Abstract
Purpose of Review
With recent cardiovascular outcome trial (CVOT) results for antihyperglycemic medications, the treatment algorithm for patients with type 2 diabetes (T2DM) and atherosclerotic vascular disease (ASCVD) requires revision.
Recent Findings
All completed CVOTs have demonstrated CV safety of the tested medications, with some trials demonstrating CV efficacy. While metformin remains the first-line recommended medication for T2DM, 18–37% of the patients enrolled in the completed CVOTs were not treated with metformin, providing substantial power to assess CV outcomes independent of metformin. The safety and tolerability of metformin are indisputable, but there are no robust data proving its efficacy for either macro or microvascular disease outcomes. We should reconsider the primacy of metformin in the management of T2DM in patients with ASCVD.
Summary
This article will review the evidence for CV effects of antihyperglycemic agents (AHAs), and propose an evidence-based treatment algorithm for patients with T2DM and ASCVD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most prevalent chronic diseases in the world, affecting over 30 million individuals, over 10% of the adult population in the USA and almost 10% of adults worldwide [1,2,3]. Cardiovascular disease (CVD) is a major complication of T2DM and cardiovascular (CV) mortality is the single most common cause of death in a patient with T2DM [4,5,6,7,8,9,10]. A major aim of T2DM therapy should therefore be to reduce CV events and deaths. For decades, this imperative was limited by a dearth of available therapies for T2DM: until the 1990s, only insulin and sulfonylureas were available in the USA and metformin elsewhere. In 1995, metformin was approved for use in the USA, and following its introduction, the number of T2DM therapies available began to grow rapidly, with a new class of T2DM medication emerging into the marketplace on average every other year over the 2 decades since. Until 2008, regulatory requirements for approval of antihyperglycemic medications (AHAs) were restricted to proving effectiveness on lowering glycated hemoglobin (HbA1c) and short-term safety: there were no trials adequately powered to evaluate cardiovascular safety or efficacy. In 2008, increasing concern over the CV safety of AHAs such as rosiglitazone [11], pioglitazone [12], tolbutamide (in fact, all sulfonylureas) [13,14,15], and muraglitazar [16] prompted the FDA to announce in December 2008 new guidance that all AHAs for T2DM must henceforth demonstrate CV safety; the European Medicines Agency (EMA) concomitantly put forward similar guidance [17,18,19].
In the wake of the 2008 change in regulatory guidance, a wealth of data has been generated with the completion of several CV safety trials, with CV safety proven for the novel therapy in each of the completed trials and with the most exciting findings being proof of superior CV outcomes with four novel AHAs [20••, 21••, 22••, 23••]. Prior to these cardiovascular outcome trials (CVOTs), for patients with T2DM and ASCVD, there were no clinical outcome data to support the use of one specific AHA over others, and the role of glucose control and most appropriate HbA1c targets for such patients remained unknown [4, 24, 25, 26•]. With a paucity of evidence for the management of hyperglycemia in patients with T2DM and ASCVD, metformin became the default first-line therapy [27•, 28•, 29], based on global availability, affordability, overall safety, and tolerability along with favorable estimates for selected CV-related outcomes observed in the United Kingdom Prospective Diabetes Study (UKPDS) randomized trial [30•]. However, more recent meta-analyses of metformin randomized comparative data support CV safety but not incremental efficacy with metformin [31]. We now have an abundance of treatment options that have proven CV safety, each with a dataset more robust from single CVOTs than for the totality of the randomized comparative metformin data, and four AHAs thus far have proven to improve CV outcomes in patients with T2DM and ASCVD. We review medications studied in the era of large T2DM CVOTs and results from those trials completed, and propose a new, evidence-based management strategy for patients with T2DM and ASCVD that may be considered in contrast to the current HbA1c-guided, metformin-as-first-line therapy approach.
Challenging the Current First-line Therapy: the Data on Metformin
In 1995, metformin was approved for the management of T2DM in the USA, though it had been available globally for some 50 years prior. Metformin was an appealing choice for many providers: prior to its availability, prescribers were limited to insulin or sulfonylureas, both associated with risk of hypoglycemia and weight gain [32, 33]. Other biguanides, phenformin and buformin, were approved but then removed from the market after evidence emerged for associated CV harm and high risk of lactic acidosis [34, 35]. Practitioners grew increasingly comfortable prescribing metformin as accumulating comparative data and meta-analyses results demonstrated that metformin had no discernable effects on the incidence of lactic acidosis, and results from the UKPDS 34 demonstrated statistically significant reductions in risk for myocardial infarction and for CV death [30•, 36, 37]. As a result, metformin has been recommended by most guidelines, both in the USA and globally, as first-line in the treatment of T2DM for decades [26•, 27•, 28•, 38•]. In recent years, use of metformin has continued to grow, especially following successful citizen petitions to the FDA that resulted in removal of boxed warnings contraindicating use in patients with heart failure (HF) in 2006, and liberalized contraindications for those with kidney disease in 2016. Metformin use in patients with kidney disease is now based on eGFR rather than creatinine, and allows patients to be initiated on metformin with eGFRs as low as 45 mL/min/1.73 m2, and to continue metformin down to an eGFR of 30 mL/min/1.73 m2 [36, 37, 39,40,41]. Metformin is now listed on the World Health Organization’s essential medicines list and is the most commonly prescribed anti-hyperglycemic worldwide [28•, 34, 42].
