Abstract
Purpose of Review
Decades after the invention of insulin pump, diabetes management has encountered a technology revolution with the introduction of continuous glucose monitoring, sensor-augmented insulin pump therapy and closed-loop/artificial pancreas systems. In this review, we discuss the significance of the 2016 Endocrine Society Guidelines for insulin pump therapy and continuous glucose monitoring and summarize findings from relevant diabetes technology studies that were conducted after the publication of the 2016 Endocrine Society Guidelines.
Recent Findings
The 2016 Endocrine Society Guidelines have been a great resource for clinicians managing diabetes in this new era of diabetes technology. There is good body of evidence indicating that using diabetes technology systems safely tightens glycemic control while managing both type 1 and type 2 diabetes.
Summary
The first-generation diabetes technology systems will evolve as we gain more experience and collaboratively work to improve them with an ultimate goal of keeping people with diabetes complication and burden-free until the cure for diabetes becomes a reality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optimizing glycemic control to reduce complications of diabetes has been the goal of diabetes management after the publication of the landmark Diabetes Control and Complications Trial (DCCT) [1]. DCCT’s formula to achieve target glycemic control was to intensify the treatment; however, the diabetes management tools were not sufficient to intensify treatment without an accompanying increased risk of hypoglycemia at the time. The fear of hypoglycemia became one of the major obstacles to reaching glycemic targets through its impact on patient’s willingness to adhere to insulin treatment and clinicians’ willingness to intensify insulin therapy. By contrast, the results of clinical trials decades after the DCCT have demonstrated that incorporating diabetes technology into management can lower hemoglobin A1c (HbA1c), glucose variability, and the risk of hypoglycemia concurrently [2, 3••]. The clear and present perils of poorly controlled diabetes from a clinical and a public health perspective made it obvious that there was a pressing need to improve diabetes management with these new tools and methods. The premise of safely tightening glycemic control galvanized the interest in diabetes technology and introduced the notion of using technology as a bridge therapy until the cure for diabetes is accomplished.
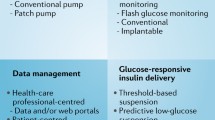
Diabetes technology has been evolving at an extraordinary rate, with new technologies being created and existing technologies improving every year. While technology impacts every aspect of our lives, using diabetes technology for treatment is still uncharted territory for many clinicians. The 2016 Endocrine Society Guidelines (ESG) of Insulin Pump Therapy and continuous glucose monitoring (CGM) have become an essential resource for clinicians to navigate the diabetes technology terrain and provided methods for a smoother transition to technology-based care. The focus has been the evidence-based recommendations itemized for insulin pump and CGM systems separately for adult patients with type 1 diabetes (T1D) and type 2 diabetes (T2D). The findings from the sensor integrated pump therapy (SIP) research are summarized; however, clinical guideline recommendations for SIP are not stated in a clear and itemized format. In addition to presenting an overview of the current status of diabetes technology, the authors outline SIP’s role for the development of semi-automated hybrid closed-loop (artificial pancreas) and give us a glimpse of the artificial intelligence driven, diabetes treatment decision-making devices of the near future. It is noteworthy that the first hybrid closed-loop system was FDA approved merely months after the publications of the ESG. The fast pace of diabetes technology has continued to gain momentum with the FDA approval of the use of the Dexcom CGM to make insulin dose adjustments [4] and CGM use with multiple daily insulin injection (MDI) therapy.
In this review, we aim to discuss the significance of 2016 ESG for managing diabetes during the era of new technology and summarize findings from the relevant diabetes technology studies that were published after the 2016 ESG [5]. The review consists of four main sections: (i) “Insulin Pump-Only Therapy” (without CGM), (ii) “Real-Time Continous Glucose Monitoring”, (iii) “Sensor (CGM) Augmented Insulin Pump Therapy”, and (iv) “CGM Use with MDI Therapy” to give a broader scope of the diabetes technology landscape in diabetes management. The key tenet of diabetes technology research with a focus on the artificial pancreas is summarized in the “What Lies Ahead in Diabetes Technology?” subsection.
Insulin Pump-Only Therapy
The insulin pump therapy pioneered a new strategy of providing an intensive adjustable insulin delivery with greater flexibility and frequent bolusing without the need for repeated injections.
