Abstract
Purpose of Review
This review focuses on the use of colchicine to target inflammation to prevent cardiovascular events among those at-risk for or with established coronary artery disease.
Recent Findings
Colchicine is an anti-inflammatory drug that reduces cardiovascular events through its effect on the IL-1β/IL-6/CRP pathway, which promotes the progression and rupture of atherosclerotic plaques. Clinical trials have demonstrated that colchicine reduces cardiovascular events by 31% among those with chronic coronary disease, and by 23% among those with recent myocardial infarction. Its ability to dampen inflammation during an acute injury may broaden its scope of use in patients at risk for cardiovascular events after major non-cardiac surgery.
Summary
Colchicine is an effective anti-inflammatory therapy in the prevention of acute coronary syndrome. Ongoing studies aim to assess when, and in whom, colchicine is most effective to prevent cardiovascular events in patients at-risk for or with established coronary artery disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last several decades, there have been tremendous gains in the reduction of mortality and morbidity from coronary artery disease (CAD). Despite the control of traditional cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes mellitus, the prevalence of CAD remains high and cardiovascular disease remains the leading cause of death worldwide. A portion of this residual risk has been attributed to inflammation.
The progression of “vulnerable plaque” is related to neutrophil infiltration of the endothelium and the associated inflammatory response, which predisposes to plaque rupture [1, 2]. Studies have shown that C-reactive protein (CRP), a marker of such inflammation, is associated with the development of CAD, even among those on statin therapy with a low density lipoprotein (LDL)-cholesterol < 70 mg/dL, and that targeting inflammation via the IL-1β/IL-6/CRP pathway reduces cardiovascular events [3, 4, 5••]. Data from both observational studies and large randomized trials demonstrate colchicine, an anti-inflammatory medication long used for gout and pericarditis, is a safe, well-tolerated medication that reduces major adverse cardiovascular events among those with stable CAD and recent acute myocardial infarction (AMI) through its effects on the IL-1β/IL-6/CRP pathway [6, 7, 8••, 9••, 10].
Inflammation, Coronary Artery Disease, and Colchicine
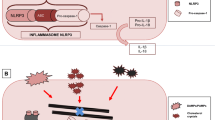
Inflammatory cells, including the most abundant white blood cells—neutrophils, play an integral role in the pathophysiology of CAD and AMI. Neutrophils are attracted to inflamed or injured endothelium via selectin molecules and adhere via integrin molecules before migrating into the endothelium. The NLRP3 inflammasome is then activated, which triggers the release of IL-1β and subsequently IL-6, the pre-curser to CRP [11]. Furthermore, serine proteases released by neutrophils inhibit the tissue factor pathway inhibitor, allowing thrombin (a potent activator of platelets) generation to go unchecked. These activated platelets may subsequently bind to other platelets or leukocytes to create aggregates within the circulation. This overall inflammatory process promotes plaque progression and rupture, and contributes to myocardial injury during ischemia and reperfusion [12].
CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was the first randomized trial to demonstrate how inhibition of the IL-1β pathway can prevent cardiovascular events. In this double-blind trial, 10,061 patients with a history of AMI and residual inflammation (high sensitivity-CRP [hs-CRP] ≥ 2 mg/L) were randomized to treatment with canakinumab (an anti-IL-1β antibody) or placebo [3]. Treatment with canakinumab led to a 15% reduction in non-fatal MI, non-fatal stroke, and cardiovascular death over 48 months of follow up, as well as reductions in IL-6 and hs-CRP concentrations. The lack of concomitant reduction in LDL concentration proved the hypothesis that the anti-inflammatory effects of the drug were responsible for the prevention in cardiovascular events. In a secondary analysis of the study, it was found that those who achieved a hs-CRP of < 2 mg/dL after receiving canakinumab had a 25% reduction in cardiovascular events, while no benefit was observed among those who did not achieve a hs-CRP < 2 mg/L [13]. This stratified analysis further supported the inflammatory hypothesis. Despite these encouraging findings, canakinumab use was also associated with an increase in fatal infections and sepsis (0.31 vs 0.18 events per 100 person-years, p = 0.02), and a cardiovascular label was never pursued by the sponsor. The connection between the IL-1β inflammatory pathway and CAD was further realized when the randomized, placebo-controlled CIRT (Cardiovascular Inflammation Reduction Trial) of 4,789 participants showed that methotrexate, which does not affect IL-1B, had no effect cardiovascular events or IL-6 or hs-CRP levels [5••].