Though there is good evidence for the efficacy of metformin on glycemic control, and millions of patient-years of clinical experience supporting overall and CV safety, any incremental CV efficacy of metformin remains unclear. A pivotal trial that underpins recommendations for widespread use of metformin is the UKPDS 34, a randomized comparison of a policy of intense glycemic control with metformin versus usual glucose management in a subset of participants of the UKPDS, a trial that enrolled patients with newly diagnosed T2DM. Patients eligible for randomization to metformin were overweight or obese at trial entry. This randomized comparison in a subset of UKPDS patients demonstrated that metformin led to a 36% reduction in all-cause mortality (RR 0.64; 95% CI 0.45–0.91) and a 39% reduction in myocardial infarction (MI) (RR 0.61; 95% CI 0.41–0.89), both achieving nominal statistical significance, when compared with conventional treatment. These analyses, however, have a number of important limitations. Only 342 trial participants were randomized to receive metformin, with total of 52 CV deaths for analysis of usual care versus metformin (36 vs. 16), and a total of 251 MIs partitioned for analyses across three groups that included patients randomized to a policy of intensive control with insulins/sulfonylureas, comprising only 39 MI events in the metformin arm. These small numbers of events yield marginal statistical precision of the comparisons. Additionally, patients were excluded from enrollment in UKPDS if they had had a recent MI, HF, or angina, excluding those with the highest risk of CVD, and limiting the generalizability of the observations across patients with T2DM at higher CVD risk. Randomized treatment in the UKPDS was not blinded, and there was no placebo group; the findings of UKPDS were further challenged by other statistical issues, more completely reviewed by Boussageon et al. [43]. Perhaps most importantly, the results of UKPDS 34 have not been replicated, and meta-analyses of the CV effects of metformin have consistently failed to show significant CV risk reduction even with the inclusion of the UKPDS data [31, 44•, 45].
For example, a meta-analysis of 13,110 patients with T2DM, 9560 of whom were treated with metformin, was unable to exclude a 23% reduction or a 31% increase in all-cause mortality, with effect estimates for CV mortality ranging from a 33% reduction to a 64% increase by the 95% confidence interval [44•]. Another meta-analysis that included only “isolated” metformin trials directly comparing metformin use with placebo or with another AHA included only four trials that directly compared metformin with placebo. Within these four trials, the number of cardiovascular events were small, and over 50% were drawn from the UKPDS 34 study [31, 46,47,48,49]. Despite these caveats, there was no statistically significant advantage of metformin in their analyses on any CV outcome [31]. Therefore, based on the totality of the data, the CV efficacy of metformin remains uncertain.
In addition to providing CV safety data on newer therapies, recent CVOTs of novel AHAs have also highlighted a high frequency of omission of metformin use among patients enrolled in these trials [20••, 21••, 22••, 23••, 50,51,52,53,54,55]. Contrary to contemporary guidance and recommendations endorsing metformin as first-line therapy for all patients with T2DM without contraindication [26•, 56], experimental drugs and placebo controls were not always added to background metformin therapy. Review of ten CVOT results with published data available demonstrated that patients entering the trials were not treated with metformin at baseline in 18–40% of trial participants, comprising from 883 to > 5000 participants in these trials (Table 1)—subsets larger than most T2DM trials prior to 2008 when regulatory guidance required CV safety assessments. For example, in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction-53 (SAVOR-TIMI 53) trial, over 5000 trial participants (30% of enrolled patients) were not treated with metformin at baseline [51]. In the Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE), a CVOT examining the CV safety of degludec versus glargine insulins in a patient population with T2DM and prevalent ASCVD or high risk for ASCVD, 40% of trial participants were not on metformin at baseline [54]. Given that such a large number of trial participants in each of these recent CVOTs were not treated with metformin at baseline, the results of these CVOTs should not be interpreted exclusively as adding the novel therapy to metformin, but instead as effects on CV outcomes independent of metformin use.
