Abstract
Mobile and smartphone (mHealth) technologies have the potential to improve diabetes care and self-management, but little is known about their effectiveness and how patients, providers, and payers currently interact with them. We conducted a systematic review and found only 20 peer-reviewed articles, published since 2010, with robust evidence about the effectiveness of mHealth interventions for diabetes. The majority of these interventions showed improvement on primary endpoints, such as HbA1c; mHealth technologies that interacted with both patients and providers were more likely to be effective. There was little evidence about persistent use by patients, use by a patient’s health care provider, or long-term effectiveness. None of the studies discussed regulatory oversight of mHealth technologies or payer reimbursement for them. No robust studies evaluated the more than 1100 publicly available smartphone apps for diabetes. More research with valid study designs and longer follow-up is needed to evaluate the impact of mHealth technologies for diabetes care and self-management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread adoption of mobile phones and smartphones provides a promising opportunity to improve diabetes care and self-management. Telehealth administered through mobile devices (mHealth) allows health providers to exchange information with patients or offer services such as direct care, education, or remote monitoring [1]. The approach may be particularly effective for patients with diabetes given that successful disease management often relies on adherence to complex care regimens such as blood glucose monitoring, medications, and secondary preventive testing, which may benefit from reminders and behavioral reinforcements.
mHealth technologies are potentially accessible to many patients; in 2014, 90 % of Americans used mobile phones and 64 % used smartphones [2]. Rates of cell phone ownership are similar across racial and ethnic groups and between communities with different levels of geographic access to health care (i.e., urban, suburban, and rural) [2]. If effective, mHealth technologies could help to reduce disparities in diabetes self-management, care, and outcomes in underserved communities [3]. Furthermore, some experts believe that telehealth has the potential to facilitate other key healthcare quality priorities such as safety, effectiveness, patient-centeredness, and timely, efficient care [4•].
The $1.3 billion mHealth industry is growing rapidly and is projected to reach $20 billion by 2018 [5]. In 2014, 19 % of US adults used at least one health-related mobile application (“app”) [5] and there are over 1100 diabetes-related smartphone apps currently available [5]. Despite their widespread availability, little is known about the effectiveness of mHealth interventions for diabetes care and self-management. Most mHealth apps are not evaluated for effectiveness and lack regulatory oversight [6, 7]. Previous reviews of the mHealth literature are outdated and largely focus on text messaging interventions rather than features currently available in smartphone apps [6]. Furthermore, little is known about the adoption and continuing use of mHealth technologies by patients, providers, and payers. Some evidence suggests that mHealth technologies are more effective when they interact with both patients and providers [8], and that providers are more likely to use mHealth if reimbursed by payers [9]. Of concern, analyses of other health monitoring mHealth technologies, such as wearable fitness trackers, have reported a high degree of discontinuation after 6 months [10••, 11].
In this article, we review the recent peer-reviewed literature on mHealth interventions for diabetes care and management. Our objectives are to summarize how patients, providers, and payers use mHealth and to examine evidence of effectiveness of current mHealth technologies. We also provide an overview of payer reimbursement for, and regulatory oversight of, mHealth technologies and recommendations for future research.
Methods
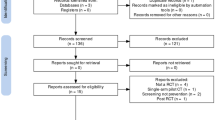
We searched PubMed for articles that included “mobile phone,” “smartphone,” or “smart phone” and “diabetes” and found 359 articles published between 2002 and April 2015. We excluded non-English articles, articles of mHealth interventions in low- and middle-income countries, study protocols, and articles that did not include an mHealth technology for diabetes (n = 77). Given the rapid advancements in technology over the last 5 years, we also excluded articles published prior to 2010 (n = 70). Our review included 212 articles.
We reviewed the abstracts of the 212 articles to provide an overview of the types of mHealth technologies for diabetes represented in the literature. For each article, we noted the year of publication and type of article (i.e., commentary/narrative, description of technology, study evaluating an mHealth technology, or review of studies). We identified which studies used a robust study design, according to the widely adopted criteria used in Cochrane Collaboration systematic reviews (i.e., randomized controlled trials (RCTs), controlled before-after studies, or interrupted time series studies with at least three time points before and after the intervention [12]), that can provide valid evidence about effectiveness. For these robust studies, we collected the following information about the mHealth technology: its description and function, including whether it functions on a mobile phone or smartphone; whether it has regulatory clearance; whether the technology is patient-facing; whether the technology interacts with providers and/or health delivery systems; whether the technology interacts with or is reimbursed by payers; the length of follow-up time and rate of discontinuation during the study; and, the reported effectiveness of the technology.
