated with increased incidence of macrosomia and neonatal hypoglycemia. Telephone or online diabetes prevention program (DPP)-based interventions for women with GDM have shown encouraging results in pilot studies. Insurance coverage remains a barrier.
Summary
Additional studies are needed to determine the safety of OAD in pregnancy. Public policy supporting DPP could help improve patient access to these proven interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a unique clinical entity that juxtaposes the health interests of mothers, babies, and communities. The American Diabetes Association (ADA) defines gestational diabetes as diabetes first diagnosed in the second or first trimester of pregnancy that is not clearly pre-existing type 1 or 2 diabetes [1••]. With dramatic increases in type 2 diabetes and obesity, identification of women with pre-existing type 2 diabetes by first trimester screening is important. In the second trimester of pregnancy, screening for GDM should be completed for all women. The foundation of GDM treatment is lifestyle interventions; medications, primarily insulin, are indicated if lifestyle measures are insufficient. Treatment of GDM reduces risk of pregnancy complications and adverse neonatal outcomes including macrosomia and shoulder dystocia [2]. After pregnancy, maternal risk for type 2 diabetes and cardiovascular disease is increased and continued follow-up and early intervention for impaired glucose intolerance are crucial. Health policy initiatives to improve insurance coverage and access to a diabetes prevention program (DPP) are an important step for preventing or delaying the onset of type 2 diabetes in women with a history of GDM. Offspring of women with GDM are at increased risk for obesity and type 2 diabetes and a vicious cycle continues [3].
Prevalence
In 2017, it was estimated that 21.3 million births (16.2%) worldwide were affected by hyperglycemia in pregnancy, and of these, 86.4% were due to GDM. There is a notable regional variation with age-adjusted prevalence ranging from 9.5% in Africa to 26.6% in Southeast Asia [4]. In the USA, according to 2016 data from the Centers for Disease Control and Prevention (CDC), the crude national prevalence of GDM was 6.0% and pre-existing diabetes in pregnancy was 0.9%. From 2012 to 2016, standardized prevalence increased for gestational diabetes (5.2 to 5.6%) and was stable for pre-existing diabetes (0.8%). Accounting for race/ethnicity, the prevalence of GDM was highest among non-Hispanic Asian women (11.1%). Accounting for BMI, prevalence of gestational diabetes ranged from 3.6% for normal-weight women (pre-pregnancy BMI 18.5–24.9) to 13.9% with class III obesity (BMI ≥ 40.0) [5•]. GDM prevalence also increases with advancing maternal age. A retrospective cohort including over 1.7 million births in women aged 40–54 between 1998 and 2014 showed that gestational diabetes increased in all racial/ethnic groups throughout the study period with the largest increases in Asian or Pacific Islander (from 13.7 to 22.4%) and Hispanic women (11.2 to 19.3%) [6].
Costs of GDM to society are significant. A 2012 US cost analysis determined that the national cost of gestational diabetes was $1.3 billion with an average cost of $5800 per case of GDM. Between 2007 and 2012, the national burden doubled for GDM (103% increase) including a 23% increase in prevalence and 65% increase in cost per case [7]. The rising prevalence and substantial costs of GDM make this a critical national and international health concern.
Pathophysiology
Physiologic adaptations to insulin secretion and sensitivity during pregnancy are crucial to maintain glucose homeostasis and provide adequate support to a growing fetus. In a pregnancy not complicated by diabetes, early in gestation insulin sensitivity may increase or remain stable to promote glucose storage in adipose tissue for use in later pregnancy. Later in pregnancy, increases in various maternal and placental hormones including estrogen, progesterone, leptin, cortisol, placental lactogen, and placental growth hormone lead to an increase in insulin resistance [8]. To maintain glucose homeostasis, hypertrophy and hyperplasia of pancreatic beta cells occur and facilitate an increase in glucose-stimulated insulin secretion. Failure of these adaptive mechanisms to maintain glucose homeostasis results in gestational diabetes. Among the factors implicated in the pathogenesis of GDM, beta cell dysfunction, chronic insulin resistance, tissue inflammation, and neurohormonal dysfunction play important roles [9].
Few studies have investigated the genetics of GDM; however, the available data suggests that type 2 diabetes susceptibility genes are also linked to GDM including variants in TCF7L2, MTNR1B, KCNJ11, IGF2BP2, CDKAL1, GCK, and KCNQ1 [10]. Studies continue to identify candidate genes for GDM including a recent finding that PAX8, a gene important in thyroid development and function, may play a role in islet survival during the stress of pregnancy [11]. Monogenic diabetes and specifically GCK-MODY may first come to medical attention during pregnancy and be misclassified as GDM [12]. Therefore, careful attention should be paid to identify atypical cases of GDM and perform genetic testing when appropriate.
Diagnosis
Pre-Pregnancy
Recent research has examined the use of pre-pregnancy biomarkers to identify women at high risk for gestational diabetes. A case control study of 256 GDM cases found that sex hormone binding globulin, glucose, adiponectin, and homeostasis model assessment-estimated insulin resistance (HOMA-IR) were independently associated with odds for GDM. Using these biomarkers, a risk score was composed which showed a better predictive value for GDM than traditional risk factors of age, ethnicity, BMI, family history of DM, and personal history of GDM (AUC 0.74 versus 0.67) [13]. These findings require further validation before they can be routinely used in clinical practice.