A number of previous studies have compared insulin pump versus MDI therapy using multiple outcome measures in different subject populations. The 2016 ESG highlight the findings and limitations of the studies and determine three major groups of patients when considering the switch from the MDI to the insulin pump; patients with T1D who have not achieved their HbA1C goal, patients experiencing severe hypoglycemia or high glucose variability despite having target HbA1c, and patients seeking more flexibility in insulin dosing and satisfaction regardless of their glycemic control. The guidelines incorporate the human factor into the final decision process while transitioning from the MDI to the insulin pump. The key determinants before making the decision to initiate insulin pump therapy are listed as patient’s willingness, interest, and the ability to use the insulin pump and availability of resources for meticulous patient monitoring and thorough patient education. The importance of utilizing multiple functions of the insulin pump are emphasized, and determining patient-customized insulin to carbohydrate ratios and insulin sensitivity factors before initiating the insulin pump are highly recommended to improve efficacy. Unfavorable factors that impact the success of insulin pump have been reported as baseline HbA1c > 10%, history of missed appointments, and mental illness.
The T1D and T2D populations represent two diverse groups of people with different disease characteristics. The impact of obesity epidemic on patients with T1D and the rising need of insulin use in the T2D population suggest that they both share and face many of the overlapping challenges with respect to achieving and maintaining glycemic control. The insulin pump use has been rising in the T2D population [6] and findings from multiple randomized control multicenter clinical trials have shown that insulin pumps provide a comparable net health benefit to multiple daily injections in patients with T2D [7,8,9,10,11,12]. The guidelines recommend using insulin pump therapy with U100 insulin if patients with T2D have not achieved their HbA1C goal despite the intensive insulin therapy, use of oral agents and other injectable drugs and lifestyle changes. The 2016 ESG recommend being cautious regarding the insulin pump treatment with concentrated insulin (U500) due to limited availability of reliable data and recommend considering insulin pump therapy with U500 for patients with a daily insulin requirement of greater than 200 units.
Inpatient stay is not an uncommon occurrence for patients with diabetes, and insulin treatment is one of the key factors to improve patient clinical outcomes, and in particular to avoid hypoglycemia. The inpatient setting could be classified into intensive care unit (ICU) and non-ICU settings. The guidelines do not differentiate between ICU and non-ICU settings and recommend insulin pump use if the inpatient facility has a well-developed protocol for management of diabetes. To date, there have been limited scientific data comparing outcome measures between inpatient insulin pump use vs. transitioning treatment to IV insulin or MDI treatment during hospital stay. There are no generalized guidelines for insulin pump use in the inpatient setting. It has been shown that hospitals with well-designed diabetes treatment protocols can efficiently manage insulin pump therapy [13,14,15,16,17,18]; however, such protocols do not exist for all inpatient care facilities. The 2016 ESG state that the decision to continue the insulin pump therapy in the inpatient setting should be evaluated thoroughly, preferably by an endocrinologist experienced in pump therapy, for potential risk factors such as the extent of medical illness, impact of co-medications, the degree of insulin resistance, and mental capacity of the patient. Institutions that do not have necessary resources to evaluate and support inpatient pump therapy may choose to transition their patients to insulin injection therapy during their hospital stay. In summary, the 2016 ESG recommend that inpatient insulin pump use decision be made on a case-by-case basis and advise the involvement of an endocrinologist for best and safest results.
Real-Time Continuous Glucose Monitor
CGMs measure glucose subcutaneously by way of interstitial fluid sampling and provide patients with a stream of glucose measurements reflective of blood glucose levels at 1–5-min intervals that can be used for adjustments of the insulin treatment regimen. The hypoglycemia and hyperglycemia alarms alert the patient of impending and real-time hypo and hyperglycemia. CGM has shown impressive scientific, technological, engineering, and clinical advances, providing benefits to many people with diabetes. Studies using CGM-specific computer algorithms have been successful in guiding dosing decisions both in the inpatient and outpatient setting [3••, 19].
The 2016 ESG recommend using real-time CGM both for patients with T1D and T2D; however, the guidelines make a distinction between these two patient populations regarding the duration and frequency of CGM use.