Colchicine as a Therapeutic Option
Given canakinumab’s safety concerns, alternative therapeutic options that targeted the IL-1β pathway were sought. Colchicine, which was primarily used for the treatment of gout and familial Mediterranean fever, became a drug of interest. Colchicine has multiple effects on the inflammatory cascade including acting as an anti-mitotic agent; preventing migration, chemotaxis, and adhesion of neutrophils; and suppressing the NLRP3 inflammasome, which decreases IL-1β and IL-18 production, resulting in the downstream reduction of IL-6 and CRP [14, 15•, 16,17,18,19,20,21,22,23]. In vitro studies have also demonstrated colchicine’s anti-proliferative effect on smooth muscle cells as well as its anti-thrombotic effect [24, 25]. In animal models, colchicine has plaque stabilizing effects and decreases the inflammatory response induced by ischemia thereby reducing infarct size [17, 18, 26,27,28].
These findings were further confirmed in humans. An open-label pilot trial of 64 patients showed that in those with an elevated hs-CRP (≥ 2 mg/L), colchicine 0.5 mg twice a day reduced hs-CRP levels in those with stable CAD already on aspirin and high-intensity statin compared with those not on colchicine [29]. This dose of colchicine inhibited neutrophil-platelet aggregates but not platelet-to-platelet aggregation, thus suggestive of an anti-thrombotic effect at the site of inflammation without an increased risk of bleeding events [30]. These findings were further supported by a prospective, observational trial of 80 patients who were hospitalized for AMI in the past 30 days, which showed that colchicine 0.5 mg daily reduced low-attenuation plaque on coronary CT and reduced hs-CRP level at 1 year, without a change in LDL level, suggesting that colchicine had plaque-stabilizing effect mediated through its effect on inflammation [31••].
Colchicine and the Prevention of Cardiovascular Events
Colchicine in Patients At-Risk of CAD
Around the time of the CANTOS trial, data emerged that colchicine use may protect against the development of cardiovascular disease when used in patients with rheumatologic conditions that poise them for higher risk of CAD. A small cross-sectional study of 290 patients with familial Mediterranean fever demonstrated a lower rate of myocardial infarctions compared to controls, a finding that was thought to be related to their use of colchicine [32]. Larger observational cohort and case-control studies supported these findings, with repeated demonstration of an association between colchicine use in patients with gout and lower prevalence of CAD and AMI [6, 7, 10].
Colchicine in Stable CAD
The first large randomized trial examining the effect of colchicine in CAD was the Low-Dose Colchicine for secondary prevention of cardiovascular disease (LoDoCo) trial [33••]. This randomized, prospective, observer blinded of the endpoint, open-label trial of 532 patients with stable CAD assessed the impact of low-dose colchicine (0.5 mg daily) on the reduction of a composite outcome of acute coronary syndrome, out-of-hospital cardiac arrest, and non-cardioembolic ischemic stroke. LoDoCo showed that colchicine reduced the rate of the composite primary outcome, largely driven by a reduction in ACS, with a number-needed-to-treat of 11 patients. These findings were subsequently validated in the larger LoDoCo-2 trial, which was a randomized, double-blind, placebo-controlled trial of 5522 patients with stable (> 6 months) CAD [9••]. In LoDoCo-2, colchicine use led to a 31% relative risk reduction in cardiovascular death, spontaneous MI, ischemic stroke, or ischemia-driven coronary revascularization from 9.6 to 6.8% on a median follow-up of 28.6 months. This finding was consistent even without the soft endpoint of ischemia-driven coronary revascularization (key secondary end point of a composite of cardiovascular death, spontaneous MI, or ischemic stroke, occurred in 4.2% in the colchicine group and in 5.7% in the placebo group with a hazard ratio, 0.72; 95% CI, 0.57 to 0.92; p = 0.007). The incidence of cardiovascular death or spontaneous MI (composite end point), and spontaneous MI were also lower with colchicine than with placebo. Patients with prior acute coronary syndrome made up 84% of the cohort, and a secondary analysis of the LoDoCo-2 trial showed that the effect of colchicine was consistent across the spectrum of patients with no history of MI, recent MI (6–24 months), remote MI (2–7 years), or very remote MI (7+ years) [34]. The mechanisms of this risk reduction were explored in the LoDoCo-2 biomarker substudy, which showed that among the 278 patients included, those treated with colchicine for 1 year had lower concentrations of extracellular vesicle NLRP3 protein, IL-6, and hs-CRP. However, NLRP3 levels did not correlate with hs-CRP levels suggesting that colchicine may lower NLRP3 and hs-CRP via independent mechanisms [35].