Early Pregnancy
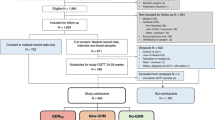
Professional societies agree that women with risk factors for type 2 diabetes should be tested at their initial prenatal visit for diabetes using standard diagnostic criteria: fasting glucose ≥ 126; 2-h, 75-g OGTT ≥ 200; A1c ≥ 6.5%; or symptoms of hyperglycemia and random glucose ≥ 200. The ADA suggests screening should be performed for overweight or obese (BMI ≥ 25 or ≥ 23 in Asian Americans) women with one or more risk factors including history of pre-diabetes or GDM, first-degree relative with diabetes, high-risk race/ethnicity (African American, Latina, Native American, Asian American, Pacific Islander), history of cardiovascular disease (CVD), hypertension, low HDL < 35, high triglyceride > 250, polycystic ovarian syndrome (PCOS), physical inactivity, or other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans) [1••]. The American College of Obstetricians and Gynecologists (ACOG) adds the additional risk factor of previously giving birth to an infant weighing 4000 g (approximately 9 lbs) or more [14••]. Screening results consistent with diabetes should be confirmed by repeat testing unless there is unequivocal hyperglycemia. If screening tests diagnose diabetes in the first trimester, women are considered to have pre-existing type 2 diabetes (or rarely, type 1 or monogenic diabetes). GDM is therefore defined as diabetes that is first diagnosed in the second or third trimester of pregnancy that is not clearly pre-existing type 1 or type 2 diabetes [1••]. Figure 1 illustrates the recommended process of screening for diabetes beginning in early pregnancy through post-partum.
“One-Step” Screening
The optimal strategy for diagnosis of GDM is debated, as different strategies and thresholds will identify different degrees of hyperglycemia and risk for adverse outcomes. The landmark Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study included 23,316 pregnant women with blinded glycemic data from 75-g, 2-h oral glucose tolerance test (OGTT) at 24–28 weeks. This study showed a continuous association between increasing maternal glucose levels and adverse maternal, fetal, and neonatal outcomes [15]. Based on these results, the International Association of Diabetes and Pregnancy Study Group (IADPSG), the ADA, and the Endocrine Society recommend the one-step 75-g OGTT for screening at 24–28 weeks [1••, 16, 17]. Thresholds for GDM diagnosis from the 75-g OGTT were defined by the average fasting, and 1-h and 2-h glucose values from the HAPO study which were associated with 1.75 times the odds of adverse outcomes compared to the study population [16]. These glucose thresholds are outlined in Table 1 and one abnormal value is sufficient for GDM diagnosis.
A secondary analysis of HAPO data using IADPSG diagnostic cutoffs confirmed that women diagnosed with GDM by IADPSG criteria had significantly increased odds ratios for birth weight > 90th percentile (OR 1.87), cord blood C-peptide > 90th percentile (OR 2.00), and primary cesarean delivery (OR 1.31) compared to women with no GDM [18•]. Using these criteria and one abnormal value for diagnosis, the incidence of GDM notably increases from 5–6% to 15–20% [19]. Societies supporting this approach prefer the one-step screening because it is evidence-based and the diagnostic criteria are derived from outcomes data, so are better suited to improve gestational outcomes. Furthermore, interventional trials have demonstrated reduced pregnancy and neonatal complications with GDM treatment in women with lower levels of hyperglycemia which overlap with IADPSG cutoffs [20, 21].
While the IADPSG cutoffs will diagnose more women with gestational diabetes, a majority (80–90%) of mild GDM cases can be managed with diet alone [20, 21]. The one-step diagnostic strategy has been modeled to be cost-effective assuming that post-delivery care reduced diabetes incidence. The incremental cost-effectiveness ratio for IADPSG screening compared to previous standard was $20,336 per quality-adjusted life year (QALY) gained (with incremental cost-effectiveness ratio (ICER) of $100,000 per QALY considered cost-effective) [22].
“Two-Step” Screening
In contrast, a National Institutes of Health (NIH) consensus group from 2013 and ACOG support a two-step approach for GDM diagnosis. A 50-g, 1-h non-fasting screen is initially performed and women who screen positive (1-h glucose > 130–140) then undergo a 100-g, 3-h OGTT for definitive diagnosis (see Table 1 for diagnostic cutoffs). The NIH consensus group concludes that this two-step approach is superior because there is a lack of evidence that the one-step strategy leads to improved maternal or fetal outcomes but is associated with increased healthcare costs, life disruption, and psychosocial burdens [23]. Various screening thresholds for the 50-g, 1-h screening OGTT and 100-g, 3-h OGTT are employed in clinical practice and two abnormal values on the 100-g, 3-h OGTT are traditionally required for diagnosis of GDM. ACOG guidelines do not specify a single cutoff to be used but recommend providers use consistent cutoffs appropriate for their patient population. The most recent ACOG guidelines additionally note that patients with one abnormal value on 100-g, 3-h OGTT have increased risk of adverse outcomes and may require a higher level of scrutiny [14••].