Furthermore, subgroup analyses of the recent CVOTs have suggested mixed results when stratified by baseline metformin use (Table 2). There appears to be a favorable interaction between dipeptidyl peptidase 4 inhibitors (DPP-4i) and metformin use, with significant reductions in both CV death and all-cause mortality (HR 0.74; 95% CI 0.57–0.95 and HR 0.75; 95% CI 0.61–0.92) with DPP-4i versus placebo in those treated with background metformin, noting the key limitation that these are crude analyses of overall trial results without adjustment for differences in patient mix and materially confounded by indication. More rigorous analyses of these trials will have to be done using patient-level data with multivariable adjustments and propensity adjustments for confirmation of this heterogeneity of DPP-4i effect [57, 58]. Although there was no statistically significant interaction between baseline metformin use and randomized trial assignment in the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME), subgroup analysis suggests that trial participants not on metformin at baseline had a possible exaggerated benefit with empagliflozin compared with those taking metformin at baseline (not on metformin HR 0.72; 95% CI 0.56–0.94 versus on metformin HR 0.92; 95% CI 0.77–1.10; interaction P = 0.14) [20••]. Subgroup analyses will be informative across all of the CVOTs of the newer AHAs to evaluate for interaction of safety and efficacy according to background metformin use.
Despite uncertainties regarding the CV efficacy of metformin and the robust CV safety and efficacy evidence of the newer therapies that have undergone formal assessment in dedicated CVOTs, metformin is still recommended as first-line treatment for patients with T2DM, even for those with prevalent ASCVD, and is the most-prescribed AHA worldwide [26•, 27•, 56]. In the absence of dedicated randomized trial data robustly demonstrating the CV safety and efficacy profile of metformin, and in the setting of multiple AHAs now with proven CV safety and some with proven CV efficacy independent of glycemic control in dedicated CVOTs with large subsets of patients not treated with metformin, unilaterally endorsing metformin as first-line medication for patients with T2DM and prevalent ASCVD and stepping down to second-line AHAs, especially for those with proven ASCVD efficacy, only when HbA1c is not at target are no longer evidence-based strategies and must be reconsidered.
Cardiovascular Outcome Trials: a Summary of CVOT Results To Date
Antihyperglycemic Therapies with Evidence for Reduction in Cardiovascular Risk
Until recently, there were no AHAs robustly proven safe or effective with regard to CV outcomes. Since the FDA and EMA mandated formal evaluation of CV safety of all new AHAs for the treatment of T2DM in 2008, results from numerous randomized trials of AHAs are now available and have demonstrated not only CV safety for all trials reported to date, but also significant CV benefit for selected therapies.
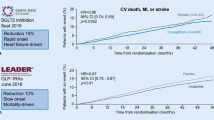
Empagliflozin is a sodium glucose co-transporter 2 inhibitor (SGLT2i) and the first AHA to demonstrate reductions in the composite outcome of CV death/non-fatal MI/non-fatal stroke, CV death, all-cause death, and HF hospitalizations [20••]. In the EMPA-REG OUTCOME randomized, placebo-controlled trial, 7020 patients with T2DM and established ASCVD were randomized to either placebo or one of two doses of empagliflozin (10 or 25 mg daily) and were followed for a median of 3.1 years. Trial participants randomized to empagliflozin had a 14% lower relative risk of the primary composite outcome of CV death, non-fatal MI, or non-fatal stroke (HR 0.86; 95% CI 0.74–0.99), driven largely by a 38% reduction in CV death (HR 0.62; 95% CI 0.49–0.77) and coincident with a 35% reduction in heart failure hospitalization. As a result, empagliflozin now carries an FDA indication for reduction of risk for CV death in patients with T2DM with concurrent ASCVD, an indication independent of glucose control [59]. Canagliflozin, another SGLT2i, was evaluated in the CANVAS Trials Program, which enrolled 10,142 patients with T2DM and with established or at high risk for ASCVD [21••]. Similar to the results from EMPA-REG OUTCOME, randomization to canagliflozin was associated with a 14% relative risk reduction in the primary composite outcome of CV death, non-fatal MI, or non-fatal stroke (HR 0.86; 95% CI 0.75–0.97). Unlike empagliflozin, canagliflozin was not associated with statistically significant decreases in CV death (HR 0.87; 95% CI 0.72–1.06) but did significantly reduce risk for heart failure hospitalization similarly to empagliflozin (HR 0.67; 95% CI 0.52–0.87).