Results
Overview of Types of mHealth Technologies for Diabetes in the Literature
The mHealth literature has grown rapidly over the last 5 years, tripling from 18 articles published in 2010 to 54 articles in 2014 (Fig. 1). The majority of the 212 articles from 2010 to mid-2015 described or evaluated patient-facing mobile phone systems and smartphone apps for patient self-management or remote monitoring, including self-monitoring of glucose, medication adherence, diet, and physical activity. Some of these technologies can interact with providers. Many of the interventions relied on automated text messaging (either on mobile or smartphones) to provide prompts for monitoring and medication, appointment reminders, and diabetes education. Many articles described or evaluated smartphone apps for self-monitoring of blood glucose, while fewer focused on apps that record other measurements such as blood pressure or insulin doses. Diet-related smartphone apps described in the literature included food/carbohydrate diaries that work either through manual entry or capturing photos of meals. Physical activity smartphone apps often made use of built-in motion sensors or accelerometers to track movement; one app also used reminders to reduce sedentary time.
Other mHealth approaches make use of specific smartphone technologies. There are a small number of provider-facing, store and forward (i.e., data are stored and can be viewed or acted upon at a later time, also known as “asynchronous”) diagnostic apps that leverage smartphone cameras for retinal screening, wound assessment, or albumin testing. Some apps run built-in algorithms that provide real-time feedback. A few articles described continuous glucose measurement systems linked with an insulin pump (i.e., artificial pancreas) that operate on a closed-loop smartphone platform, or bolus calculator apps in development, which allow patients to self-titrate insulin doses. Very few articles described the use of real-time (i.e., synchronous) video-conferencing capabilities on smartphones.
Current Evidence on the Effectiveness of mHealth Interventions for Diabetes
Most of the 212 articles included in our review did not provide robust evidence on the effectiveness of the diabetes mHealth technologies they described (Fig. 2). A total of 91 (43 %) articles were descriptive in nature, including 71 (33 %) that described a technology or app and/or tested its accuracy, and 20 (9 %) that provided a narrative overview of mHealth technologies. While nearly half (n = 97, 46 %) of the articles were studies that examined the effectiveness of mHealth interventions for improving diabetes outcomes, only 20 (9 % of all articles and 21 % of studies) used robust study designs—all 20 were small- to medium-sized randomized controlled trials (sample sizes ranged from 30 to 276, median = 118). The remaining studies utilized nonexperimental cross-sectional, post-only, or pre-post with no control designs, or they used pre-post with control designs but had biased control groups (i.e., subjects self-selected into treatment or control groups) and/or were pilot studies with a very small sample size (n < 30). A surprisingly large number (24, 11 %) of articles described reviews of studies. However, only nine of these reviews limit inclusion to studies that used robust research designs and the majority of articles included in these reviews were published prior to 2010, when the mHealth industry was substantially different from today.
Number of diabetes mHealth articles by type of article (n = 212). “Robust studies” utilized one of the following study designs: randomized controlled trials (RCTs), controlled before-after (CBA) studies, or interrupted time series (ITS) studies with at least three time points before and after the intervention
Robust Studies
Sixteen unique mHealth interventions were evaluated in the 20 robust studies (Appendix). Half (n = 7 interventions, evaluated in ten studies) of the 16 interventions were for patient self-management of blood glucose [13–22]; all consisted of systems that analyzed patients’ self-measured glucose and most provided real-time feedback via text messaging. The remaining studies tested interventions for general diabetes education and self-management (n = 3) [23–25]; medication adherence (n = 1 intervention evaluated in 2 studies) [26, 27]; self-management of weight (n = 1) [28]; self-management of depression (n = 1) [29]; self-management of blood pressure (n = 1) [30], self-management of blood glucose, blood pressure, body weight, and physical activity (n = 1) [31]; and text reminders for gestational diabetes patients to receive a post-partum oral glucose tolerance test (OGTT) (n = 1) [32]. Most (n = 9) of the 16 interventions were smartphone-based; the remaining 6 included text-based interventions that could be utilized on mobile or smartphones. Only 5 of the 16 technologies were studied in the USA.