Real-time CGM use is suggested for adults with T1D regardless of the glycemic control status who are willing and able to use the devices on a daily basis. The rationale behind the recommendation has concrete findings from multiple randomized control clinical trials demonstrating a significant reduction in HbA1c in patients with T1D utilizing CGM without an increase in hypoglycemia and sustained benefit in HbA1c improvement during the following 6-month observation period [2, 20, 21]. The real benefit of CGM use for patients who are already in good glycemic control has been in reducing episodes of severe hypoglycemia and time spent in biochemical hypoglycemic range [22].
Previously published studies have shown benefits of CGM for patients with T2D [23]. The 2016 ESG recommend real-time CGM use on an intermittent, short-term basis for poorly controlled patients with T2D (defined as HbA1c ≥ 7%) who are not on prandial insulin and are willing and able to use the device. The exclusion of patients on prandial insulin stems from lack of studies conducted for patients with T2D on prandial insulin rather than any existing data against the CGM use. A series of studies published after the release of 2016 ESG could potentially change this recommendation as will be discussed in the section titled “CGM Use with MDI Therapy” [20, 21, 23, 24••, 25, 26••].
The 2016 ESG do not elaborate on the inpatient CGM use. There are multiple categories of inpatient CGM devices that are currently in-use or in development. In addition to commercially available, non-invasive real-time CGM devices, intravascular CGMs have been used in the inpatient hospital or research settings. Discussion of CGMs other than the FDA approved subcutaneous real-time devices is not within the scope of this review paper. Intermittent blood glucose measurements relying on point-of-care glucometer devices have been the most commonly used method to monitor blood glucose at the inpatient setting. CGM use has been tested in hospitals largely in the ICU for patients with and without diabetes. Moreover, there has been significant debate over safe target glycemic control ranges for the hospital setting. CGM has been used to compare the efficacy of different insulin infusion protocols in achieving target blood glucose levels and reducing glycemic variability in the medical ICU and has been described as a reliable method to assess glucometric parameters given the increased availability of continuous measurements with patterns. When two groups of subjects with similar mean glucose levels were compared during a study conducted in the ICU, a significant reduction in rates of severe hypoglycemia was shown in the CGM (intervention) group highlighting the potential risk of missing imminent hypoglycemia by intermittent blood glucose checks [27]. A recently published multicenter trial conducted in the pediatric ICU utilized CGM to guide insulin administration and compared groups with different target glucose ranges [28]. The investigators found no significant difference between the two groups in terms of the primary outcome (number of ICU-free days to day 28) and found higher rates of healthcare-associated infections and severe hypoglycemia in the lower-target (blood glucose 80–110 mg/dL) group as compared to the higher target (150–180 mg/dL) group prompting early termination of the trial for safety concerns. The CGM values were monitored specifically to prevent hypoglycemic episodes and were verified with arterial blood glucose levels demonstrating CGM’s role in preventing hypoglycemia by early detection; however, the CGM accuracy during critical illness has not been clearly established yet and abnormal levels should be verified by measurement of blood glucose [29].
Sensor (CGM)-Augmented Insulin Pump Therapy
The integration of CGM into insulin pump therapy became a reality almost four decades after the invention of insulin pump therapy [30].
The landmark multicenter clinical trial by Bergenstal et al. [3••] clearly demonstrated the efficacy of using sensor-augmented insulin pump therapy (SAP), described as sensor incorporated insulin pump therapy (SIP) in 2016 ESG, in improving glycemic controls as compared to MDI. The evolution of current pump and CGM technologies continued as sophisticated insulin delivery decision-making algorithms have been incorporated into the insulin pumps. The early version of such systems which worked by suspending insulin delivery when the CGM value reaches a set value has been used in patients with nocturnal hypoglycemia and has been found to reduce nocturnal hypoglycemia by 31.8% when compared to SIP [31]. The next version SAP/SIP use with a predicted hypoglycemic function has shown similar results with reduction in hypoglycemia and has been approved in Europe and Australia [32]. CGM trend markers with up and down arrows indicating the rate of increase or drop in blood glucose level have been used to optimize insulin treatment [33,34,35] and are useful tools to recognize abnormal blood glucose patterns for patients with diabetes.