Liraglutide is a glucagon-like peptide 1 receptor analogue (GLP-1 RA) administered subcutaneously once daily, with CV safety assessed versus placebo in a completed CVOT. In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, 9341 patients with T2DM and established ASCVD or at high risk for CVD were randomized to receive either liraglutide or placebo. Randomization to liraglutide was associated with a 13% relative risk reduction in the primary composite outcome of CV death, non-fatal MI, or non-fatal stroke (HR 0.87; 95% CI 0.78–0.97) and a significant reduction in CV death (HR 0.78; 95% CI 0.66–0.93) with trends for improvement in non-fatal MI (HR 0.88; 95% CI 0.75–1.03) and non-fatal stroke (HR 0.89; 95% CI 0.72–1.11) [22••]. These pivotal findings led to approval by the FDA for liraglutide to be indicated for the reduction of risk of major adverse cardiovascular events, CV death, MI, and stroke in individuals with T2DM and established ASCVD, an indication independent of glucose control [60]. The CV safety of semaglutide, another GLP1 RA that is administered once weekly, was evaluated in the SUSTAIN-6 trial, and demonstrated a significant reduction in the same primary composite CV outcome as in LEADER (HR 0.74; 95% CI 0.58–0.95), but did not demonstrate statistical differences in CV mortality [23••]. SUSTAIN-6 was a smaller trial than LEADER and designed primarily to demonstrate non-inferiority and accumulated only 254 primary composite outcome events for analysis (contrasted with 1302 such events in LEADER). Its statistically significant differences in CV outcomes support an FDA-approved claim of CV safety without claim of statistical superiority.
Pioglitazone, a thiazolidinedione and PPARγ receptor agonist, was studied in the PROactive trial [61]. This was a randomized controlled trial of 5238 study participants with T2DM and established ASCVD who were randomized to receive either pioglitazone 45 mg daily or placebo. The primary composite outcome in this CVOT was different than those described above and included a composite of all-cause death, non-fatal MI (including silent MI), stroke, acute coronary syndrome, coronary or leg artery revascularization, or above the ankle amputation. The prioritized secondary composite outcome was a composite of all-cause death, non-fatal MI (excluding silent MI), or non-fatal stroke. There was no significant difference in the primary composite outcome for pioglitazone versus placebo (HR 0.90; 95% CI 0.80–1.02), though there was a nominally significant 16% relative risk reduction for the prioritized secondary composite outcome (HR 0.84; 95% CI 0.72–0.98). The frequency of HF leading to hospitalization was higher in those patients randomized to pioglitazone versus placebo (HR 1.45; 95% CI 1.06–2.0) [61, 62]. The interpretation of these selected favorable results are tempered by the fact that overall, PROactive failed to meet its primary outcome. In aggregate, it appears that pioglitazone is safe from an ASCVD perspective noting cautions about HF, but not superior for CV outcomes. For the gold-standard 3-point major adverse cardiovascular event (MACE) composite outcome used in most contemporary CVD trials, PROactive results demonstrated superiority, a finding that has since been supported by a meta-analysis of data from 16,390 patients, which yielded a similar estimate of relative risk reduction for the composite of death, MI, or stroke with pioglitazone (HR 0.82; 95% CI 0.72–0.94) [63].
These five medications have demonstrated not only CV safety, but an actual reduction in risk for future CV events, each independent of effects on glucose control. We therefore propose that they should be considered first-line therapies in patients with concomitant T2DM and ASCVD, at least in the absence of systolic heart failure or advanced kidney disease with CVOTs underway of SGLT2i in these latter two high-risk subsets.
Therapies with Evidence for Non-inferiority Versus Placebo for Cardiovascular Risk
Among the CVOTs of AHAs for T2DM that have been completed since the change in regulatory guidance by the FDA and EMA in 2008, most have failed to demonstrate CV benefits, but all have met non-inferiority criteria proving CV safety of the novel therapies versus placebo.
Results of large-scale randomized CVOTs of 3 DPP4i’s, saxagliptin, alogliptin, and sitagliptin have demonstrated non-inferiority regarding their primary CV endpoints meeting the regulatory guidance proving cardiovascular safety, though results of each trial failed to prove superiority versus placebo. The Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial and the SAVOR-TIMI 53 trial investigated alogliptin and saxagliptin, respectively, versus placebo in CVOTs with similar primary composite outcomes of CV death, non-fatal MI, or non-fatal stroke [50, 51]. The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) studied sitagliptin versus placebo and used a similar endpoint, but additionally included hospitalization for unstable angina in the primary composite outcome [52]. For all three of these trials, results robustly proved CV safety but with no suggestion of incremental CV efficacy.
Importantly, from these three trials, there is demonstration of heterogeneous effects of the three DPP4i’s on HF outcomes [64]. In the SAVOR-TIMI 53 trial, saxagliptin was associated with a significant 27% higher risk of HF hospitalization compared with placebo [65]. In the EXAMINE trial, the parameter estimate for HF was a 19% increased relative risk associated with alogliptin that did not achieve statistical significance [66]. In TECOS, the analysis of HF risk of sitagliptin versus placebo was completely neutral [64]. The discrepancy in HF outcomes among DPP4i warrants additional study.