The 16 mHealth interventions that were evaluated were all patient-facing. Although study participants were usually recruited in outpatient or hospital settings, the technologies were all intended to be used by patients in the community, and not in medical settings. While over half (n = 10) of the interventions allowed interaction with providers (e.g., diabetes educators, diabetes nurses, nurse practitioners, or physicians), none of the studies involved use of mobile or smartphones by providers. Communication or feedback from providers was mainly via web-based servers, presumably accessed on a computer and not a mobile device. Provider interaction in these studies generally did not reflect real world settings—eight out of the ten technologies with provider interaction involved providers from the study team. While four of the technologies had the capability to interact with a patient’s primary care physician via data reports or alerts for abnormal values from self-measurement, there was no evidence provided on whether and how real-world physicians actually used the technologies. None of the studies mentioned provider reimbursement from payers, although one intervention did explicitly pay primary care providers ($250 per patient enrolled in the study) for their time to interact with the technology. None of the studies mentioned regulatory approval (e.g., FDA) for the technologies.
The majority (n = 11, represented in 14 studies) of the 16 mHealth interventions were effective in improving primary outcomes over follow-up periods that were generally less than a year (median = 7 months, range = 2–24 months). HbA1c was evaluated as the primary outcome for ten interventions (represented in 12 studies); seven of these interventions (represented in eight studies) found that the mHealth intervention significantly reduced HbA1c levels by an average of 0.83 % (range 0.2 to 1.4 %), from a baseline average A1c level of 8.9 % (range 7.1 to 9.9 %) (Table 1). However, these interventions aimed primarily at glucose self-management were rarely successful at improving other outcomes, such as patient-reported self-efficacy, quality of life, self-care, depression, or other clinical endpoints (e.g., blood pressure, BMI, cholesterol). The interventions to improve medication adherence, blood pressure, depressive symptoms, and general diabetes education and self-management all reported statistically significant impacts on primary outcomes, but the weight management intervention and the text reminder system for patients with gestational diabetes did not. As with the glucose self-management interventions, very few of these interventions had an impact on secondary outcomes. While most studies (n = 17 out of 20) reported participant attrition (i.e., loss-to-follow-up or drop out), the persistence of mHealth technology use during the follow-up period was generally not reported. The three studies which examined actual use of the mHealth technology during the study period found high rates of discontinuation among participants: 32 % discontinued use within 2 months [24], 66 % were not active users for at least 6 months [17], and 38 % did not complete all six counseling sessions [29].
Of the 11 interventions that reported a positive impact on primary outcomes, 8 (73 %) actively engaged patients (via self-measurement, self-help education tutorials, or remote monitoring) and provided real-time feedback or reminders via text messaging and 8 (73 %) involved active interaction with study team providers or provided an avenue of communication with the patients’ primary care provider. Conversely, of the five interventions (six studies) that showed no impact, only three (60 %) actively engaged patients and one (20 %) involved interaction with providers (another intervention had very limited interaction—health counselors were available via text if patient initiated). Of the five interventions that showed no impact, two were smartphone-based and three used text messaging available on mobile or smartphones.