Management
Lifestyle Interventions
Once a GDM diagnosis is established, the mainstays of therapy include medical nutrition therapy, physical activity, weight management, and glucose monitoring. As previously mentioned, GDM can be managed with lifestyle interventions alone in up to 80–90% of women, depending on diagnostic criteria, can be managed with lifestyle interventions alone [20, 21]. Little data is published on the effect of specific dietary interventions on maternal and fetal outcomes. The ADA does not provide specific dietary recommendations but refers to the Dietary Reference Intakes (DRI) for pregnant women with a minimum of 175 g of carbohydrates, 71 g of protein, and 28 g of fiber [24•]. The Endocrine Society provides more specific guidance to limit carbohydrates to 35–45% of total calories, distributed in 3 small- to moderate-sized meals and 2–4 snacks including an evening snack [17]. A systematic review and meta-analysis in 2014 examined the effects of dietary interventions in GDM on maternal and newborn outcomes. Among the findings, a low glycemic index diet was the only dietary intervention associated with improved outcomes including less frequent insulin use (RR 0.767) and lower newborn weight (− 161.9 g) based on data from four randomized controlled trials. No differences in outcomes were seen with total energy restriction diets or low-carbohydrate diets [25]. Regarding weight management, the Institute of Medicine guidelines provide recommendations for gestational weight gain based on BMI [26].
Glucose Monitoring
Self-monitoring of blood glucose (SMBG) should initially be performed at least four times daily: fasting and 1–2 h post-prandial. Glycemic targets include fasting glucose < 95 mg/dL (or < 90 if achieved without hypoglycemia), 1-h post-prandial glucose < 140, and 2-h post-prandial glucose < 120 [17, 24, 27]. Post-prandial glucose measurement is preferred based on older data which showed improved glycemic control and neonatal outcomes for women with GDM who adjusted insulin based on post-prandial glucose values rather than pre-prandial values [28]. For women with GDM well controlled with diet alone, frequency of SMBG may be reduced but generally should still be performed at least twice a day. Hemoglobin A1c may be used as a secondary measure for glycemic control with a target of < 6–6.5% if not contraindicated due to underlying hemoglobinopathy [24•]. Physiologic changes in pregnancy including dilutional effects from increased blood volume and increased red blood cell turnover cause a decrease in hemoglobin A1c and are taken into consideration [29].
Insulin
Pharmacologic therapy is indicated in cases of significant initial hyperglycemia or if SMBG shows glucose values consistently above pregnancy targets. Table 2 summarizes the options for medical therapy of GDM. Insulin is the first-line therapy for GDM requiring pharmacologic intervention and can be administered as a single bedtime injection, multiple daily injections (MDI), or by continuous subcutaneous insulin infusion (CSII). A meta-analysis in 2016 showed no difference in pregnancy outcomes for women with pre-existing diabetes treated with insulin administered by CSII or MDI [30]. Insulin therapy can be targeted to a specific abnormal glucose pattern. For example, isolated fasting hyperglycemia can be managed with bedtime neutral protamine Hagedorn (NPH) alone. If consistent fasting and post-prandial hyperglycemia is present, basal/bolus regimens using up to 0.7–1.0 units/kg may be required in a ratio of approximately 50% long or intermediate acting and 50% short or rapid acting [14••]. Though historically NPH and regular insulin were the preferred insulin choices for GDM, recent studies have shown insulin analogues to be safe as well [31•, 32, 33]. Insulins assigned a pregnancy category B include regular insulin (U-100 and U-500), insulin aspart, insulin lispro (U-100 and U-200), NPH, and insulin detemir. The pregnancy category B classification indicates that the US Food and Drug Administration (FDA) has sufficient human data to consider these medications low risk in pregnancy. Pregnancy category C designation has been assigned to insulin glulisine, insulin degludec, and inhaled human insulin because there is no data during pregnancy. Insulin glargine (U-100 and U-300) does not carry a pregnancy category and its package insert indicates that there are “no well-controlled clinical studies in pregnant women” [34•].
Oral Antidiabetic Medications
Oral antidiabetic medications (OADs) are not approved by the FDA for treatment of GDM and their use remains an area of controversy. The two classes of medications historically used for GDM are metformin and sulfonylureas (commonly glyburide). A meta-analysis in 2015 of 18 studies showed no significant differences in glycemic measures between insulin, metformin, and glyburide for gestational diabetes [35••]. However, up to 50% of patients initially treated with metformin ultimately require insulin for glycemic control [36] and maternal glycemic measures are not the only consideration.
Both metformin and sulfonylureas are known to cross the placenta. A pharmacokinetic study in 2015 demonstrated that transplacental transfer of glyburide does occur with significant variability between subjects but 7/19 neonates (37%) had higher glyburide levels than maternal samples [37•]. The previously mentioned meta-analysis observed higher infant birth weight and increased incidence of macrosomia and neonatal hypoglycemia with glyburide compared with insulin [35••]. Metformin has also been documented to cross the placenta with levels similar to maternal levels [38]. Metformin compared to insulin was associated with less maternal weight gain and lower risk of neonatal hypoglycemia, though with slightly higher risks of prematurity [35••]. Long-term safety data and offspring metabolic outcomes with OAD therapies are unknown. Taking this evidence into account, the ADA and ACOG both recommend insulin as the preferred treatment for GDM. ACOG guidelines add that metformin is a reasonable alternative for a patient with GDM who declines insulin therapy or is unable to safely administer or afford insulin [14••, 24].