Exenatide extended-release (ER) and lixisenatide are GLP-1RAs studied in the CVOTs, the Exenatide Study of Cardiovascular Events (EXSCEL), and the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) that demonstrated CV safety, but did not demonstrate a reduction in CV events, with either agent [53, 55]. Exenatide ER was non-inferior to placebo with regard to the primary composite CV outcome of CV death, non-fatal MI, and non-fatal stroke, but failed to show superiority (HR 0.91; 95% CI 0.83–1.00; P < 0.001 for non-inferiority; P = 0.06 for superiority). All-cause death was lower in individuals randomized to exenatide ER (HR 0.86; 95% CI 0.77–0.97) but this finding was not considered statistically significant because of the hierarchical testing plan controlling for multiple comparisons. Lixisenatide is a once-daily administered GLP-1 RA that was compared with placebo in the ELIXA trial, which enrolled patients with T2DM post ACS events to daily lixisenatide versus placebo and demonstrated CV non-inferiority but not superiority for the primary composite CV outcome of CV death, MI, stroke, or hospitalization for unstable angina (HR 1.02; 95% CI 0.89–1.17).
Acarbose is an alpha-glucosidase inhibitor that was studied in the Acarbose Cardiovascular Evaluation (ACE) trial in which Chinese patients with impaired glucose tolerance and CVD were randomized to receive either placebo or acarbose [67]. Although there was a reduction in risk for incident T2DM in those individuals randomized to receive acarbose versus placebo, there was no difference in the primary composite CV outcome of CV death, non-fatal MI, non-fatal stroke, or hospitalization for unstable angina or heart failure (HR 0.98; 95% CI 0.86–1.11) between the two groups.
The FDA does not provide specific guidance regarding CV safety assessment for insulin therapies, though there are two CVOTs examining longer-acting basal insulins. The ORIGIN trial of over 12,000 patients with T2DM or high risk for T2DM randomized to insulin glargine versus usual care found no difference in CV outcomes when comparing insulin glargine to standard therapy, demonstrating CV safety but not incremental efficacy of insulin glargine [68].
The DEVOTE trial evaluated degludec insulin, an ultra-long-acting once-daily basal insulin, versus once daily insulin glargine in a patient population with T2DM with or at high risk for ASCVD and found that degludec was non-inferior to glargine for the primary CV safety composite outcome of CV death, non-fatal MI, and non-fatal stroke (HR 0.91; 95% CI 0.78–1.06) [54].
Antihyperglycemic Therapies Without Cardiovascular Outcome Trial Assessment
There are no dedicated CVOTs available for sulfonylureas, but a large ongoing randomized CVOT is comparing the CV effects of the sulfonylurea glimepiride with the DPP4i linagliptin [69]. Sulfonylureas have several undesirable side effects including weight gain and increased risk for hypoglycemia, and meta-analyses question the cardiovascular safety of these agents as a class [70,71,72,73]. Based on the observation of increased CV and all-cause mortality with tolbutamide in the University Group Diabetes Program randomized trial [14], US product labels to date have a warning that oral hypoglycemic medications have been associated with increased cardiovascular mortality, a warning unique to sulfonylurea medications. Contemporary observational analyses using state-of-the-art analytical strategies support an ongoing concern regarding potential adverse effects of sulfonylureas on CV outcomes [15].
Short-acting insulins have not been shown to affect CV risk but have not been robustly evaluated in CVOTs [74]. Given the lack of regulatory requirement to prove CV safety of insulins, there is little to no incentive to study the CV safety and efficacy of short-acting insulins in dedicated CVOTs and their role in the management of patients with T2DM and prevalent ASCVD remains uncertain.
Challenging the Use of HbA1c in the Hierarchy of Management for Patients with T2DM and ASCVD
Contemporary T2DM CVOTs have been designed for glycemic equipoise targeting the same HbA1c in both placebo and active comparator arms. HbA1c goals in the trials were determined by individual clinical providers and based on local/regional guidelines. In the trials reported to date, the placebo versus active comparator arms achieved minimal differences in HbA1c between the groups, averaging 0.2–0.4% difference across the trials. In each of the trials, the differences in HbA1c diminished over the course of the trial and were unrelated to observed CV effects. Consequently, for those agents with proven CV efficacy, the CV benefits are demonstrated to be unrelated to their effects on HbA1c [75]. In this context, initiation of an SGLT2i and/or a GLP-1 RA in patients with T2DM and ASCVD should not be contingent upon inadequate HbA1c control once treated with lifestyle interventions and metformin, as recommended by contemporary guidance [26•, 56], but rather indicated irrespective of HbA1c level. A similar argument, with slightly less robust data, can be made for consideration of pioglitazone therapy. Here, we propose a new clinical algorithm, in which AHAs with established CV superiority are used upstream (and in favor of metformin) and are also considered as potential add-on therapies even if HbA1c is controlled with other therapies, since their cardiovascular benefits are not related to HbA1c control.