Robust Review Articles
Evidence from the nine robust review articles is mixed and generally outdated; most studies in the reviews were published before 2010 and therefore focus solely on mobile phone, and not smartphone, interventions. Unlike our review, these reviews did not exclude studies of technologies from low- and middle-income countries where the context of mHealth is quite different. Cochrane Collaboration members have done three reviews on diabetes mHealth interventions and found that these interventions have limited effectiveness. A 2012 Cochrane review of mobile phone messaging for facilitating management of chronic illnesses found that text-messaging interventions for diabetes (n = 4 studies, all published prior to 2010) had no impact on clinical outcomes (e.g., HbA1c, diabetic complications, and weight) or disease knowledge, but did improve self-management capacity and the number of glucose test results reported to the provider [33]. Another Cochrane review in 2012 on the use of mobile phone messaging for preventive health found no robust studies that evaluated the use of text messaging for prevention of diabetes [34]. A 2013 Cochrane review and associated meta-analysis found that mobile phone self-management interventions for adults with type 2 diabetes (n = 3 studies, all published in 2011 or earlier) improved HbA1c levels, but had no impact on other outcomes such as depression, quality of life, blood pressure, serum lipids, or weight [35•, 36]. Two other reviews examining the impact of mobile phone interventions on glycemic control (n = 22 studies, all published in 2010 or earlier [37], and n = 10, six published in 2010 or earlier [38], respectively) found that the interventions significantly reduced HbA1c levels, and that the interventions with the greatest impact involved patient feedback and a role for providers [8]. A 2013 review of mobile phone and text messaging interventions to promote physical activity (n = 3 studies, all published in 2011 or earlier) found that the interventions had no significant impact [39]. To date, there have been no other reviews of smartphone app effectiveness (as distinct from mobile phone interventions) for diabetes self-management and care.
Discussion
Mobile phone and smartphone (mHealth) technologies to improve diabetes outcomes are increasingly available and generally include tools for glucose self-monitoring, medication adherence, diet, or physical activity. The results of the few (n = 20) robust studies of mHealth technologies for diabetes show promising short-term results—11 of the 16 mHealth interventions evaluated in these studies showed significant improvement on primary endpoints, including HbA1c (seven interventions) and medication adherence, depressive symptoms, blood pressure, or self-management tasks (one intervention each). Although HbA1c was the most common outcome in the studies we reviewed, and many studies showed clinically significant improvement in HbA1c level, there is debate about whether glycemic control is appropriate as a sole endpoint because changes might not reflect important patient-centered outcomes [17]. Most of the glycemic control interventions in our review were not effective at improving secondary endpoints such as quality of life or depression. However, the few interventions that targeted other outcomes (e.g., depression and medication adherence) were generally effective. Essential evidence about persistence in use of mHealth technologies for diabetes and their long-term impacts is missing.
Similar to an earlier review by Mulvaney et al. [8], we found that mHealth interventions with active patient engagement and a role for providers were more likely to be effective. However, provider interaction with mHealth technologies in the studies we reviewed was largely limited to providers on the study team. There is a general lack of evidence regarding use of these technologies by providers in the community and none of the articles mentioned payers’ role in reimbursing providers to use the mHealth technologies. The use of mHealth for care and management is quite low in real-world clinical settings and provider reimbursement (i.e., payment) is a major barrier to the adoption of telehealth technologies [1, 9]. A recent Commonwealth Fund survey of community health centers and clinics in the USA found that one quarter (27 %) of centers used cell phones in care delivery (not specific to diabetes) [9] and that cell phones were primarily used for appointment reminders rather than disease management or health promotion activities. Community health center providers’ top four barriers to implementation of mobile phone interventions for these functions were as follows: lack of resources to finance the initial investment in mHealth technologies; limited human and technical resources; challenges of integration with electronic health records and other health information technology infrastructure; and lack of incentives or reimbursement for adoption and use [9].
Payer Reimbursement of mHealth Technologies
Medicare, the largest public payer in the USA and the standard-setter for many private payers, provides very limited reimbursement of telehealth services and has no mechanisms to pay providers for interacting with the vast majority of the mHealth technologies in this review. Medicare has expanded telehealth coverage over the last two decades [4•] but still almost completely limits reimbursement to “face-to-face”/“clinic-to-clinic” (i.e., real time) video consultations for patients from rural geographical areas [40–42]. If passed, the 2015 Telehealth Enhancement Act (H.R. 2066) would reduce geographic restrictions for telehealth and cover services received in a patient’s home [4•, 43, 44]. The bill would provide reimbursement for remote monitoring, evaluation, and management of diabetes as well as congestive heart failure and chronic obstructive pulmonary disease. Depending on implementation, the law may allow for reimbursement for care delivered through smartphone apps and non-real-time “store and forward” technologies.
States also play a large role in the reimbursement of telehealth. Forty-five states provide reimbursement for certain telehealth services through Medicaid [45], and 20 states mandate that private insurers in the state cover telehealth service [46]. Half (25) of states allow patients to be at home while receiving telehealth services [47] and most (41) do not place geographical restrictions. Like Medicare, coverage of face-to-face video conferencing is widely reimbursed; however, there is substantial variation in coverage for remote monitoring services and store and forward technologies [45, 47].
