Abstract
Diabetic nephropathy (DN) is a major complication of diabetes mellitus (DM) affecting individuals with type 1 or type 2 DM and is the leading cause of chronic kidney disease and end-stage kidney disease (ESKD) in the USA. Estimates of disease burden are projected to increase, with prevalence of nearly one in five adults by 2050. The role of renin-angiotensin-aldosterone system (RAAS) inhibition in delaying the progression of DN utilizing angiotensin-converting enzyme inhibitors or angiotensin receptor blockers has been well established in multiple controlled trials. Given greater reduction of proteinuria with dual RAAS blockade compared to monotherapy alone, the potential benefit of dual therapy on progression of DN has been tested in three large randomized clinical trials. Unfortunately, results from these studies demonstrated lack of benefit of dual blockade on renal or cardiovascular outcomes in patients with diabetes. The overall objectives of this review are to provide both the rationale for dual blockade as potential therapy as well as review the literature of its use in patients with DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of diabetes mellitus (DM) has reached epidemic proportions [1]. Disease estimates are projected to increase, affecting nearly one in five US adults by 2050 [2]. Patients with DM suffer from both micro- and macro-vascular complications, such as cardiovascular disease, retinopathy, and nephropathy. Diabetic nephropathy (DN) is a major complication of DM affecting individuals with type 1 or type 2 DM and is the leading cause of chronic kidney disease and end-stage kidney disease (ESKD) in the USA [3]. Those diagnosed with DN are faced with a mean survival of 5–7 years, with excess mortality mainly attributed to cardiovascular complications [4]. Approximately 39 % of patients with chronic kidney disease have DM and 41–44 % of all new cases of ESKD are due to DN. The 5-year survival of diabetic patients on dialysis is only 34 % [5].
Early diagnosis of DM and early intervention are critical in delaying progression to ESKD [2]. Despite increases in the prevalence of DM in the last decade, it is reassuring that the incidence of DN has been declining since 2006 [5]. The recent decline in the incidence of DN has been attributed to better glycemic management, blood pressure control, and widespread use of renin-angiotensin-aldosterone system (RAAS) blockade [4, 6]. Given the underlying pathophysiology, there was hope that dual RAAS blockade could reduce progression even further. In this report, we review the role of RAAS in DN and examine the results of recent trials that evaluated the effect of dual RAAS blockade on hard clinical outcomes in patients with DM.
The Role of RAAS in Diabetic Nephropathy
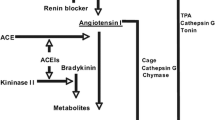
The pathogenesis of DN is complex and involves several mechanisms. The RAAS has a range of both hemodynamic and non-hemodynamic effects that contribute to the development of DN (Fig. 1). Renal hemodynamics are altered in DN [7], in large part due to activation of RAAS. Despite the decrease in plasma renin activity in DN, evidence suggests local activation of RAAS that is independent of systemic regulation [8–10]. Sustained hyperglycemia and advanced glycation end products (AGEs) stimulate the generation of tissue angiotensin II (Ang II). Ang II exerts its vasoconstrictive effects on the afferent and the efferent arterioles with an effect greater at the efferent arteriole. This leads to increase in intraglomerular pressure and a concomitant increase in hyperfiltration. In addition to its intraglomerular hemodynamic effects, Ang II enhances proximal sodium reabsorption, increases adrenal aldosterone production, and stimulates production of fibrogenic cytokine transforming growth factor beta (TGF-β) [11, 9, 12]. Ang II is central to the pathogenesis of DN and provides a therapeutic target.
Hemodynamic and non-hemodynamic effects of the RAAS system contribute to diabetic nephropathy (adapted from Tylicki L, Lizakowski S, Rutkowski B. Renin-angiotensin-aldosterone system blockade for nephroprotection: current evidence and future directions. J Nephrol. 2012 Nov-Dec;25(6):900–10) [42]
The non-hemodynamic consequences of RAAS activation in DN have been demonstrated in animal [10, 13] and human studies [9]. DM stimulates proximal tubule renin mRNA expression in streptozotocin-induced diabetic rats [10]. Independent of its effect on Ang II production, renin binds to mesenchymal and endothelial cell receptors and stimulates the production of TGF-β [8, 14]. Beyond its hemodynamic effects, increased production of Ang II [10] coupled with upregulation of angiotensin I receptors on the podocytes of diabetic kidneys results in loss of nephrin, podocyte hypertrophy, and at later stage apoptosis [15, 16]. In addition, Ang II alters the anionic charge of the glomerular basement membrane in DN by modulating heparin sulfate proteoglycan synthesis of the podocytes [17]. In the tubule, Ang II induces tubular cell hypertrophy [18] and tubular epithelial-myofibroblast transdifferentiation [19].