Evidence-Based Algorithm for the Management of Patients with T2DM and ASCVD
New cardiology guidelines acknowledge the data on CV protection that has emerged from the recent CVOTs of AHAs. Such guidance is not predicated on concomitant use of metformin, challenging the contemporary T2DM management guidance and instead buttressed by the CVOTs’ evidence. While the American Diabetes Association (ADA) continues to recommend metformin as the preferred pharmacologic agent for T2DM management, it recognizes that GLP-1 RA and SGLT2i should be considered, especially in the setting of comorbid ASCVD but only as second line after metformin and contingent upon not achieving target HbA1c, neither of which were criteria for the completed CVOTs [29]. The European Society of Cardiology similarly still gives preference to metformin in patients with concomitant HF and T2DM, but also notes the potential role for empagliflozin in treating HF [76]. The most recent American Association of Clinical Endocrinologists (AACE) guidance radically shifted their recommendations in the initial approach to glycemic management and equated GLP-1 RA (liraglutide), SGLT2i (empagliflozin and canagliflozin), and DPP4i with metformin, citing all four classes as potential first-line agents [27•]. These recommendations, though perhaps in varying degrees, reflect the shift in T2DM management strategies in light of new data from CVOTs considering CV outcome data along with implied microvascular disease risk mitigation with intensive glycemic control.
Here, we propose an evidence-based algorithm for the management of patients with comorbid T2DM and ASCVD (Fig. 1). It is imperative to note that (a) this proposed algorithm is limited to those patients with T2DM with prevalent ASCVD, (b) this algorithm excludes patients with reduced ejection fraction heart failure (HFrEF, “systolic”), and (c) this algorithm excludes those with severe diabetic kidney disease (i.e., eGFR < 45 ml/kg/1.73 m2)—CVOTs in these high-risk populations with SGLTi are ongoing. Along with lifestyle interventions and optimization of other CV risk factors such as blood pressure and LDL-cholesterol, AHAs with proven CV efficacy should be incorporated irrespective of HbA1c targets. In this paradigm, practitioners would start first with medications shown to reduce CV complications and death, adding additional medications stepwise from other classes with proven CV benefits. These medications would be started and titrated to target doses studied in randomized trials, irrespective of HbA1c levels, since the CV benefits of these drugs are not attributable to glycemic control. After prescription of medications at doses targeted in the CVOTs that demonstrated CV benefits, HbA1c levels can then be checked to assess if at patient-specific target. If HbA1c levels are above individualized goal, additional medications that have proven CV safety can be considered, including DPP4i’s and acarbose on similar footing as metformin. If at any point, improved glycemic control is deemed necessary, long-acting basal insulin with either degludec or glargine could be considered. There are many caveats and details regarding this proposed algorithm that are outside of the scope of this paper, including but not limited to the uncertainty around whether the combination of SGLT2i and GLP1 RA will result in additive or incremental efficacy, the serious considerations of cost of these new medications and availability/accessibility for the average patient, and the criteria for most trials requiring HbA1c > 7% for trial eligibility. Safety and cost implications will remain paramount as management strategies are further developed.
This algorithm is proposed for individuals with T2DM and prevalent ASCVD; however, it should be emphasized that there is a lack of data regarding the safety and efficacy of these newer agents in patients with HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), as well as uncertain incremental CV efficacy among subsets at high risk for but without prevalent ASCVD. Some of the medications discussed here should be used with caution in those with systolic HF. Liraglutide has been evaluated in two randomized clinical trials with results from each raising concerns about its safety in the setting of systolic HF. In the effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic HF patients with and without diabetes (LIVE) trial, an investigator-initiated randomized clinical trial, liraglutide did not impair left ventricular ejection fraction compared with placebo but was associated with serious adverse cardiac events and higher heart failure rates (HR 3.9; 95% CI 1.1 to 13.8) [77]. The Functional Impact of GLP-1 for Heart Failure (FIGHT) trial, which examined the effects of liraglutide versus placebo in patients with HFrEF who were recently hospitalized for HF, similarly found that liraglutide had an adverse signal for HF hospitalization, though it did not meet statistical significance [78]. Saxagliptin and pioglitazone have also been associated with increased HF hospitalizations in patients with T2DM with or at high risk for ASCVD [51, 61]. The increased HF risk seen with saxagliptin and a similar trend toward increased HF hospitalization risk with alogliptin does not appear to be a class effect, as sitagliptin had no discernable effect on HF outcomes [51, 52, 61, 64]. Encouragingly, empagliflozin and canagliflozin led to reductions in HF hospitalizations in patients with and without prior history of HF, though they did not affect HF-related deaths [20••, 21••]. The empagliflozin outcome trials in patients with chronic heart failure (EMPEROR) are currently examining the use of empagliflozin in patients with and without T2DM and HFrEF and HFpEF. These studies will investigate empagliflozin as a possible treatment for patients with HFrEF or HFpEF, both with and without pre-existing T2DM [79]. Similarly, the Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure (DAPA-HF) is evaluating the effect of dapagliflozin on heart failure incidence or CV death in patients with HFrEF, regardless of whether or not they have T2DM [80]. The results of those trials will likely inform future recommendations. Additional studies are necessary to further understand the safety profile of these medications in the setting of concomitant HF.