Colchicine in AMI
The efficacy of colchicine was also assessed in the acute phase of CAD with trials in patients with AMI. The first of these trials was a randomized, double-blind, placebo-controlled pilot trial of 151 patients with STEMI who were randomized to receive a loading dose of colchicine 1.5 mg before percutaneous coronary intervention (PCI) followed by standard dose maintenance (0.5 mg twice daily) for 5 days. Randomization to the colchicine arm was associated with a smaller infarct size as measured by biomarkers (AUC for CK 3,144 ng/h/mL vs 6,184 ng/h/mL, p < 0.001) and also by MRI-late gadolinium enhancement (18.8 mL vs 25.1 mL, p = 0.0019) [36]. Those randomized to receive colchicine peri-infarct also experienced a significantly greater reduction in the maximum CRP after infarct [36].
Thereafter, larger randomized trials sought to explore if colchicine treatment impacted clinical events in those with recent AMI. The COPS trial randomized participants hospitalized with ACS to receive colchicine 0.5 mg twice a day for 1 month followed by colchicine 0.5 mg daily for 11 months [37••]. In this small (n = 795) study, randomization to colchicine therapy did not reduce the primary composite outcome of death from any cause, ACS, ischemia-driven urgent revascularization, or non-cardioembolic stroke at 1 year compared to placebo, although events were lower in the colchicine versus placebo arm (24 events vs. 38 events, p = 0.09). However, there was a signal towards higher total mortality in those treated with colchicine, and a post hoc analysis of the composite endpoint using cardiovascular (rather than all-cause) mortality demonstrated an associated lower event rate with colchicine (5.0% vs. 9.5%, HR 0.51 [95% CI 0.29–0.89], p = 0.019) [37••]. Furthermore, a 2-year follow up of this study showed a reduction in the primary composite outcome among those administered colchicine (8.1%) compared to placebo (13.5%, p = 0.02), also suggesting that a longer time horizon may be needed to evaluate the benefit of colchicine [38••].
In contrast to COPS, the Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blind, placebo-controlled trial of 4,745 patients, demonstrated benefit of colchicine in a population of patients who had experienced MI in the past 30 days. In COLCOT, those randomized to colchicine 0.5 mg daily experienced a 23% reduction in CV death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring PCI (5.5% vs 7.1%) over a median of 22 months. Unlike prior observational studies in the gout and stable CAD populations, however, this effect was largely driven by a reduction in stroke rather than AMI [8••]. A subanalysis of the COLCOT trial found that early initiation of low-dose colchicine within the first 3 days of AMI was associated with a 48% reduction in risk of primary endpoint when compared to placebo, driven by reduction in AMI, stroke, and urgent hospitalization for angina. There was no significant effect noted among those who started colchicine > 3 days after AMI [39]. Given the early benefit of colchicine seen in the COLCOT trial, the ongoing CLEAR-SYNERGY trial (NCT03048825) is designed to assess the impact of colchicine versus placebo, as well as spironolactone versus placebo, on cardiovascular outcomes when given within 72 h of large MI as part of a randomized, 2 × 2 factorial design trial of 7,000 participants.
Colchicine in Context of Percutaneous Coronary Interventions
CAD remains a systemic disease process, but about half of all follow-up cardiovascular events in patients who undergo PCI for acute coronary syndrome are attributable to the original culprit lesion [40]. Colchicine has been studied as an agent that can potentially reduce restenosis and myocardial injury following PCI. Some of the early studies in the use of colchicine in coronary disease evaluated its role in the prevention of restenosis after plain old balloon angioplasty and disappointingly found limited effect [41,42,43]. However, a small randomized, double-blind, placebo-controlled trial of 197 patients with diabetes undergoing PCI with a bare-metal stent, colchicine was found to reduce rates of in-stent restenosis by 52% [44]. Furthermore, biomarker trials of those undergoing PCI showed colchicine to reduce levels of IL-6 and hs-CRP, as well as neutrophil extracellular trap release [14, 15•, 45].