As noted in the guidelines, there has been a significant shift from insulin pump only treatment to CGM-augmented insulin pump therapy. These more advanced systems transform insulin pump’s function from a delivery tool to a treatment decision-making device with predictive models to suspend the insulin delivery in advance of a hypoglycemic episode and with real-time adjustable insulin infusion capability to mitigate hyperglycemia. While the comparison between the smart insulin pumps and the MDI creates an additional challenge, the CGM use in conjunction with the MDI opens a new assessment arena for the two major players of insulin treatment.
CGM Use with MDI Therapy
The impact of CGM use on glycemic control has been investigated predominantly for insulin pump users rather than the MDI users. The limited available observational data suggestive of the glycemic benefit for patients on MDI and CGM therapy has been replicated by the T1D Exchange registry 2015 data demonstrating similar mean HbA1c level in the adult MDI and insulin pump users who also use CGM [36••]. Moreover, patients who are non-CGM users and on MDI treatment had significantly higher HbA1c levels as compared to CGM users. Three multicenter randomized clinical trials published after the 2016 ESG solidified previous findings by demonstrating the improvement in HbA1c for subjects with T1D on MDI therapy [24••, 25, 26••, 36••]. Subjects were patients with inadequately controlled T1D (HbA1c ≥ 7.5%). The study by Beck et al. demonstrated a mean HbA1c level reduction from baseline of 1.1% at 12 weeks and 1.0% at 24 weeks in the CGM group and 0.5 and 0.4% in the control group, respectively (P < 0.001). The adjusted treatment group difference in mean change in HbA1c level was a clinically and statistically significant reduction of – 0.6% (95% CI, − 0.8 to − 0.3%; P < 0.001) at 24 weeks [24••]. The CGM group spent less time in the hypoglycemic range and less time in the hyperglycemic range only during daytime as compared to the control group based on exploratory analysis. The subset analysis from the full dataset revealed benefits of CGM use for older adult population (≥ 60 years of age) with T1D and T2D and on MDI treatment. The HbA1c reduction from baseline was greater in the CGM group than the control group (− 0.9 ± 0.7% vs − 0.5 ± 0.7%) at 24 weeks with an adjusted difference in mean change of − 0.4 ± 0.1% (P < 0.001) [26••]. Similar to findings from the previously published CGM studies in pump patients, the study by Lind et al. highlighted the importance of compliance for the CGM use [25]. The significant HbA1c reduction by 0.46% (0.31–0.61%) has been shown only in patients who were using the CGM sensor more than 70% of the time with no significant difference in HbA1c for those using the CGM sensor for less than 70% of the time [25]. The quality of life measure evaluations favored CGM use with subjects on CGM reporting a significant improvement in treatment satisfaction and well-being in comparison to the conventional treatment group [25]. The satisfaction survey results of the ≥ 60-year-old age subgroup favored the CGM use indicating high-perceived benefits and only few perceived hassles of using CGM [26••].
The results from these studies indicated that the potential benefit of CGM use on improving glycemic control in people with poorly controlled diabetes has been comparable for MDI and insulin pump users and thus expanded the target patient population that can benefit from the CGM use.
What Lies Ahead in Diabetes Technology?