Obstetric Considerations
Monitoring of fetal well-being is initiated at 32 weeks in women with GDM and poor glycemic control or those requiring treatment with insulin or OAD. Typical surveillance includes twice weekly non-stress test and amniotic fluid index, a strategy which has been shown to reduce stillbirth in pregnancies with pre-gestational or GDM [39]. The specific time to initiate surveillance and frequency of monitoring have not been studied and vary by institution. For women with GDM well controlled on nutritional therapy alone, no increase in stillbirth has been observed and additional antenatal screening may not be necessary [40, 41]. Many practitioners perform third trimester ultrasound in all women with GDM to assess fetal weight and identify cases where elective induction or cesarean section may be indicated to prevent birth complications. While this approach may be beneficial in reducing rates of shoulder dystocia and brachial plexus injury, current ultrasound measurement techniques have poor sensitivity and specificity for identifying macrosomic infants [42, 43].
Timing of delivery in pregnancies affected by GDM must weigh the risks of later delivery (stillbirth and delivery complications from excessive fetal growth) versus risks of earlier induction (risks of prematurity and possible increase in cesarean section). Studies examining the impact of early delivery versus expectant management have yielded conflicting results. Older studies and a 2008 meta-analysis reported reduced rates of macrosomia and shoulder dystocia with elective induction of labor at 38–39 weeks versus expectant management [44, 45]. A recent multicenter randomized controlled trial randomized 425 women with uncomplicated GDM to induction of labor at 38–39 weeks versus expectant management and found no difference in incidence of cesarean section or maternal or fetal complications (though intended sample size was not achieved) [46•]. ACOG recommends that for women with GDM well controlled on nutritional therapy alone, delivery should not be induced before 39 weeks and expectant management up to 40 6/7 weeks is reasonable with appropriate monitoring. For women with GDM requiring medication, delivery between 39 0/7 and 39 6/7 weeks is recommended [14••].
In preparation for scheduled cesarean section or induction of labor, the night before surgery or induction, the long-acting insulin dose should be reduced by 50%. The morning of surgery or induction, insulin or OAD should be held. Intrapartum glycemic control can be maintained by a rotating fluid protocol which uses a 5% dextrose in normal saline (D5NS) infusion for maternal glucose ≤ 100, lactated Ringers or normal saline (NS) for glucose > 101, and rapid-acting infusion for glucose > 140 titrated to goal glucose 100 [47]. After delivery of the placenta, insulin resistance drops rapidly and medication can often be discontinued. Monitoring of fasting glucose for 24–72 h has been suggested to identify patients with ongoing hyperglycemia [17].
Post-Partum Follow-Up
Breastfeeding has been shown to reduce the risk of type 2 diabetes and should be encouraged for all women including those with GDM. In the Nurses’ Health Study (NHS) and NHS II, each additional year of lactation resulted in a decrease in the risk of diabetes of 14–15% when controlling for other risk factors for T2DM [48]. A population-based study in Korea in 2018 showed lower rates of T2DM in women who ever breastfed (OR 0.60) and a trend toward better glycemic control with longer breastfeeding in women with T2DM [49]. Breastfeeding has additional short- and long-term beneficial effects on offspring metabolic health as described in a recent review [50]. Routine assessment for post-partum depression should be performed because post-partum depression is nearly twice as common in women with pre-gestational diabetes and GDM compared to women without diabetes [51].
All patients with GDM should undergo post-partum OGTT at 4–12 weeks to identify persistent glucose intolerance or overt diabetes [14••, 24]. If post-partum OGTT is normal, routine screening should continue every 1–3 years, and in subsequent pregnancies early screening for recurrent GDM is indicated [24•]. Persistent glucose intolerance is present in up to 20% of women at post-partum follow-up and subsequent risk for type 2 diabetes is significantly increased [52, 53]. Studies have demonstrated that women with a history of GDM have up to seven times the risk of type 2 diabetes as women without GDM and that the cumulative incidence of type 2 diabetes is as high as 70%, depending on the population and length of follow-up [54, 55]. Based on NHANES data, subsequent diabetes diagnosis after GDM is more common in older women (age ≥ 65, OR 5.81), > 20 years since GDM diagnosis (OR 5.10) with BMI ≥ 30 (OR 4.75), and family history of diabetes (OR 3.83) [56•]. Risk of recurrence of GDM in a subsequent pregnancy is 41.3%, compared to 4.2% in women without previous GDM [57].