The COLCHICINE-PCI trial sought to study the effects of peri-procedural colchicine on PCI-related myocardial injury. The randomized, double-blind, placebo-controlled single center trial of 714 patients referred for PCI in the setting of ACS or stable CAD-loaded patients with colchicine 1.2 mg 1–2 h before PCI followed by colchicine 0.6 mg immediately pre-procedure. PCI-related myocardial injury or 30-day major adverse cardiovascular events, defined as all-cause death, non-fatal MI, or target vessel revascularization, was not reduced with colchicine [15•]. An extended follow-up analysis looking at cardiovascular events over a median of 3.3 years also did not demonstrate a difference between the colchicine and placebo groups [46].
However, the COLCHICINE-PCI trial did show, for the first time, that colchicine does dampen the inflammatory response to iatrogenic injury within 22–24 h [15•]. The randomized, double-blind, placebo-controlled COPE-PCI pilot trial then replicated the COLCHICINE-PCI trial but loaded patients up to 24 h pre-PCI and demonstrated a reduction in both major procedural myocardial injury (31% vs 54%) and minor procedural injury (58% vs 85%) with colchicine compared to placebo [47••]. One of the only other populations in which myocardial injury or cardiovascular events can be foreseen is among those undergoing high-risk non-cardiac surgery. The ongoing POPCORN (PeriOPerative Colchicine to Reduce Negative Events, NCT05618353), a randomized, placebo-controlled trial of 700 participants, aims to determine the effect of colchicine on cardiovascular events among those with established CAD undergoing intermediate- or high-risk non-cardiac surgery.
Safety of Colchicine
Multiple analyses, including a large meta-analysis of randomized control trials including 8659 pooled participants, have also shown the overall safety of using colchicine in the prevention of cardiovascular disease [48,49,50]. Studies have demonstrated that in addition to preventing adverse cardiovascular events, colchicine does not increase all cause death or cause significant renal dysfunction, liver dysfunction, or myotoxicity. There was a numerical increase in non-cardiovascular death with colchicine observed in COPS and LoDoCo2 (pooled hazard ratio of 1.38 [95% CI 0.99–1.93]). However, no consistent underlying cause could be identified, with no difference in infection, pneumonia, or cancer. There is an association between colchicine and gastrointestinal symptoms, such as diarrhea, but there were no differences in hospitalization rates for gastrointestinal symptoms between colchicine and placebo, indicating the symptoms to be mild. Furthermore, these symptoms dissipate with time in most patients and occur at lower rates among those receiving lower doses [51].
It is important to highlight that notable patient populations were excluded from many of these trials, including those with moderate to severe kidney disease, severe liver disease or who are pregnant, as well as those taking other immunosuppressive medications or potent CYP3A4 inhibitors. Therefore, the results of the trials discussed in this review may not be extrapolated to these populations.
Cost-Effectiveness of Colchicine
A recent analysis of the cost-effectiveness of colchicine in patients with recent MI, from a Canadian health system perspective, utilizing data from the COLCOT trial demonstrated that the addition of colchicine to optimal medical therapy was cost-effective [52]. However, this analysis did not include data from the US where colchicine prices are higher. Before the creation of the US Food and Drug Administration (FDA), colchicine was available for prescription in unregulated forms. However, with the FDA’s Unapproved Drug Initiative in 2006, the FDA partnered with the pharmaceutical industry to conduct pharmacokinetic studies and determine the appropriate dosing of colchicine in acute gout flares, while bringing the medication under FDA approval. The pivotal study led by Terkeltaub allowed the dosing regimen to change from unlimited dosing until the achievement of diarrhea to the currently used dosing of 0.5 mg once or twice daily, which was associated with markedly lower rates of diarrhea [53]. With the limited patent, in 2009, the FDA-approved Colcrys entered the US market at $5.00/pill compared with the previous $0.50/pill. In an analysis of Medicare and Medicaid drug-spending data, spending on colchicine rose 2833% between 2008 and 2017 from $2.1 million to $32 million [54]. It was estimated that 58% of this spending increase was attributed to the price increase after FDA approval of Colcrys. Therefore, cost-effectiveness analyses focused on the US are needed to assess the economic impact of colchicine use in cardiovascular disease. Of note, there are no known differences in efficacy between the 0.5 mg and 0.6 mg doses as 0.6 mg has been routinely used for gout therapy in the US for more than a decade.