The MiniMed 670G system with background insulin infusion adjustments based on CGM glucose values requiring manual meal bolus input was approved by the FDA on September 28, 2016 [37]. The FDA approval of the first artificial pancreas system has been a game changer in diabetes treatment and already opened new avenues to improve diabetes care. The Medtronic 670G system was approved based on data from a 3-month, multicenter study with 124 participants who used the device for 12 weeks during free-living conditions following a 2-week baseline assessment [38••]. The results from the study have shown that time with glucose concentration in target range increased by 5·6% from baseline through a reduction in both time in hyperglycemia and time in hypoglycemia with the Medtronic 670G system use as compared to sensor-augmented pump use at baseline proving that the system can safely and effectively control glucose in people with T1D [38••]. It also should be noted that there are many other artificial pancreas systems undergoing clinical testing for the FDA approval [39, 40]. The artificial pancreas system studies aim to test the feasibility and efficacy of each system before their implementation to clinical care. The findings from these studies have been remarkably consistent demonstrating a significant reduction in hypoglycemia and an increase in time spent within target blood glucose range with the artificial pancreas use as compared to standard of care treatment [40,41,42,43]. The artificial pancreas systems have made great progress during the past decade; however, there are still areas to improve before a fully automated artificial pancreas system is implemented in diabetes management. Just like every other new treatment and paradigm shift in medicine, there will be a diabetes technology learning curve for our patients, caregivers and clinicians regarding the interpretation and use of diabetes technology tools and data to help modify behaviors, enhance the ability to self-adjust therapy, and help our patients decide when to seek medical assistance. The system will continuously evolve to match the needs of clinicians and people with diabetes and to reduce the burden of disease. The highly adaptable systems will allow patient-centered, customized diabetes treatment defying the one-size fits all approach. Moreover, the systems will adapt not only to the patient’s characteristics but also to the external disruptors such as exercise. The new generation ultra-fast acting insulins [44, 45••, 46] and their incorporation into the artificial pancreas system (also known as the closed-loop system or bionic pancreas) will be an essential step to achieve target after meal blood glucose control and will ultimately lower the HbA1c levels to a desirable target range. The ongoing research to miniaturize insulin delivery and glucose detection systems suggests that technology will continue to offer boundless potential to improve diabetes management.
Conclusions
The importance of stringent glycemic control to reduce acute and chronic complications of diabetes has been well proven and accepted. However, despite the knowledge of its importance, it remains an unattainable goal with 70% of patients with T1D failing to achieve target glycemic control [47•]. There is an urgent need to improve diabetes management with new tools and methods. Technology has transformed the way we live, and its effect on our daily life has been increasing exponentially. Isolating diabetes management from technology in this era is an unrealistic and short-sighted expectation.
Recent advances in diabetes technology with smart insulin pumps and CGM engineered around patient and clinician needs have the potential to overcome the diabetes treatment challenge for clinicians and patients. Data from a multinational database registry demonstrated that on average the HbA1c levels were significantly lower by 0.5 points among patients receiving insulin pump therapy as compared to patients on MDI supporting research studies with real-life clinical data [48]. The results from the SAP therapy and CGM use with MDI therapy have already demonstrated significant improvements in glycemic control and reduction in hypoglycemia for patients with diabetes. In addition, diabetes technology early hypoglycemia detection and prevention function and could be a key safety net for people with hypoglycemia unawareness especially in the high-risk older age patients with diabetes [49,50,51].
There has been a significant increase in CGM users for the past 5 years [36••], and the numbers are expected to increase even further after the FDA approval of the first hybrid closed-loop system. The 2016 ESG have been an essential resource for clinicians highlighting key factors and pragmatic methods in implementing insulin pumps and CGMs. The importance of patient involvement, motivation, education and overall support in making the decision to transition from MDI to the insulin pump are highlighted, emphasizing the role of team management in diabetes. The recommendations by the ESG broaden the treatment scope of insulin pumps and CGM to both T1D and T2D management and to inpatient and outpatient settings.
The integration of insulin pump, CGM, and algorithm-based insulin delivery to adjust the insulin dose based on CGM values, namely the artificial pancreas system, has become the new generation insulin treatment and caused a paradigm shift in diabetes management. The first-generation diabetes technology systems will evolve as we gain more experience and collaboratively work to improve them over the course of years with an ultimate goal of keeping people with diabetes complication and burden-free until the cure becomes a reality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76.
•• Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–20. The results of the landmark clinical trial demonstrated that sensor-augmented pump therapy improves glycemic control significantly in adult and pediatric subjects with inadequately controlled type 1 diabetes as compared to insulin therapy.
Panel FCCaCTD. FDA Executive Summary. Dexcom G5 Mobile Continuous Glucose Monitoring System, Dexcom, Inc. July 21, 2016 meeting of the Clinical Chemistry and Clinical Toxicology Devices Panel P120005/S041.
Peters AL, Ahmann AJ, Battelino T, Evert A, Hirsch IB, Murad MH, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3922–37.
Reznik Y, Cohen O. Insulin pump for type 2 diabetes: use and misuse of continuous subcutaneous insulin infusion in type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S219–25.