Racial/Ethnic Differences
There are notable racial and ethnic differences in GDM prevalence, outcomes, and possibly subsequent risk of type 2 diabetes. Studies of large US cohorts have shown GDM prevalence is higher is Filipina, other Asian, and Hispanic women compared to African-American and non-Hispanic white women. Increasing BMI is associated with an increased risk of GDM in all ethnicities; however, the effect of higher BMI is somewhat diminished in the highest risk ethnicities. In a 2012 study of over 120,000 women with GDM, the adjusted OR of GDM for BMI ≥ 35 compared to normal BMI (18.5–24.9) was 6.0 for non-Hispanic white women versus 2.60 for Asian women. This study observed that the increased risk of GDM with higher BMI in Filipina and other Asian women appeared to plateau at a BMI of 28, while risk continued to increase with each BMI category for non-Hispanic white, African-American, and Hispanic women [58]. Obesity prevalence and gestational weight gain also vary by ethnicity. A retrospective study of > 1000 African-American and Latina women with GDM from a large Detroit health system observed that 47% of African-American women and 37% of Latina women were overweight or obese and excessive weight gain in pregnancy occurred for 53% of African-American and 38% of Latina women [59].
Recent data has suggested that healthcare utilization and cesarean delivery rates for women with GDM vary by race/ethnicity. Data from a multiethnic cohort showed higher rates of cesarean delivery in Hispanic, non-Hispanic white, and Asian Indian women compared to Southeast and East Asian women, and Hispanic women had the highest rates of ED utilization during pregnancy (OR 3.21) [60]. Data about subsequent diabetes after GDM have not shown consistent differences in risk based on maternal race/ethnicity, though one study found that Latina women were less likely to complete post-partum screening [61]. A recent study found that subsequent diabetes after GDM was less common in women with more education; high school/GED was associated with OR 0.44 and > high school with OR 0.46 [56•].
Barriers to GDM care for minority and low-income women have been explored in small cohorts with focus groups and communication, personal/environmental barriers, and type and quality of healthcare were identified as areas for improvement. Data from providers about the risks of GDM were described by participants as both “vague” and “overwhelming” and women reported poor communication from their providers about community resources for GDM and the benefits of breastfeeding [62•].
Health Policy
Identification of women with glucose intolerance after GDM is critical because effective interventions can reduce the risk of type 2 diabetes. The DPP study found that intensive lifestyle therapy and metformin reduce type 2 diabetes incidence by approximately 50% compared with placebo for women with impaired glucose tolerance and GDM history [63]. Unfortunately, only 18.5 to 61.0% of women with GDM receive appropriately timed post-partum OGTT [64] and the opportunity to prevent or delay type 2 diabetes may be lost. Studies have shown that older women with higher education level and income are more likely to complete post-partum screening [65, 66]. Obesity, higher parity, and Latina ethnicity have been associated with lower screening rates [61].
Even if appropriately timed screening is performed to identify glucose intolerance, referral rates for DPP in general are very low. A 2016 survey found that only 4.2% of eligible adults were referred to a 12-month DPP, while 26.2% of those who were not referred said they would be interested in diabetes prevention programming [67]. Pilot studies of targeted interventions based on the DPP for women with GDM during pregnancy and post-partum using a telephone-based program and lactation support have shown encouraging results for improving post-partum weight loss [68]. However, other programs have found challenges in enrolling and retaining post-partum women in these programs due to difficulties with time commitments and cultural, linguistic, and socioeconomic barriers [69]. The ideal time for DPP interventions post-partum has not been studied, but in most cases women with a history of GDM should be referred to a DPP by 6 months to 1 year post-partum. Interventions as early as 6 weeks post-partum could have added benefits (breastfeeding assistance, post-partum weight loss) and may be more feasible for new mothers if administered by telephone or online programs [68, 70]. Recent interventions with a specific focus on culturally appropriate and peer-led DPP-based lifestyle intervention are promising [71].
Insurance access for GDM care and DPP remains challenging, despite recent policy changes to improve accessibility. The Affordable Care Act requires that gestational diabetes screening at 24–28 weeks of pregnancy and diabetes screening for women with a history of GDM be covered as preventive services without charging copayment or coinsurance [72]. However, diabetes prevention programs for these patients may not be covered. In 2018, Medicare began reimbursing for eligible patients with Medicare Part B to participate in Medicare Diabetes Prevention Programs (MDPP). Commercial insurers including Anthem and Cigna have similarly expanded programs to cover DPP. In 2018, the DPP was offered as a Medicaid benefit in 11 states as a covered benefit (California, Montana, Minnesota, New Jersey), waiver program (Texas, New York, Vermont), pilot project (Arkansas, Pennsylvania), or demonstration project (Oregon, Maryland) [73].
Conclusions
The prevalence of GDM in the USA is increasing and the worldwide burden of GDM is a substantial public health concern. Diagnostic strategies for GDM continue to be debated as some groups advocate for lower glycemic cutoffs to identify pregnancies where even a small risk of complications from hyperglycemia can be reduced by appropriate treatment. Lifestyle interventions including medical nutrition therapy, physical activity, and weight management are first-line therapies for all women with GDM. If medication is required, insulin is the preferred therapy and insulin analogues have been shown to be safe and effective. OADs including metformin and sulfonylureas are known to cross the placenta and lack long-term outcomes data for exposed offspring, though recent studies have shown increased risk of macrosomia and neonatal hypoglycemia with sulfonylurea treatment. Additional studies to confirm these findings and evaluate long-term outcomes are needed to further assess the safety of OADs for GDM.