Conclusion
Metformin is still considered first-line therapy for the management of T2DM in patients with CVD for most contemporary recommendations, despite a paucity of evidence that it offers long-term CV benefits. New AHAs have been shown, in dedicated CV outcomes trials, to reduce the risk for CV death and other CV complications in patient populations with or at high risk for ASCVD. Targeting lower HbA1c levels with intensive therapies has not been shown to improve macrovascular outcomes, with some suggestion of harm in certain settings. Furthermore, AHAs that lower the risk for CV death appear to do so independent of their glycemic effects. Therefore, we recommend abandoning an HbA1c-biomarker-guided approach for patients with T2DM and prevalent ASCVD. Instead, we propose a strategy for the management of comorbid T2DM and ASCVD using agents proven to improve CV outcomes irrespective of HbA1c level. Based on this evidenced-based algorithm, metformin should no longer be the first-line agent.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615.
National Diabetes Statistics Report. | Data & Statistics | Diabetes | CDC. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 24 Mar 2018.
Roglic G, World Health Organization Global report on diabetes. World Health Organization, Geneva, Switzerland. http://www.who.int/diabetes/global-report/en/. Accessed 24 Mar 3018.
Patel A, MacMahon S, on behalf of the ADVANCE Collaborative Group, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 358:2560–72.
Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care. 2002;25:471–5.
Gæde P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91.
Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21:1138–45.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 352:837–53.
Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson A-M, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–18.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71.
Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36.
Gore MO, McGuire DK. Resolving drug effects from class effects among drugs for type 2 diabetes mellitus: more support for cardiovascular outcome assessments. Eur Heart J. 2011;32:1832–4.
Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 19:Suppl:789–830.
Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32:1900–8.
Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–6.
Nathan DM. Finding new treatments for diabetes—how many, how fast . . . how good? N Engl J Med. 2007;356:437–40.
Fleming A. FDA approach to the regulation of drugs for diabetes. Am Heart J. 1999;138:S338–45.
Gore MO, McGuire DK. Cardiovascular disease and type 2 diabetes mellitus: regulating glucose and regulating drugs. Curr Cardiol Rep. 2009;11:258–63.
•• Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 373:2117–28. CVOT showing CV superiority for empagliflozin, an SGLT2i
•• Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 377:644–57. CVOT showing CV superiority for canagliflozin, an SGLT2i
•• Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 375:311–22. CVOT showing CV superiority for liraglutide, a GLP-1RA
•• Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 375:1834–44. CVOT showing CV superiority for semaglutide, a GLP-1RA.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 358:2545–59.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
• Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 Diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 38:140–9. Current Guidelines on T2DM management by the American Diabetes Association and European Association for the Study of Diabetes
• AACE/ACE Comprehensive Type 2 Diabetes Management Algorithm 2018. In: Am. Assoc. Clin. Endocrinol. https://www.aace.com/publications/algorithm. Accessed 2 Apr 2018 Current Guidelines on T2DM management by the American Association of Endocrinologists and American College of Endocrinology.
• International Diabetes Federation. Global Guideline for Type 2 Diabetes. https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes. Accessed 6 Apr 2018 Current Guidelines on T2DM management by the International Diabetes Federation.
American Diabetes Association 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care. 2017;41:S73–85.
• UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet Lond Engl. 1998;352:854–65. Study that suggested that there was a decrease in MI and CV mortality rate in patients randomized to metformin. This study played a large role in metformin’s ubiquity
Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60:1620–9.
McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin N Am. 2012;41:57–87.
Klein-Schwartz W, Stassinos GL, Isbister GK. Treatment of sulfonylurea and insulin overdose. Br J Clin Pharmacol. 2016;81:496–504.
Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566–76.
Seltzer HS. A summary of criticisms of the findings and conclusions of the University Group Diabetes Program (UGDP). Diabetes. 1972;21:976–9.
Salpeter SR, Greyber E, Pasternak GA, Salpeter Posthumous EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev CD002967.
Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–75.