Reznik Y. Continuous subcutaneous insulin infusion (CSII) using an external insulin pump for the treatment of type 2 diabetes. Diabetes Metab. 2010;36(6 Pt 1):415–21.
Reznik Y, Huang S. Group OmS. Reductions in A1C with pump therapy in type 2 diabetes are independent of C-peptide and anti-glutamic acid decarboxylase antibody concentrations. Diabetes Technol Ther. 2014;16(11):816–8.
Reznik Y, Cohen O, Aronson R, Conget I, Runzis S, Castaneda J, et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet. 2014;384(9950):1265–72.
Pickup JC, Reznik Y, Sutton AJ. Glycemic control during continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 2 diabetes: individual patient data meta-analysis and meta-regression of randomized controlled trials. Diabetes Care. 2017;40(5):715–22.
(ICER) IFCaER. Controversies in the Management of Patients with Type 2 Diabetes. 2014.
Berthe E, Lireux B, Coffin C, Goulet-Salmon B, Houlbert D, Boutreux S, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224–9.
Boyle ME, Seifert KM, Beer KA, Apsey HA, Nassar AA, Littman SD, et al. Guidelines for application of continuous subcutaneous insulin infusion (insulin pump) therapy in the perioperative period. J Diabetes Sci Technol. 2012;6(1):184–90.
Boyle ME, Seifert KM, Beer KA, Mackey P, Schlinkert RT, Stearns JD, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol. 2012;6(5):1016–21.
Morviducci L, Di Flaviani A, Lauria A, Pitocco D, Pozzilli P, Suraci C, et al. Continuous subcutaneous insulin infusion (CSII) in inpatient setting: unmet needs and the proposal of a CSII unit. Diabetes Technol Ther. 2011;13(10):1071–4.
Houlden RL, Moore S. In-hospital management of adults using insulin pump therapy. Can J Diabetes. 2014;38(2):126–33.
Lansang MC, Modic MB, Sauvey R, Lock P, Ross D, Combs P, et al. Approach to the adult hospitalized patient on an insulin pump. J Hosp Med. 2013;8(12):721–7.
Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol. 2012;6(5):995–1002.
Kopecký P, Mráz M, Bláha J, Lindner J, Svačina S, Hovorka R, et al. The use of continuous glucose monitoring combined with computer-based eMPC algorithm for tight glucose control in cardiosurgical ICU. Biomed Res Int. 2013;2013:186439.
O'Connell MA, Donath S, O'Neal DN, Colman PG, Ambler GR, Jones TW, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–7.
Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2.
Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795–800.
Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32–8.
•• Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–8. The randomized clinical multi-center trial results demonstrated the improvement in glycemic control for subjects with T1D using MDI therapy with CGM as compared to no CGM use
Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–87.
•• Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C, Group DS. Continuous Glucose Monitoring in Older Adults With Type 1 and Type 2 Diabetes Using Multiple Daily Injections of Insulin: results From the DIAMOND Trial. J Diabetes Sci Technol. 2017:1932296817704445. The subgroup analysis from the DIAMOND Trial showed favorable impact of CGM use in improving HbA1c in subjects with T1D and T2D on MDI as compared to non-CGM use while managing diabetes.
Holzinger U, Warszawska J, Kitzberger R, Wewalka M, Miehsler W, Herkner H, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33(3):467–72.
Agus MS, Wypij D, Hirshberg EL, Srinivasan V, Faustino EV, Luckett PM, et al. Tight glycemic control in critically ill children. N Engl J Med. 2017;376(8):729–41.
Dadlani V, Kudva YC. Continuous glucose monitor use and accuracy in hospitalized patients. Diabetes Technol Ther. 2016;18(8):449–51.
Cengiz E, Sherr JL, Weinzimer SA, Tamborlane WV. New-generation diabetes management: glucose sensor-augmented insulin pump therapy. Expert Rev Med Devices. 2011;8(4):449–58.
Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–32.
Buckingham BA, Raghinaru D, Cameron F, Bequette BW, Chase HP, Maahs DM, et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38(7):1197–204.
Pettus J, Edelman SV. Differences in use of glucose rate of change (ROC) arrows to adjust insulin therapy among individuals with type 1 and type 2 diabetes who use continuous glucose monitoring (CGM). J Diabetes Sci Technol. 2016;10(5):1087–93.