In addition to broader reimbursement for telehealth by states and Medicare, the movement toward outcomes-based payment models, such as accountable care organizations (ACOs) and patient-centered medical homes (PCMH), might encourage investment in mHealth in an attempt to promote efficient, high quality care and management for chronically ill patients [1].
Regulatory Oversight of mHealth Technologies
Provider use and payer reimbursement for mHealth interventions may also increase if a regulatory agency (e.g., the US Food and Drug Administration) validates the safety and effectiveness of these technologies. None of the robust studies in our review mentioned regulatory clearance or approval for the mHealth technologies. Most of the technologies in our review fall under the FDA’s definition of “mobile apps” and not “medical mobile apps” which are considered medical devices requiring FDA approval [48]. According to the FDA, “mobile apps” can help patients self-manage their disease, provide tools to track health information, provide health-related information, and communicate their condition with health care providers, but they cannot provide specific treatment suggestions [48]. While personalized feedback and prompting behavior “appear to be critical components of behavior change” [36], it was uncommon for the apps to use self-monitoring data to provide tailored feedback to patients (e.g., insulin dosing in response to glucose levels) likely because that capability would qualify the app as a medical mobile app requiring regulatory oversight. As of 2013, there was only one FDA-approved app for diabetes management [6].
Research on mHealth Technologies for Diabetes: Challenges and Future Directions
Payers and providers would be more likely to endorse mHealth technologies if there was strong evidence of effectiveness and cost savings. Our review found that the vast majority (79 %) of peer-reviewed studies published in the last 5 years did not provide adequate evidence about mHealth effectiveness. There were no robust studies on the impact of provider-facing apps (i.e., diagnostic apps that use smartphone cameras), and there were no articles that described or evaluated provider-facing decision support tools or clinical information resources that are available on smartphones (e.g., UpToDate®). Also, although over 1100 consumer-facing diabetes smartphone apps are on the market, none of these stand-alone apps have been rigorously evaluated; only one robust study evaluated a publicly available app (Glucose Buddy), and the app was evaluated in conjunction with a provider engagement intervention.
It will be especially important to evaluate the impact of mHealth apps in disadvantaged populations. Despite the narrowing of disparities in ownership of mobile and smartphones, disparities in use of these technologies for health persist—racial/ethnic minorities, non-English speakers, older people, and people with lower levels of education are less likely to download mobile apps for health [49]. Only one of the 20 robust studies in our review evaluated the impact of an mHealth technology in a low-income, urban population and only two focused on older adults.
There are many challenges to assessing the impact of mHealth technologies for diabetes in real-world settings. Selection bias, which occurs when participants self-select into an intervention or control group, was a common problem in the majority of the nonrobust studies we reviewed. Participants who actively choose to use mHealth may have unique characteristics (e.g., greater motivation, faster pre-intervention acceleration of A1C levels, higher education level, or worse health status) that impact outcomes. Self-selection bias can be minimized by using robust study designs such as RCTs, interrupted time series, or controlled before and after designs [50]. Regression to the mean was another potential problem in the nonrobust studies we reviewed. For instance, many studies of glycemic control interventions select patients with high HbA1c levels. Without a valid control group, it is impossible to know if HbA1c reductions are due to the intervention or regression to the mean. Even with robust controlled study designs, problems such as high participant attrition, contamination (i.e., participants in the control group get the intervention, which may be particularly problematic for publicly available apps), and concurrent interventions may bias study results. For instance, in a study of text reminders for OGTT sent to post-partum women with gestational diabetes, the majority of intervention and control group members also received postal reminders for post-partum OGTT from the Australian National Diabetes Registry program. Finally, authors and journals might be more likely to publish reports of positive intervention effects (i.e., publication bias [51]), so the generally positive effects reported in this review may overestimate the overall impact of mHealth technologies for diabetes.