Other consequences of RAAS also contribute to the development of DN. Prorenin plasma levels are elevated in diabetic patients [20]. Prorenin is believed to contribute to glomerulosclerosis and renal fibrosis via stimulation of mitogenic and fibrotic factors independent of Ang II [21]. Aldosterone also has a pathogenic role in DN and DM is associated with increased aldosterone production [22]. In addition to its profibrotic properties, which involve stimulation of extracellular matrix production through increased TGF-β production [8] and collagen IV deposition [23], aldosterone is incriminated in the pathogenesis of inflammation in DN. In animal models, aldosterone infusion induces tubulointerstitial and glomerular leukocyte infiltration through upregulation of inflammatory cytokines, including monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and interleukin-1beta (IL-1β) [24], and generation of reactive oxygen species [25]. Treatment with aldosterone receptor blockers attenuates the renal fibrotic and inflammatory processes in animal models [26] and provides evidence of the pathogenic role of aldosterone in DN.
Dual RAAS Inhibition in Animals
Animal and human studies have demonstrated that RAAS blockade with angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) reduces proteinuria, controls hypertension, and slows progression of renal disease. However, ACEi and ARB monotherapy resulted in incomplete inhibition of the RAAS [27]. Experimental studies showed that chronic ACEi or ARB use was associated with an increase in aldosterone level, a concept called “aldosterone escape,” and a concomitant rise in Ang II production through ACE- independent pathways [28, 29].
Given the “aldosterone escape” observed with monotherapy, the effect of dual RAAS blockade on disease progression was evaluated in animal models of DN. In hypertensive rats, Ménard et al. first reported a synergistic effect between ACEi (benazepril) and ARB (valsartan) on left ventricular hypertrophy, diastolic dysfunction, and survival [29]. Cao et al. reported that combination therapy with ACEi (captopril) and ARB (irbesartan), in diabetic hypertensive rats, was more effective in reducing albuminuria, glomerulosclerosis, and hypertension, compared to monotherapy with these agents [30]. A recent study by Whaley-Connell et al. described a beneficial effect of combination with a renin inhibitor (aliskiren) and an ARB (irbesartan) on podocyte integrity, tubular injury, and markers of oxidative stress in transgenic rats [31]. In total, dual RAAS blockade decreased blood pressure, reduced albuminuria, and attenuated glomerulosclerosis and podocyte injury. However, the fidelity of these results in humans was unknown.
Dual Blockade—Preliminary Human Studies
Clinical trials in humans have also demonstrated potential benefits to dual RAAS blockade. Early clinical trials of dual RAAS blockade in diabetic patients showed a greater reduction in proteinuria compared to monotherapy with ACEi or ARB [32–34]. More recently, Mehdi et al. randomized 81 patients already receiving lisinopril 80 mg once daily to placebo, losartan 100 mg daily, or spironolactone 25 mg daily [35]. After 48 weeks, clinical and ambulatory blood pressures were similar between groups. Compared with placebo, urine albumin-to-creatinine ratio decreased by 34 % (P = 0.007) in the spironolactone group and by 17 % (P = 0.20) in the losartan group [35]. In a review of 49 randomized clinical trials of combination therapy with ACEi and ARB, in diabetic and non-diabetic proteinuric patients, combination therapy was more effective in reducing proteinuria compared to monotherapy alone [11]. Whether reductions in proteinuria utilizing dual blockade would translate into improved patient-level outcomes was yet to be realized.
Dual RAAS Blockade—Recent Large Clinical Trials
Despite the evidence that dual RAAS blockade decreases proteinuria, the question of whether this enhanced antiproteinuric effect would translate into better renal outcomes was unanswered until recently. Data on renal outcomes of dual RAAS blockade comes from three large randomized controlled trials (Table 1). The Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET), a large multicenter randomized placebo-control trial, randomized 25,584 patients with cardiovascular disease or DM to the ARB telmisartan, ACEi ramipril, or dual blockade with a combination of telmisartan and ramipril [36]. After a median follow-up of 56 months, there was no difference between the dual blockade arm and the ramipril arm with regard to the primary cardiovascular composite outcome [36]. However, dual blockade was associated with an increased risk for renal impairment despite greater reduction in proteinuria with dual blockade [36]. Dual blockade was also associated with increased risk for hyperkalemia [36]. Results for the primary renal outcome were similar between those with (N = 6982) and without DM [37]. It is worth noting that only 516 ONTARGET participants had overt DN [37].