Wolpert HA. The nuts and bolts of achieving end points with real-time continuous glucose monitoring. Diabetes Care. 2008;31(Suppl 2):S146–9.
Wolpert H. Establishing a continuous glucose monitoring program. J Diabetes Sci Technol. 2008;2(2):307–10.
•• Foster NC, Miller KM, Tamborlane WV, Bergenstal RM, Beck RW, TID Exchange Network. Continuous glucose monitoring in patients with type 1 diabetes using insulin injections. Diabetes Care. 2016;39(6):e81–2. The TIDExchange Registry data suggest that CGM can be beneficial for insulin injection users across all age-groups to achieve optimized metabolic control of TID.
Administration USFD. The 670G system—P160017—approval letter. 2016.
•• Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–63. The study by Garg et al. presents findings from the first FDA approved hybrid closed-loop system in the USA
Jazowski SA, Winn AN. The role of the FDA and regulatory approval of new devices for diabetes care. Curr Diab Rep. 2017;17(6):40.
Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–12.
Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2016.
Thabit H, Elleri D, Leelarathna L, Allen J, Lubina-Solomon A, Stadler M, et al. Unsupervised overnight closed loop insulin delivery during free living: analysis of randomised cross-over home studies in adults and adolescents with type 1 diabetes. Lancet. 2015;385(Suppl 1):S96.
Brown SA, Breton MD, Anderson SM, Kollar L, Keith-Hynes P, Levy CJ, et al. Overnight closed loop control improves glycemic control in a multicenter study of adults with type 1 diabetes. J Clin Endocrinol Metab. 2017.
Cengiz E. Closer to ideal insulin action: ultra fast acting insulins. Panminerva Med. 2013;55(3):269–75.
•• Cengiz E, Bode B, Van Name M, Tamborlane WV. Moving toward the ideal insulin for insulin pumps. Expert Rev Med Devices. 2016;13(1):57–69. The overview of insulin analog use in pumps, findings from the innovative ultra-fast acting insulin analog research and their integration in to the closed-loop system are summarized
Cengiz E. Undeniable need for ultrafast-acting insulin: the pediatric perspective. J Diabetes Sci Technol. 2012;6(4):797–801.
• Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8. The T1D Exchange Registry data by Miller et al. investigated the current status of glycemic control in subjects with T1D in the USA and revealed that 70% of patients have inadequate glycemic control
Blackman SM, Raghinaru D, Adi S, Simmons JH, Ebner-Lyon L, Chase HP, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15(8):564–72.
Weinstock RS, Xing D, Maahs DM, Michels A, Rickels MR, Peters AL, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. 2013;98(8):3411–9.
Weinstock RS, Schütz-Fuhrmann I, Connor CG, Hermann JM, Maahs DM, Schütt M, et al. Type 1 diabetes in older adults: comparing treatments and chronic complications in the United States T1D Exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract. 2016;122:28–37.
DuBose SN, Weinstock RS, Beck RW, Peters AL, Aleppo G, Bergenstal RM, et al. Hypoglycemia in older adults with type 1 diabetes. Diabetes Technol Ther. 2016;18(12):765–71.
Acknowledgments
This manuscript is made possible by grants from International Society for Pediatric and Adolescent Diabetes (ISPAD), Juvenile Diabetes Research Association International (JDRF), and The Leona M. and Harry B. Helmsley Charitable Trust. We would like to thank Ela Pinar for her assistance in text formatting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alfonso Galderisi received research device support from Dexcom, Inc., USA.
Elise Schlissel has no conflict of interest.
Eda Cengiz is a speaker for NovoNordisk and serves on the advisory board for Novo Nordisk, MannKind, Adocia, and Lexicon.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Galderisi, A., Schlissel, E. & Cengiz, E. Keeping Up with the Diabetes Technology: 2016 Endocrine Society Guidelines of Insulin Pump Therapy and Continuous Glucose Monitor Management of Diabetes. Curr Diab Rep 17, 111 (2017). https://doi.org/10.1007/s11892-017-0944-6
Published:
DOI: https://doi.org/10.1007/s11892-017-0944-6