With a national epidemic of obesity and type 2 diabetes, GDM presents an important opportunity to identify women at risk for developing type 2 diabetes and metabolic syndrome and intervene to mitigate those risks. DPP is proven to reduce incidence of type 2 diabetes in women with a history of GDM. However, significant barriers remain to their effective use as many women with a history of GDM do not receive appropriately timed post-partum OGTTs and fewer are referred to DPP. Improving insurance coverage for DPP is one step that can improve the accessibility of these programs. Future efforts to engage women with a history of GDM in post-partum care must account for time constraints, family commitments, and social/cultural expectations in this population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–27. https://doi.org/10.2337/dc18-S002. Key recommendations from an ADA multidisciplinary expert committee for classification and diagnosis of diabetes, updated yearly.
Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Aktary WM, et al. Screening and diagnosing gestational diabetes mellitus. Evidence report/technology assessment no. 210. (prepared by the University of Alberta Evidence-based Practice Center under contract no. 290–2007-10021-I.) AHRQ Publication No. 12(13)-E021-EF. Agency for Healthcare Research and Quality: Rockville; 2012. www.effectivehealthcare.ahrq.gov/reports/final.cfm
Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30(Suppl 2):S169–74. https://doi.org/10.2337/dc07-s211 Review. Erratum in: Diabetes Care. 2007 Dec;30(12):3154.
International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. http://www.diabetesatlas.org. Accessed 10 December 2018
• Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth — United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201–7. https://doi.org/10.15585/mmwr.mm6743a2. This report analyzes US vital statistics data to provide estimates about the overall prevalence of GDM in 2016 and prevalence for different races/ethnicities and BMI categories.
Booker WA, Gyamfi-Bannerman C, Sheen JJ, Wright JD, Siddiq Z, D'Alton ME, et al. Maternal outcomes by race for women aged 40 years or older. Obstet Gynecol. 2018;132(2):404–13. https://doi.org/10.1097/AOG.0000000000002751.
Dall TM, Yang W, Halder P, Pang B, Massoudi M, Wintfeld N, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37(12):3172–9. https://doi.org/10.2337/dc14-1036.
Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–9 Erratum in: Diabetes Care. 2007 Dec;30(12):3154.
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11). https://doi.org/10.3390/ijms19113342.
Kleinberger JW, Maloney KA, Pollin TI. The genetic architecture of diabetes in pregnancy: implications for clinical practice. Am J Perinatol. 2016;33(13):1319–26.
Martin-Montalvo A, López-Noriega L, Jiménez-Moreno C, Herranz A, Lorenzo PI, Cobo-Vuilleumier N, et al. Transient PAX8 expression in islets during pregnancy correlates with β-cell survival, revealing a novel candidate gene in gestational diabetes mellitus. Diabetes. 2019;68(1):109–18. https://doi.org/10.2337/db18-0285.
Chakera AJ, Steele AM, Gloyn AL, Shepherd MH, Shields B, Ellard S, et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015;38(7):1383–92. https://doi.org/10.2337/dc14-2769.
Badon SE, Zhu Y, Sridhar SB, Xu F, Lee C, Ehrlich SF, et al. A pre-pregnancy biomarker risk score improves prediction of future gestational diabetes. J Endocr Soc. 2018;2(10):1158–69. https://doi.org/10.1210/js.2018-00200.
•• Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64. https://doi.org/10.1097/AOG.0000000000002501. Key guidelines from ACOG for GDM diagnosis and management with specific updates on pharmacologic treatment of GDM.
HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. https://doi.org/10.1056/NEJMoa0707943.
International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. https://doi.org/10.2337/dc09-1848.
Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227–49. https://doi.org/10.1210/jc.2013-2465.
• Waters TP, Dyer AR, Scholtens DM, Dooley SL, Herer E, Lowe LP, et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes Care. 2016;39(12):2204–10. A secondary analysis of HAPO study participants which observed higher rates of adverse pregnancy outcomes for women diagnosed with GDM based on IADPSG criteria compared to women with no GDM.
Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35(3):526–8. https://doi.org/10.2337/dc11-1641.
Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–48. https://doi.org/10.1056/NEJMoa0902430.
Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86.
Werner EF, Pettker CM, Zuckerwise L, Reel M, Funai EF, Henderson J, et al. Screening for gestational diabetes mellitus: are the criteria proposed by the international association of the Diabetes and Pregnancy Study Groups cost-effective? Diabetes Care. 2012;35(3):529–35. https://doi.org/10.2337/dc11-1643.
Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29(1):1–31.
• American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S137–43. https://doi.org/10.2337/dc18-S013. Key recommendations from an ADA multidisciplinary expert committee for management of type 1, type 2, and GDM in pregnancy and post-partum, updated yearly.
Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37(12):3345–55. https://doi.org/10.2337/dc14-1530.
Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009. Schwartz RA, Rosenn B, Aleksa K, Koren G. Glyburide transport across the human placenta. Obstet Gynecol 2015 Mar;125(3):583–8. doi: https://doi.org/10.1097/AOG.0000000000000672
Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. https://doi.org/10.2337/dc07-s225 Erratum in: Diabetes Care. 2007 Dec;30(12):3154.
de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237–41.
Nielsen LR, Ekbom P, Damm P, Glümer C, Frandsen MM, Jensen DM, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27(5):1200–1.
Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev. 2016;6:CD005542. https://doi.org/10.1002/14651858.CD005542.pub3.