Future Directions
Colchicine is a promising addition to our arsenal of medications used to prevent major adverse cardiovascular events, and the low dose of colchicine recently received a cardiovascular label indication by the FDA for patients with established atherosclerotic disease or with multiple risk factors for cardiovascular disease. The ongoing CLEAR-SYNERGY trial and the COLCARDIO-ACS (Colchicine Cardiovascular Outcomes In Acute Coronary Syndrome, ACTRN12616000400460) trial will expand our understanding of the impact of colchicine in those with AMI. New directions for the use of colchicine include its use in preventing adverse events after intermediate- or high-risk non-cardiac surgery, which will be explored in the ongoing POPCORN trial, as well as its use in preventing stent thrombosis in conjunction with single anti-platelet therapy as was shown in the MACT pilot trial [55]. Other areas of ongoing investigation include the CLEAR-SYNERGY Biomarker substudy (NCT03874338), which will use an integrated profile of demographic, clinical, anatomical, and biomarker characteristics, including the use of genome wide associated studies, to identify heterogeneity in treatment response (those who would benefit most from the use of colchicine after AMI). Although a pharmacogenetic study of the COLCOT population did not show any significant genes associated with colchicine efficacy, the study had limited power based on small sample size [56].
Conclusions
Targeting inflammation is an effective strategy to reduce risk of cardiovascular events, even among those already receiving optimal medical therapy for CAD and who have achieved target traditional risk factor goals. Given (1) the lack of reduction in AMI in COLCOT, (2) the benefit in COLCOT being largely driven by urgent revascularization (a soft cardiovascular endpoint) and stroke (an endpoint not previously shown to be consistently reduced in other cardiovascular trials), and (3) the increase in non-cardiovascular death observed in COPS, the European guidelines on acute coronary syndrome and the AHA/ACC guidelines on chronic coronary disease give colchicine a class 2b indication for the use of low-dose colchicine in the secondary prevention of AMI. The cardiovascular community eagerly awaits the results of the 7000-patient CLEAR-SYNERGY trial. For now, the abundance of data that demonstrate the benefit of colchicine on a background of optimal medical therapy in patients at-risk of or with established CAD is consistently driven by reductions in MI and cardiovascular death and should be added to a patient’s regimen barring contraindications. However, in the setting of a patient’s negative perspective of polypharmacy and/or risk of drug interactions, and until more data are available on which demographic, clinical, and/or genomic subgroups would more likely benefit from the addition of colchicine, it is reasonable to prescribe colchicine to patients with hs-CRP levels ≥ 2 mg/L or with recurrent cardiovascular events despite control of traditional risk factors. As we begin to utilize this ancient medication for new purposes, ongoing trials in both clinical efficacy, safety, and cost-effectiveness will help determine the spectrum of uses for colchicine in clinical practice.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894–900. https://doi.org/10.1161/01.cir.0000042674.89762.20.
Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur Heart J. 2015;36(22):1394–404. https://doi.org/10.1093/eurheartj/ehv044.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–301. https://doi.org/10.1016/s0140-6736(23)00215-5.
•• Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–62. https://doi.org/10.1056/NEJMoa1809798. The CANTOS randomized control trial demonstrated that the IL-1B inhibitor canakinumab reduced cardiovascular events among those with AMI and elevated hs-CRP with a concurrent increase in fatal infections.
Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39(7):1458–64. https://doi.org/10.3899/jrheum.111533.
Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: A cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis. 2016;75(9):1674–9. https://doi.org/10.1136/annrheumdis-2015-207984.
•• Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505. https://doi.org/10.1056/NEJMoa1912388. The COLCOT randomized control trial demonstrated that colchicine reduced cardiovascular events by 23% among those with recent AMI.
•• Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–47. https://doi.org/10.1056/NEJMoa2021372. LoDoCo-2 randomized control trial showed that colchicine reduced cardiovascular events by 31% among those with chronic coronary disease.
Shah B, Toprover M, Crittenden DB, Jeurling S, Pike VC, Krasnokutsky S, et al. Colchicine use and incident coronary artery disease in male patients with gout. Can J Cardiol. 2020;36(11):1722–8. https://doi.org/10.1016/j.cjca.2020.05.026.
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. https://doi.org/10.1161/circulationaha.110.982777.