The second large study to evaluate dual blockade was the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE) study, which randomized 8561 patients already receiving an ACEi or ARB to a renin inhibitor, aliskiren 300 mg daily or placebo [38••]. Over 33 months of follow-up, dual blockade with aliskiren and ACEi or ARB did not reduce the risk for a composite of ESRD, death from renal causes, or a doubling of serum creatinine. Treatment with aliskiren did increase risk for hyperkalemia and hypotension [38••]. As in ONTARGET, dual blockade in ALTITUDE resulted in a significant reduction in albuminuria compared to the control group (14 %, 95 % CI 11 to 17 %) [38••].
The most recent large randomized controlled trial to evaluate dual blockade was the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial [39••]. Patients with diabetes and a urine albumin-to-creatinine ratio of >300 mg/g were initiated on losartan 100 mg daily and then randomized to lisinopril or placebo [40]. The study was stopped after a median follow-up of 2.2 years in 1448 participants due to increased risk for hyperkalemia (hazard ratio [HR] 2.8, 95 % CI 1.8 to 4.3) and acute kidney injury (HR 1.7, 95 % CI 1.3 to 2.2) in the combination arm compared to the placebo arm [39••]. Again, no benefit to dual blockade was observed with regard renal or cardiovascular outcomes. VA NEPHRON-D results confirm the lack of benefit to dual blockade observed in ONTARGET and ALTITUDE but this time in a population with significant proteinuria.
Conclusions
Dual RAAS blockade is an attractive therapeutic strategy for patients with DN: The RAAS is involved in the pathogenesis of DN; there is aldosterone escape with monotherapy and dual therapy with reduced albuminuria and multiple markers of renal damage in animal models and short-term human trials. However, recent large randomized clinical trials have failed to demonstrate a benefit to dual blockade with regard to renal and cardiovascular outcomes. Additionally, dual blockade has increased risk for hyperkalemia and acute kidney injury. New agents to treat hyperkalemia may increase the feasibility of dual blockade, but further research is needed [41]. Finally, additional studies are needed to identify subgroups of patients who may benefit from dual blockade without the increased risk for adverse effects observed in these large trials.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi:10.1016/j.diabres.2009.10.007.
Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi:10.1186/1478-7954-8-29.
Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61(1 Suppl 1):A7, e1-476. doi:10.1053/j.ajkd.2012.11.031.
Andresdottir G, Jensen ML, Carstensen B, Parving HH, Hovind P, Hansen TW, et al. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney Int. 2015;87(2):417–26. doi:10.1038/ki.2014.206.
Cooper WC, Wong O, Graebner R. Mortality of workers in two Minnesota taconite mining and milling operations. J Occup Med. 1988;30(6):506–11.
Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med. 1994;330(1):15–8. doi:10.1056/NEJM199401063300103.
Anderson S, Vora JP. Current concepts of renal hemodynamics in diabetes. J Diabetes Complications. 1995;9(4):304–7.
Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70(11):1914–9. doi:10.1038/sj.ki.5001846.
Hollenberg NK, Price DA, Fisher ND, Lansang MC, Perkins B, Gordon MS, et al. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003;63(1):172–8. doi:10.1046/j.1523-1755.2003.00701.x.
Zimpelmann J, Kumar D, Levine DZ, Wehbi G, Imig JD, Navar LG, et al. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int. 2000;58(6):2320–30. doi:10.1046/j.1523-1755.2000.00416.x.
Rahimi Z, Moradi M, Nasri H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J Res Med Sci. 2014;19(11):1090–8.
Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest. 2005;35(5):330–6. doi:10.1111/j.1365-2362.2005.01497.x.
Nicholas SB, Mauer M, Basgen JM, Aguiniga E, Chon Y. Effect of angiotensin II on glomerular structure in streptozotocin-induced diabetic rats. Am J Nephrol. 2004;24(5):549–56. doi:10.1159/000082001.
Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III up-regulates genes involved in kidney damage in mesangial cells and renal interstitial fibroblasts. Kidney Int Suppl. 1998;68:S41–5.
Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15(6):1475–87.
Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294(4):F830–9. doi:10.1152/ajprenal.00266.2007.
Brinkkoetter PT, Holtgrefe S, van der Woude FJ, Yard BA. Angiotensin II type 1-receptor mediated changes in heparan sulfate proteoglycans in human SV40 transformed podocytes. J Am Soc Nephrol. 2004;15(1):33–40.