• Herrera KM, Rosenn BM, Foroutan J, Bimson BE, Al Ibraheemi Z, Moshier EL, et al. Randomized controlled trial of insulin detemir versus NPH for the treatment of pregnant women with diabetes. Am J Obstet Gynecol. 2015;213(3):426.e1–7. https://doi.org/10.1016/j.ajog.2015.06.010. This RCT demonstrated that insulin detemir was non-inferior to NPH for glycemic control and perinatal/neonatal outcomes.
Koren R, Toledano Y, Hod M. The use of insulin detemir during pregnancy: a safety evaluation. Expert Opin Drug Saf. 2015;14(4):593–9. https://doi.org/10.1517/14740338.2015.1013533.
Lv S, Wang J, Xu Y. Safety of insulin analogs during pregnancy: a meta-analysis. Arch Gynecol Obstet. 2015;292(4):749–56. https://doi.org/10.1007/s00404-015-3692-3.
• Blum AK. Insulin use in pregnancy: an update. Diabetes Spectr. 2016;29(2):92–7. https://doi.org/10.2337/diaspect.29.2.92 Erratum in: Diabetes Spectr. 2016 Aug;29(3):191. Review of the use of insulin in pregnancy and safety of different insulin formulations including FDA pregnancy categories.
•• Jiang YF, Chen XY, Ding T, Wang XF, Zhu ZN, Su SW. Comparative efficacy and safety of OADs in management of GDM: network meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2015;100(5):2071–80. https://doi.org/10.1210/jc.2014-4403. A meta-analysis of 18 RCTs which showed similar glycemic control with insulin, metformin, and glyburide but increased incidence of neonatal hypoglycemia and macrosomia with glyburide.
Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003–15. https://doi.org/10.1056/NEJMoa0707193 Erratum in: N Engl J Med. 2008 Jul 3;359(1):106.
• Schwartz RA, Rosenn B, Aleksa K, Koren G. Glyburide transport across the human placenta. Obstet Gynecol. 2015;125(3):583–8. https://doi.org/10.1097/AOG.0000000000000672. Prospective study which demonstrated transplacental transfer of glyburide occurs and is highly variable among patients.
Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83(5):1575–8.
Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL. Antepartum surveillance in diabetic pregnancies: predictors of fetal distress in labor. Am J Obstet Gynecol. 1995;173(5):1532–9.
Gabbe SG, Mestman JG, Freeman RK, Anderson GV, Lowensohn RI. Management and outcome of class A diabetes mellitus. Am J Obstet Gynecol. 1977;127(5):465–9.
Casey BM, Lucas MJ, Mcintire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90(6):869–73.
Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276(18):1480–6.
Johnstone FD, Prescott RJ, Steel JM, Mao JH, Chambers S, Muir N. Clinical and ultrasound prediction of macrosomia in diabetic pregnancy. Br J Obstet Gynaecol. 1996;103(8):747–54.
Lurie S, Insler V, Hagay ZJ. Induction of labor at 38 to 39 weeks of gestation reduces the incidence of shoulder dystocia in gestational diabetic patients class A2. Am J Perinatol. 1996;13(5):293–6.
Witkop CT, Neale D, Wilson LM, Bass EB, Nicholson WK. Active compared with expectant delivery management in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):206–17. https://doi.org/10.1097/AOG.0b013e31818db36f.
• Alberico S, Erenbourg A, Hod M, Yogev Y, Hadar E, Neri F, et al. Immediate delivery or expectant management in gestational diabetes at term: the GINEXMAL randomised controlled trial. BJOG. 2017;124(4):669–77. https://doi.org/10.1111/1471-0528.14389. Multicenter RCT which showed no difference in rates of cesarean section or complications for women with uncomplicated GDM randomized to induction of labor at 38–39 weeks versus expectant management (note that intended sample size was not achieved).
Rosenberg VA, Eglinton GS, Rauch ER, Skupski DW. Intrapartum maternal glycemic control in women with insulin requiring diabetes: a randomized clinical trial of rotating fluids versus insulin drip. Am J Obstet Gynecol. 2006;195(4):1095–9.
Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–10.
Nam GE, Han K, Kim DH, Huh Y, Han B, Cho SJ, et al. Associations between Breastfeeding and Type 2 Diabetes Mellitus and Glycemic Control in Parous Women: A Nationwide, Population-Based Study. Diabetes Metab J. 2019;43(2):236-241. https://doi.org/10.4093/dmj.2018.0044. Accessed 21 Dec 2018.
Horta BL, de Lima NP. Breastfeeding and type 2 diabetes: systematic review and meta-analysis. Curr Diab Rep. 2019;19(1):1. https://doi.org/10.1007/s11892-019-1121-x.
Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA. 2009;301(8):842–7. https://doi.org/10.1001/jama.2009.201.
Kjos SL, Buchanan TA, Greenspoon JS, Montoro M, Bernstein GS, Mestman JH. Gestational diabetes mellitus: the prevalence of glucose intolerance and diabetes mellitus in the first two months post partum. Am J Obstet Gynecol. 1990;163(1 Pt 1):93–8.