Shah B, Baber U, Pocock SJ, Krucoff MW, Ariti C, Gibson CM, et al. White blood cell count and major adverse cardiovascular events after percutaneous coronary intervention in the contemporary era: Insights from the PARIS Study (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients Registry). Circ Cardiovasc Interv. 2017;10(9). https://doi.org/10.1161/circinterventions.117.004981.
Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–28. https://doi.org/10.1016/s0140-6736(17)32814-3.
Martínez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, et al. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc. 2015;4(8):e002128. https://doi.org/10.1161/jaha.115.002128.
• Shah B, Pillinger M, Zhong H, Cronstein B, Xia Y, Lorin JD, et al. Effects of acute colchicine administration prior to percutaneous coronary intervention: COLCHICINE-PCI randomized trial. Circ Cardiovasc Interv. 2020;13(4):e008717. https://doi.org/10.1161/circinterventions.119.008717. This randomized control trial demonstrated for the first time that colchicine can dampen the inflammatory response to acute injury.
Toprover M, Shah B, Oh C, Igel TF, Romero AG, Pike VC, et al. Initiating guideline-concordant gout treatment improves arterial endothelial function and reduces intercritical inflammation: A prospective observational study. Arthritis Res Ther. 2020;22(1):169. https://doi.org/10.1186/s13075-020-02260-6.
Fujisue K, Sugamura K, Kurokawa H, Matsubara J, Ishii M, Izumiya Y, et al. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ J. 2017;81(8):1174–82. https://doi.org/10.1253/circj.CJ-16-0949.
Bakhta O, Blanchard S, Guihot AL, Tamareille S, Mirebeau-Prunier D, Jeannin P, et al. Cardioprotective role of colchicine against inflammatory injury in a rat model of acute myocardial infarction. J Cardiovasc Pharmacol Ther. 2018;23(5):446–55. https://doi.org/10.1177/1074248418763611.
Forrat R, Sebbag L, Ferrera R, Hadour G, Canet E, Tabib A, et al. Effect of colchicine on circulating and myocardial neutrophils and on infarct size in a canine model of ischemia and reperfusion. J Cardiovasc Pharmacol. 1996;27(6):876–83. https://doi.org/10.1097/00005344-199606000-00016.
Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96(2):994–1002. https://doi.org/10.1172/jci118147.
Yang M, Lv H, Liu Q, Zhang L, Zhang R, Huang X, et al. Colchicine Alleviates cholesterol crystal-induced endothelial cell pyroptosis through activating AMPK/SIRT1 pathway. Oxid Med Cell Longev. 2020;2020:9173530. https://doi.org/10.1155/2020/9173530.
Meyer-Lindemann U, Mauersberger C, Schmidt AC, Moggio A, Hinterdobler J, Li X, et al. Colchicine impacts leukocyte trafficking in atherosclerosis and reduces vascular inflammation. Front Immunol. 2022;13:898690. https://doi.org/10.3389/fimmu.2022.898690.
Kiraz S, Ertenli I, Arici M, Calgüneri M, Haznedaroglu I, Celik I, et al. Effects of colchicine on inflammatory cytokines and selectins in familial Mediterranean fever. Clin Exp Rheumatol. 1998;16(6):721–4.
Bauriedel G, Heimerl J, Beinert T, Welsch U, Höfling B. Colchicine antagonizes the activity of human smooth muscle cells cultivated from arteriosclerotic lesions after atherectomy. Coron Artery Dis. 1994;5(6):531–9.
Abu-Fanne R, Stepanova V, Litvinov RI, Abdeen S, Bdeir K, Higazi M, et al. Neutrophil α-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood. 2019;133(5):481–93. https://doi.org/10.1182/blood-2018-07-861237.
Mori H, Taki J, Wakabayashi H, Hiromasa T, Inaki A, Ogawa K, et al. Colchicine treatment early after infarction attenuates myocardial inflammatory response demonstrated by (14)C-methionine imaging and subsequent ventricular remodeling by quantitative gated SPECT. Ann Nucl Med. 2021;35(2):253–9. https://doi.org/10.1007/s12149-020-01559-3.
Aimo A, Martinez-Falguera D, Barison A, Musetti V, Masotti S, Morfino P, et al. Colchicine added to standard therapy further reduces fibrosis in pigs with myocardial infarction. J Cardiovasc Med (Hagerstown). 2023;24(11):840–6. https://doi.org/10.2459/jcm.0000000000001554.