A randomized controlled trial of a popular calorie counting and weight loss app (MyFitnessPal), in a primary care setting demonstrated the difficulty of evaluating apps in the real world. This app, which is not specific to diabetes and was therefore not included in our review, had no impact on weight, blood pressure, and most self-reported behaviors, likely due to low participant utilization [10••]. Almost half of participants stopped using the app after 1 month, two thirds were no longer using the app after 6 months, and those who continued to use the app did so with less frequency over time. In addition, 13 % of participants assigned to the control group used the app during the trial, including the person who used the app the most and lost the most weight. It is likely that rates of discontinuation are also high for diabetes-specific mHealth technologies—the few (n = 3) robust studies in our review that examined data on utilization showed high rates of abandonment. However, mHealth interventions may be a useful tool for highly motivated participants and utilization may increase with a clinician’s recommendation [10••].
Conclusions
Mobile and smartphone (mHealth) technologies are likely to have an important, but still undetermined role, in diabetes care and self-management. More research is needed to determine the effectiveness of these interventions across a range of possible clinical and patient-centered endpoints. The quality of research on the effectiveness of mHealth interventions for diabetes is poor—only 21 % of the studies in the literature provided valid evidence about the effectiveness of an mHealth intervention. However, the majority of the 20 robust studies in our review found that the mHealth interventions for diabetes were effective at improving short-term primary endpoints. Successful mHealth interventions will likely involve active patient engagement and a role for providers. If robust research demonstrates the long-term effectiveness of mHealth technologies in real-world populations, provider reimbursement or incentives to use mHealth technologies should be implemented as a general strategy for improving diabetes outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. 2012. http://iom.nationalacademies.org/Reports/2012/The-Role-of-Telehealth-in-an-Evolving-Health-Care-Environment.aspx.
Pew Research Center. Mobile Technology Fact Sheet. Oct 2014. http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
White RO, Beech BM, Miller S. Health care disparities and diabetes care: practical considerations for primary care providers. Clin Diabetes. 2009;27(3):105–12.
Daschle T, Dorsey ER. The return of the housecall. Ann Intern Med. 2015;162:587–8. This article describes the potential of telehealth and key barriers to its adoption by providers (reimbursement and licensure). Although the focus is on virtual visits, these issues are relevant to other mHealth inteventions for diabetes.
Istepanian R. Professor Robert Istepanian is a Guest Speaker at 75th Annual Scientific Sessions of the American Diabetes Association, Boston, MA. 2015. istepanian.co.uk/professor-robert-istepanian-is-a-guest-speaker-at-75th-annual-scientific-sessions-of-the-american-diabetes-association-boston-ma/.
Eng DS, Lee JM. The promise and peril of mobile health applications for diabetes and endocrinology. Pediatr Diabetes. 2013;14:231–8.
Gray LJ, Leigh T, Davies MJ, Patel N, Stone M, Boner M, et al. Systematic review of the development, implementation and availability of smart-phone applications for assessing type 2 diabetes risk. Diabet Med. 2013;30:758–60.
Mulvaney SA, Ritterband LM, Bosslet L. Mobile intervention design in diabetes: review and recommendations. Curr Diab Rep. 2011;11:486–93.
The Commonwealth Fund. Mobile Health and Patients Engagement in the Safety Net: A Survey of Community Health Centers and Clinics. 2015. http://www.commonwealthfund.org/publications/issue-briefs/2015/may/mobile-health-and-patient-engagement-in-the-safety-net.
Laing BY, Mangione CM, Tseng C-H, Leng M, Vaisberg E, Mahida M, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014;161:S5–12. Although not specific to diabetes, this article provides an example of a robust evaluation of an mHealth technology and describes challenges relevant to the evaluation of diabetes mHealth interventions.
Ledger D. Inside wearables – part 2. Endevour Partners. July 2014. http://endeavourpartners.net/assets/Endeavour-Partners-Inside-Wearables-Part-2-July-2014.pdf.
Higgins JPT, Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934–42. doi:10.2337/dc11-0366.
Lim S, Kang SM, Shin H, Lee HJ, Won Yoon J, Yu SH, et al. Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system. Diabetes Care. 2011;34:308–13. doi:10.2337/dc10-1447.
Charpentier G, Benhamou PY, Dardari D, Clergeot A, Franc S, Schaepelynck-Belicar P, et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial(TeleDiab 1 Study). Diabetes Care. 2011;34:533–9.
Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. 2013;15:e235. doi:10.2196/jmir.2588.
Holmen H, Torbjornsen A, Wahl AK, Jenum AK, Smastuen MC, Arsand E, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, Part 2: one-year results from the Norwegian randomized controlled trial renewing health. JMIR Mhealth Uhealth. 2014;2:e57. doi:10.2196/mhealth.3882.
Torbjørnsen A, Jenum AK, Smastuen MC, Arsand E, Holmen H, Wahl AK, et al. A low-intensity mobile health intervention with and without health counseling for persons with type 2 diabetes, Part 1: baseline and short-term results from a randomized controlled trial in the Norwegian part of renewing health. JMIR Mhealth Uhealth. 2014;2:e52. doi:10.2196/mhealth.3535.
Quinn CC, Sareh PL, Shardell ML, Terrin ML, Barr EA, Gruber-Baldini AL. Mobile diabetes intervention for glycemic control: impact on physician prescribing. J Diabetes Sci Technol. 2014;8:362–70.
Quinn CC, Shardell MD, Terrin ML, Barr EA, Park D, Shaikh F, et al. Mobile diabetes intervention for glycemic control in 45-to 64-year-old persons with type 2 diabetes. J Appl Gerontol. 2014.
Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med. 2014;63:745–54.e6. doi:10.1016/j.annemergmed.2013.10.012.
Skrovseth SO, Arsand E, Godtliebsen F, Joakimsen RM. Data-driven personalized feedback to patients with type 1 diabetes: a randomized trial. Diabetes Technol Ther. 2015;17:482–9. doi:10.1089/dia.2014.0276.
Noh JH, Cho YJ, Nam HW, Kim JH, Kim DJ, Yoo HS, et al. Web-based comprehensive information system for self-management of diabetes mellitus. Diabetes Technol Ther. 2010;12:333–7. doi:10.1089/dia.2009.0122.
Bell AM, Fonda SJ, Walker MS, Schmidt V, Vigersky RA. Mobile phone-based video messages for diabetes self-care support. J Diabetes Sci Technol. 2012;6:310–9.
Huang JS, Terrones L, Tompane L, Dillon L, Pian M, Gottschalk M, et al. Preparing adolescents with chronic disease for transition to adult care: a technology program. Pediatrics. 2014;133:e1639–46. doi:10.1542/peds.2013-2830.
Vervloet M, van Dijk L, Santen-Reestman J, van Vlijmen B, van Wingerden P, Bouvy ML, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81:594–604. doi:10.1016/j.ijmedinf.2012.05.005.
Vervloet M, van Dijk L, de Bakker DH, Souverein PC, Santen-Reestman J, van Vlijmen B, et al. Short- and long-term effects of real-time medication monitoring with short message service (SMS) reminders for missed doses on the refill adherence of people with Type 2 diabetes: evidence from a randomized controlled trial. Diabet Med. 2014;31:821–8. doi:10.1111/dme.12439.
Patrick K, Norman GJ, Davila EP, Calfas KJ, Raab F, Gottschalk M, et al. Outcomes of a 12-month technology-based intervention to promote weight loss in adolescents at risk for type 2 diabetes. J Diabetes Sci Technol. 2013;7:759–70.
Nobis S, Lehr D, Ebert DD, Baumeister H, Snoek F, Riper H, et al. Efficacy of a web-based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: a randomized controlled trial. Diabetes Care. 2015;38:776–83. doi:10.2337/dc14-1728.
Logan AG, Irvine MJ, McIsaac WJ, Tisler A, Rossos PG, Easty A, et al. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60:51–7. doi:10.1161/HYPERTENSIONAHA.111.188409.
Waki K, Fujita H, Uchimura Y, Omae K, Aramaki E, Kato S, et al. DialBetics: a novel smartphone-based self-management support system for type 2 diabetes patients. J Diabetes Sci Technol. 2014;8:209–15.
Van Ryswyk EM, Middleton PF, Haque WM, Crowther CA. Postpartum SMS reminders to women who have experienced gestational diabetes to test for Type 2 diabetes: the DIAMIND randomized trial. Diabet Med. 2015. doi:10.1111/dme.12769.
de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12:CD007459.
Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database Syst Rev. 2012;12:CD007457. doi:10.1002/14651858.CD007457.pub2.
Pal K, Eastwood SV, Michie S, Farmer AJ, Barnard ML, Peacock R, et al. Computer-based diabetes self-management interventions for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;3:CD008776. doi:10.1002/14651858.CD008776.pub2. This Cochrane Review (and the associated meta-analysis [36]) provides the most robust previous review of mHealth technologies for diabetes. It includes mobile phone interventions but does not evaluate more recent smartphone technologies.
Pal K, Eastwood SV, Michie S, Farmer A, Barnard ML, Peacock R, et al. Computer-based interventions to improve self-management in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2014;37:1759–66. doi:10.2337/dc13-1386.
Liang X, Wang Q, Yang X, Cao J, Chen J, Mo X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28:455–63. doi:10.1111/j.1464-5491.2010.03180.x.
Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes. 2014;8:275–85. doi:10.1016/j.pcd.2014.03.004.
Connelly J, Kirk A, Masthoff J, MacRury S. The use of technology to promote physical activity in type 2 diabetes management: a systematic review. Diabet Med. 2013;30:1420–32. doi:10.1111/dme.12289.
Centers for Medicare & Medicaid Services. Department of Health and Human Services: Medicare Program; Revisions to payment policies under the physician fee schedule, clinical laboratory fee schedule & revisions to part B for CY 2014; Final rule. Federal Register. 2013. 78; Part II: 74229.
U.S Department of Health and Human Services. Health Information Technology: what are the reimbursement issues for telehealth? 2011. http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Telehealth/whatarethereimbursement.html.
Centers for Medicare & Medicaid Services. Physician fee schedule: details for title: CMS- 1600-FC. 2013. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1600-FC.html.
Discussion Draft: a Bill to amend title XVIII of the Social Security Act to provide for a phased in expansion of telehealth coverage under the Medicare program. 2014. https://s3.amazonaws.com/s3.documentcloud.org/documents/1225617/thomca-062-xml.pdf.
American Telemedicine Association. Rep. Thompson’s New Bill Supporting Medicare Telehealth Parity to Be Introduced July 7. 2015. http://www.americantelemed.org/news-landing/2015/07/01/rep.-thompson-s-new-bill-supporting-medicare-telehealth-parity-to-be-introduced-july-7#.VZribUaVmq4.
Robert J Waters: Center For Telehealth & e-Health Law. Medicaid Reimbursement: 2014 CteL Medicare Reimbursement Checklist for TelehealthProfessional Fees and Originating Site Facility Fees. 2014. http://ctel.org/expertise/reimbursement/medicaid-reimbursement/.
NCSL. State coverage for Telehealth Services. 2014. http://www.ncsl.org/research/health/state-coverage-for-telehealth-services.aspx.
Thomas L, Capistrant G. American Telemedicine Association: State Telemedicine Gaps Analysis. http://www.americantelemed.org/docs/default-source/policy/50-state-telemedicine-gaps-analysis---coverage-and-reimbursement.pdf?sfvrsn=10.
U.S. Food and Drug Administration. Mobile Medical Applications. Medical devices 2014.http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ConnectedHealth/MobileMedicalApplications/ucm255978.htm.
Bender MS, Choi J, Arai S, Paul SM, Gonzalez P, Fukuoka Y. Digital technology ownership, usage, and factors predicting downloading health apps among Caucasian, Filipino, Korean, and Latino Americans: the digital link to health survey. JMIR Mhealth Uhealth. 2014;2:e43.
Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. 2nd ed. Boston: Houghton Mifflin; 2001.
Chan A-W, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291(20):2457–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Laura F. Garabedian and Dennis Ross-Degnan report personal fees from the Hospital Corporation of America (HCA).
J. Frank Wharam declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Health Care Delivery Systems and Implementation in Diabetes
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
(DOCX 22.6 kb)
Rights and permissions
About this article
Cite this article
Garabedian, L.F., Ross-Degnan, D. & Wharam, J.F. Mobile Phone and Smartphone Technologies for Diabetes Care and Self-Management. Curr Diab Rep 15, 109 (2015). https://doi.org/10.1007/s11892-015-0680-8
Published:
DOI: https://doi.org/10.1007/s11892-015-0680-8