• Diabetes Canada | Clinical Practice Guidelines - 2018 Full Guidelines. http://guidelines.diabetes.ca/fullguidelines. Accessed 9 May 2018. Current guidelines on T2DM management by Diabetes Canada.
Inzucchi SE, Masoudi FA, McGuire DK. Metformin in heart failure. Diabetes Care. 2007;30:e129.
Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to moderate renal insufficiency. Diabetes Care. 2011;34(6):1431–7.
U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed 26 Apr 2018.
WHO | WHO Model Lists of Essential Medicines. In: WHO. http://www.who.int/medicines/publications/essentialmedicines/en/. Accessed 27 Apr 2018.
Boussageon R, Gueyffier F, Cornu C. Metformin as firstline treatment for type 2 diabetes: are we sure? BMJ. 2016;352:h6748.
• Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, Boissel J-P, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med. 2012;9:e1001204. A meta-analysis of metformin trials (including UKPDS 34) that was unable to exclude reductions or increases in all-cause mortality or CV mortality associated with metformin use.
Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–51.
Chiasson JL, Naditch L, Miglitol Canadian University Investigator Group. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care. 2001;24:989–94.
DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter Metformin Study Group. N Engl J Med. 1995;333:541–9.
Hällsten K, Virtanen KA, Lönnqvist F, Sipilä H, Oksanen A, Viljanen T, et al. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes. 2002;51:3479–85.
Horton ES, Clinkingbeard C, Gatlin M, Foley J, Mallows S, Shen S. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000;23:1660–5.
White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–32.
Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39.
Standards of Medical Care in Diabetes | American Diabetes Association. https://professional.diabetes.org/content-page/standards-medical-care-diabetes. Accessed 9 May 2018.
Bergmark BA, Bhatt DL, McGuire D, et al. Abstract 16764: metformin use and clinical outcomes among patients with diabetes mellitus and heart failure or kidney dysfunction—observations from the SAVOR-TIMI 53 trial. Circulation. 2016;134:A16764.
Crowley MJ, Williams JW, Kosinski AS, D’Alessio DA, Buse JB. Metformin use may moderate the effect of DPP-4 inhibitors on cardiovascular outcomes. Diabetes Care. 2017; https://doi.org/10.2337/dc17-1528.
U.S. Food and Drug Administration. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed 31 Mar 2018.
U.S. Food and Drug Administration. Victoza (liraglutide [rDNA origin] injection. Package Insert.
Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet Lond Engl. 2005;366:1279–89.
Rydén L, Thráinsdóttir I, Swedberg K. Adjudication of serious heart failure in patients from PROactive. Lancet. 2007;369:189–90.
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–8.
McGuire DK, de Werf FV, Armstrong PW, et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126–35.
Scirica BM, Braunwald E, Raz I et al. Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR - TIMI 53 Randomized Trial. Circulation CIRCULATIONAHA.114.010389.
Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–76.
Holman RR, Coleman RL, Chan JCN, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:877–86.
Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28.
Rosenstock J, Perkovic V, Alexander JH, et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and renal microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17:39.
Forst T, Hanefeld M, Jacob S, Moeser G, Schwenk G, Pfützner A, et al. Association of sulphonylurea treatment with all-cause and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Diab Vasc Dis Res. 2013;10:302–14.
Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304–11.
Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157:601–10.
Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:938–53.
Schnell O, Standl E, Catrinoiu D, Genovese S, Lalic N, Skra J, et al. Report from the 1st cardiovascular outcome trial (CVOT) summit of the Diabetes & Cardiovascular Disease (D&CVD) EASD Study Group. Cardiovasc Diabetol. 2016; https://doi.org/10.1186/s12933-016-0357-x.
Patel KV, de Albuquerque Rocha N, McGuire DK. Diabetes medications and cardiovascular outcome trials: lessons learned. Cleve Clin J Med. 2017;84:759–67.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77.
Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–8.
Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–6. https://doi.org/10.1016/j.jjcc.2017.12.004.
Study to evaluate the effect of dapagliflozin on the incidence of worsening heart failure or cardiovascular death in patients with chronic heart failure - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03036124. Accessed 10 May 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Josephine L. Harrington, Natalia de Albuquerque Rocha, and Kershaw V. Patel declare that they have no conflict of interest.
Subodh Verma has received support from Amgen, AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi.
Darren K. McGuire has received support for clinical trial leadership from AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, Esperion; and honoraria for consultancy from AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Pfizer, Metavant, and Novo Nordisk.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Harrington, J.L., de Albuquerque Rocha, N., Patel, K.V. et al. Should Metformin Remain First-Line Medical Therapy for Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease? An Alternative Approach. Curr Diab Rep 18, 64 (2018). https://doi.org/10.1007/s11892-018-1035-z
Published:
DOI: https://doi.org/10.1007/s11892-018-1035-z