Schwarz N, Fernando S, Chen YC, Salagaras T, Rao SR, Liyanage S, et al. Colchicine exerts anti-atherosclerotic and -plaque-stabilizing effects targeting foam cell formation. Faseb J. 2023;37(4):e22846. https://doi.org/10.1096/fj.202201469R.
Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol. 2007;99(6):805–7. https://doi.org/10.1016/j.amjcard.2006.10.039.
Shah B, Allen N, Harchandani B, Pillinger M, Katz S, Sedlis SP, et al. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: A pilot study in healthy subjects. Inflammation. 2016;39(1):182–9. https://doi.org/10.1007/s10753-015-0237-7.
•• Vaidya K, Arnott C, Martínez GJ, Ng B, McCormack S, Sullivan DR, et al. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: A CT coronary angiography study. JACC Cardiovasc Imaging. 2018;11(2 Pt 2):305–16. https://doi.org/10.1016/j.jcmg.2017.08.013. This observational trial showed that colchicine reduced low-attenuation plaque burden on coronary CT and reduced hs-CRP at one year among those with recent AMI.
Langevitz P, Livneh A, Neumann L, Buskila D, Shemer J, Amolsky D, et al. Prevalence of ischemic heart disease in patients with familial Mediterranean fever. Isr Med Assoc J. 2001;3(1):9–12.
•• Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61(4):404–10. https://doi.org/10.1016/j.jacc.2012.10.027. LoDoCo showed that colchicine reduced the rate of cardiovascular events, driven by reduction in AMI, with a number-needed-to-treat of 11 patients.
Opstal TSJ, Fiolet ATL, van Broekhoven A, Mosterd A, Eikelboom JW, Nidorf SM, et al. Colchicine in patients with chronic coronary disease in relation to prior acute coronary syndrome. J Am Coll Cardiol. 2021;78(9):859–66. https://doi.org/10.1016/j.jacc.2021.06.037.
Silvis MJM, Fiolet ATL, Opstal TSJ, Dekker M, Suquilanda D, Zivkovic M, et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: A LoDoCo2 biomarker substudy. Atherosclerosis. 2021;334:93–100. https://doi.org/10.1016/j.atherosclerosis.2021.08.005.
Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: A pilot study. Circulation. 2015;132(15):1395–403. https://doi.org/10.1161/circulationaha.115.017611.
•• Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, et al. Colchicine in patients with acute coronary syndrome: The Australian COPS randomized clinical trial. Circulation. 2020;142(20):1890–900. https://doi.org/10.1161/circulationaha.120.050771.The COPS randomized control trial did not demonstrate a reduction in cardiovascular events at one year when colchicine was administered to those with recent AMI but did show a previously unobserved increase in non-cardiovascular deaths.
•• Tong DC, Bloom JE, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, et al. Colchicine in patients with acute coronary syndrome: Two-year follow-up of the Australian COPS randomized clinical trial. Circulation. 2021;144(19):1584–6. https://doi.org/10.1161/circulationaha.121.054610. This two year follow up of the COPS trial showed that colchicine reduced cardiovascular events among those with recent AMI.
Bouabdallaoui N, Tardif JC, Waters DD, Pinto FJ, Maggioni AP, Diaz R, et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J. 2020;41(42):4092–9. https://doi.org/10.1093/eurheartj/ehaa659.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35. https://doi.org/10.1056/NEJMoa1002358.
O’Keefe JH Jr, McCallister BD, Bateman TM, Kuhnlein DL, Ligon RW, Hartzler GO. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J Am Coll Cardiol. 1992;19(7):1597–600. https://doi.org/10.1016/0735-1097(92)90624-v.
Rab ST, King SB 3rd, Roubin GS, Carlin S, Hearn JA, Douglas JS Jr. Coronary aneurysms after stent placement: A suggestion of altered vessel wall healing in the presence of anti-inflammatory agents. J Am Coll Cardiol. 1991;18(6):1524–8. https://doi.org/10.1016/0735-1097(91)90685-3.
Freed M, Safian RD, O’Neill WW, Safian M, Jones D, Grines CL. Combination of lovastatin, enalapril, and colchicine does not prevent restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1995;76(16):1185–8. https://doi.org/10.1016/s0002-9149(99)80334-8.
Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol. 2013;61(16):1679–85. https://doi.org/10.1016/j.jacc.2013.01.055.
Vaidya K, Tucker B, Kurup R, Khandkar C, Pandzic E, Barraclough J, et al. Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention. J Am Heart Assoc. 2021;10(1):e018993. https://doi.org/10.1161/jaha.120.018993.
Shah B, Smilowitz NR, Xia Y, Feit F, Katz SD, Zhong J, et al. Major adverse cardiovascular events after colchicine administration before percutaneous coronary intervention: Follow-up of the colchicine-PCI trial. Am J Cardiol. 2023;204:26–8. https://doi.org/10.1016/j.amjcard.2023.07.029.
•• Cole J, Htun N, Lew R, Freilich M, Quinn S, Layland J. Colchicine to prevent periprocedural myocardial injury in percutaneous coronary intervention: The COPE-PCI pilot trial. Circ Cardiovasc Interv. 2021;14(5):e009992. https://doi.org/10.1161/circinterventions.120.009992. This randomized control pilot trial showed that colchicine administered peri-procedurally among those undergoing percutaneous coronary intervention reduced the incidence of myocardial injury when given 6-24 hours in advance.
Samuel M, Tardif JC, Bouabdallaoui N, Khairy P, Dubé MP, Blondeau L, et al. Colchicine for secondary prevention of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Can J Cardiol. 2021;37(5):776–85. https://doi.org/10.1016/j.cjca.2020.10.006.
Kofler T, Kurmann R, Lehnick D, Cioffi GM, Chandran S, Attinger-Toller A, et al. Colchicine in patients with coronary artery disease: A systematic review and meta-analysis of randomized trials. J Am Heart Assoc. 2021;10(16):e021198. https://doi.org/10.1161/jaha.121.021198.
Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: A systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42(28):2765–75. https://doi.org/10.1093/eurheartj/ehab115.
Robinson PC, Terkeltaub R, Pillinger MH, Shah B, Karalis V, Karatza E, et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. 2022;135(1):32–8. https://doi.org/10.1016/j.amjmed.2021.07.025.
Samuel M, Tardif JC, Khairy P, Roubille F, Waters DD, Grégoire JC, et al. Cost-effectiveness of low-dose colchicine after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J Qual Care Clin Outcomes. 2021;7(5):486–95. https://doi.org/10.1093/ehjqcco/qcaa045.
Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–8. https://doi.org/10.1002/art.27327.
McCormick N, Wallace ZS, Yokose C, Jorge A, Sacks CA, Hsu J, et al. Prolonged increases in public-payer spending and prices after unapproved drug initiative approval of colchicine. JAMA Intern Med. 2021;181(2):284–7. https://doi.org/10.1001/jamainternmed.2020.5017.
Lee SY, Jeong YH, Yun KH, Cho JY, Gorog DA, Angiolillo DJ, et al. P2Y(12) Inhibitor monotherapy combined with colchicine following PCI in ACS patients: The MACT Pilot Study. JACC Cardiovasc Interv. 2023;16(15):1845–55. https://doi.org/10.1016/j.jcin.2023.05.035.
Dubé MP, Legault MA, Lemaçon A, Lemieux Perreault LP, Fouodjio R, Waters DD, et al. Pharmacogenomics of the efficacy and safety of colchicine in COLCOT. Circ Genom Precis Med. 2021;14(2):e003183. https://doi.org/10.1161/circgen.120.003183.
Author information
Authors and Affiliations
Contributions
DB and MM conducted literature review and wrote the main manuscript BS developed manuscript outline, assisted with literature review, and edited and reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Banco and Dr. Mustehsan have nothing to disclose. Dr. Shah receives funding outside this paper—from the NIH and from the VA Office of Research and Development for the CLEAR-SYNERGY biospecimen substudy and POPCORN trials, respectively; serves on the steering committee for the NovoNordisk-funded ARTEMIS trial, CIHR-funded CLEAR SYNERGY Trial, and the Philips Volcano-funded IMPROVE Trial; serves as an advisory board member for Philips Volcano; and serves as a Board of Trustee for the Society of Cardiovascular Angiography and Interventions.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banco, D., Mustehsan, M. & Shah, B. Update on the Role of Colchicine in Cardiovascular Disease. Curr Cardiol Rep 26, 191–198 (2024). https://doi.org/10.1007/s11886-024-02026-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-024-02026-5