Wolf G, Ziyadeh FN. Renal tubular hypertrophy induced by angiotensin II. Semin Nephrol. 1997;17(5):448–54.
Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12(1):25–9. doi:10.1097/01.mnh.0000049812.98789.97.
Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312(22):1412–7. doi:10.1056/NEJM198505303122202.
Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, et al. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69(1):105–13. doi:10.1038/sj.ki.5000011.
Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LM, et al. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65(4):1435–9. doi:10.1111/j.1523-1755.2004.00524.x.
Kang YS, Ko GJ, Lee MH, Song HK, Han SY, Han KH, et al. Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol Dial Transplant. 2009;24(1):73–84. doi:10.1093/ndt/gfn448.
Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63(5):1791–800. doi:10.1046/j.1523-1755.2003.00929.x.
Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16(10):2906–12. doi:10.1681/ASN.2005040390.
Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17(5):1362–72. doi:10.1681/ASN.2005111196.
van den Meiracker AH, Man in ’t Veld AJ, Admiraal PJ, van Eck HJ R, Boomsma F, Derkx FH, et al. Partial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: does it exist and does it affect the antihypertensive response? J Hypertens. 1992;10(8):803–12.
Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70(12):2116–23. doi:10.1038/sj.ki.5001854.
Ménard J, Campbell DJ, Azizi M, Gonzales MF. Synergistic effects of ACE inhibition and Ang II antagonism on blood pressure, cardiac weight, and renin in spontaneously hypertensive rats. Circulation. 1997;96(9):3072–8.
Cao Z, Bonnet F, Davis B, Allen TJ, Cooper ME. Additive hypotensive and anti-albuminuric effects of angiotensin-converting enzyme inhibition and angiotensin receptor antagonism in diabetic spontaneously hypertensive rats. Clin Sci (Lond). 2001;100(6):591–9.
Whaley-Connell A, Habibi J, Nistala R, Hayden MR, Pulakat L, Sinak C, et al. Combination of direct renin inhibition with angiotensin type 1 receptor blockade improves aldosterone but does not improve kidney injury in the transgenic Ren2 rat. Regul Pept. 2012;176(1–3):36–44. doi:10.1016/j.regpep.2012.03.002.
Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440–4.
Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63(5):1874–80. doi:10.1046/j.1523-1755.2003.00940.x.
Sengul AM, Altuntas Y, Kürklü A, Aydin L. Beneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res Clin Pract. 2006;71(2):210–9. doi:10.1016/j.diabres.2005.06.010.
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2641–50. doi:10.1681/ASN.2009070737.
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–59. doi:10.1056/NEJMoa0801317.
Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–53. doi:10.1016/S0140-6736(08)61236-2.
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–13. doi:10.1056/NEJMoa1208799. The ALTITUDE study evaluated the effect of aliskiren on cardiovascular and renal outcomes compared to placebo in type 2 diabetic patients. This study demonstrates that aliskiren may increase risk for cardiovascular events, does not affect renal outcomes, but does increase risk for hyperkalemia.
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–903. doi:10.1056/NEJMoa1303154. This study involving type 2 diabetic patients with significant albuminuria was stopped early due to increased risk for hyperkalemia and acute kidney injury in the dual blockade arm. Dual blockade did not reduce risk for renal outcomes compared to losartan alone.
Fried LF, Duckworth W, Zhang JH, O’Connor T, Brophy M, Emanuele N, et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clin J Am Soc Nephrol. 2009;4(2):361–8. doi:10.2215/CJN.03350708.
Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–31. doi:10.1056/NEJMoa1411487.
Tylicki L, Lizakowski S, Rutkowski B. Renin-angiotensin-aldosterone system blockade for nephroprotection: current evidence and future directions. J Nephrol. 2012;25(6):900–10.
Compliance with Ethics Guidelines
Conflict of Interest
Boutros El-Haddad, Scott Reule, and Paul E. Drawz declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Microvascular Complications—Nephropathy
Rights and permissions
About this article
Cite this article
El-Haddad, B., Reule, S. & Drawz, P.E. Dual Renin-Angiotensin-Aldosterone System Inhibition for the Treatment of Diabetic Kidney Disease: Adverse Effects and Unfulfilled Promise. Curr Diab Rep 15, 70 (2015). https://doi.org/10.1007/s11892-015-0640-3
Published:
DOI: https://doi.org/10.1007/s11892-015-0640-3