Catalano PM, Vargo KM, Bernstein IM, Amini SB. Incidence and risk factors associated with abnormal postpartum glucose tolerance in women with gestational diabetes. Am J Obstet Gynecol. 1991;165(4 Pt 1):914–9.
Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–8.
Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. https://doi.org/10.1016/S0140-6736(09)60731-5.
• Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent type 2 diabetes among U.S. women. Diabetes Res Clin Pract. 2018;141:200–8. https://doi.org/10.1016/j.diabres.2018.05.010. A study of NHANES data which assessed prevalence of GDM and subsequent diabetes and identified associated sociodemographic and health-related characteristics associated with these outcomes.
Getahun D, Fassett MJ, Jacobsen SJ. Gestational diabetes: risk of recurrence in subsequent pregnancies. Am J Obstet Gynecol. 2010;203(5):467.e1–6. https://doi.org/10.1016/j.ajog.2010.05.032.
Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35(7):1492–8. https://doi.org/10.2337/dc11-2267.
Kieffer EC, Carman WJ, Gillespie BW, Nolan GH, Worley SE, Guzman JR. Obesity and gestational diabetes among African-American women and Latinas in Detroit: implications for disparities in women’s health. J Am Med Womens Assoc (1972). 2001;56(4):181–7 196.
Singh H, Soyoltulga K, Fong T, Billimek J. Delivery outcomes, emergency room visits, and psychological aspects of gestational diabetes: results from a community hospital multiethnic cohort. Diabetes Educ. 2018;44(5):465–74. https://doi.org/10.1177/0145721718795589.
Stasenko M, Cheng YW, McLean T, Jelin AC, Rand L, Caughey AB. Postpartum follow-up for women with gestational diabetes mellitus. Am J Perinatol. 2010;27(9):737–42. https://doi.org/10.1055/s-0030-1253557.
• Oza-Frank R, Conrey E, Bouchard J, Shellhaas C, Weber MB. Healthcare experiences of low-income women with prior gestational diabetes. Matern Child Health J. 2018;22(7):1059–66. https://doi.org/10.1007/s10995-018-2489-y. This study conducted focus groups with a diverse sample of women with history of GDM to identify barriers to GDM care, management, and follow-up.
Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–9. https://doi.org/10.1210/jc.2008-0772.
Pastore I, Chiefari E, Vero R, Brunetti A. Postpartum glucose intolerance: an updated overview. Endocrine. 2018;59(3):481–94. https://doi.org/10.1007/s12020-017-1388-0.
Ferrara A, Peng T, Kim C. Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: a report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2009;32(2):269–74. https://doi.org/10.2337/dc08-1184.
Lawrence JM, Black MH, Hsu JW, Chen W, Sacks DA. Prevalence and timing of postpartum glucose testing and sustained glucose dysregulation after gestational diabetes mellitus. Diabetes Care. 2010;33(3):569–76. https://doi.org/10.2337/dc09-2095.
Venkataramani M, Pollack CE, Yeh HC, Maruthur NM. Prevalence and Correlates of Diabetes Prevention Program Referral and Participation. Am J Prev Med. 2019;56(3):452-457. https://doi.org/10.1016/j.amepre.2018.10.005. Accessed 17 Jan 2019.
Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP Jr, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care. 2011;34(7):1519–25. https://doi.org/10.2337/dc10-2221.
Gómez ML, Hieronymus LB, Ashford KB, Barnett JM, Renn TA. Linking postpartum and parenting women with a National Diabetes Prevention Program: recruitment efforts, challenges, and recommendations. Diabetes Spectr. 2018;31(4):324–9. https://doi.org/10.2337/ds18-0013.
• Handley MA, Harleman E, Gonzalez-Mendez E, Stotland NE, Althavale P, Fisher L, et al. Applying the COM-B model to creation of an IT-enabled health coaching and resource linkage program for low-income Latina moms with recent gestational diabetes: the STAR MAMA program. Implement Sci. 2016;11(1):73. https://doi.org/10.1186/s13012-016-0426-2. This paper describes an intervention for Latina women with a history of GDM using a bilingual, telephone-based health coaching program adapted from the DPP.
McCurley JL, Fortmann AL, Gutierrez AP, Gonzalez P, Euyoque J, Clark T, et al. Pilot test of a culturally appropriate diabetes prevention intervention for at-risk Latina women. Diabetes Educ. 2017;43(6):631–40. https://doi.org/10.1177/0145721717738020.
Preventive care benefits for women. HealthCare.gov. https://www.healthcare.gov/preventive-care-women/. Accessed 31 Jan 2019.
Medicaid Coverage. National Diabetes Prevention Program Coverage Toolkit. https://coveragetoolkit.org/medicaid-agencies/medicaid-coverage-2/ (2019). Accessed 1 Feb 2019.
Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13(8):677–84. https://doi.org/10.1111/j.1463-1326.2011.01395.x.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant number T32 DK0070110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Economics and Policy in Diabetes
Rights and permissions
About this article
Cite this article
Dickens, L.T., Thomas, C.C. Updates in Gestational Diabetes Prevalence, Treatment, and Health Policy. Curr Diab Rep 19, 33 (2019). https://doi.org/10.1007/s11892-019-1147-0
Published:
DOI: https://doi.org/10.1007/s11892-019-1147-0