Abstract
Purpose of Review
Finerenone, an FDA-approved nonsteroidal mineralocorticoid receptor (MR) antagonist, has been evaluated in context of chronic kidney disease (CKD) and associated cardiovascular disease (CVD). In this review, we summarize pre-clinical and clinical studies focused on the impact of finerenone on these disease processes.
Recent Findings
Activation of the MR upregulates genes encoding for facilitators of tissue damage. Finerenone binding to a helix domain in this receptor inhibits receptor function. Studies in murine models of kidney disease, heart failure, hypertension, and vascular injury demonstrate significant protective effects of finerenone against further disease progression, as well as association with reduced oxidative stress, inflammation, and fibrosis. Phase 1–3 clinical trials with finerenone show safety and efficacy in improving renal and cardiovascular outcomes in patients with CKD.
Summary
Research thus far encourages the addition of finerenone to the standard of care for certain CKD patients, especially those especially at risk for or with pre-existing cardiovascular disease. Continued study of the effect of finerenone in diverse patient populations and different disease states is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: Mineralocorticoid Receptors and Their Ligands—a Focus on Finerenone
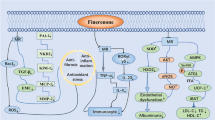
Mineralocorticoids such as aldosterone, deoxycorticosterone, and cortisol exert cellular responses through binding to mineralocorticoid receptors (MRs). These receptors are members of the nuclear receptor superfamily and function as ligand-dependent transcription factors activated by an endogenous ligand (aldosterone). More specifically, once bound to aldosterone, MRs translocate to the nucleus and as homodimers bind to specific sequences in gene promoters to activate transcription of various genes [1]. MRs are readily detected in cardiomyocytes, endothelial cells, smooth muscle cells, fibroblasts, and inflammatory cells, the activation of which could lead to deposition of a dense extracellular matrix, oxidative stress, and upregulation of various inflammatory cytokines [1]. For instance, MRs expressed on vascular smooth muscle cells mediate signaling towards coronary and left ventricular dysfunction post myocardial infarction [2]. Activation of these receptors also activates the NFkappaB transcription factor signaling pathway [3] and upregulates the expression of genes encoding proteins that control blood sodium level, such as the epithelial Na + /K + pump, leading to blood pressure augmentation. Together, these effects lead to tissue damage, including endothelial dysfunction, fibrosis of the myocardium, and glomerular and tubular dysfunction (reviewed in [4]) (Fig. 1).
Finerenone’s effects on mineralocorticoid receptor (MR)-induced cellular pathways. MRs are expressed on cardiomyocytes, fibroblasts, endothelial cells, smooth muscle cells, and inflammatory cells. Upon aldosterone binding, the receptors homodimerize and translocate to the nucleus, where they bind specific hormone response elements to initiate gene transcription. Finerenone, binding to the MR within its helix 12 domain, indirectly inhibits agonist binding and receptor translocation to the nucleus, thereby preventing activation of tissue-damaging pathways
The importance of regulating the function of MRs has prompted synthesis of both agonists and antagonists (reviewed in [5]). A known synthetic agonist approved for clinical use is fludrocortisone. Steroidal synthetic antagonists include drospirenone, spironolactone, canrenone, and eplerenone [1]. Spironolactone and eplerenone reduce hospitalization and mortality in patients with heart failure with reduced ejection fraction (HFrEF) [6]. However, these classic steroidal mineralocorticoid receptor antagonists (MRAs) can elicit side effects, including hyperkalemia and reduced renal function, as well as hormonal perturbances such as gynecomastia [7, 8]. This spurred the synthesis of non-steroidal antagonists, including finerenone (BAY 94–8862; also known as Kerendia), AZD9977, apararenone, KBP-5074, LY-2623091, and esaxerenone.
Of these compounds, finerenone is among the most extensively studied, and several large clinical studies paint an emerging picture of relative potency and selectivity [9, 10••, 11••]. The current review focuses on surveying studies demonstrating the effects of finerenone on both cardiovascular and renal systems, as examined in experimental models and in human cohorts.
Pharmacology of Finerenone
Finerenone (ChemSpider ID 28,669,387), the molecular formula of which is C21H22N4O3, has an average mass of 378.424 Da and a monoisotopic mass of 378.169189 Da. The unique structure of finerenone allows its binding to a helical site within the MR and engaging in allosteric modulation. Helix 12 plays a key role in the activation of the receptor through the binding of important coactivators. While most MRAs competitively inhibit MRs by directly binding to the ligand binding domain of the receptor, finerenone’s side chain binds to the receptor helix 12 domain, leading to conformational changes that inhibit the receptor translocation to the nucleus and downstream signaling [12] (Fig. 1).
Several studies investigated the pharmacokinetics of finerenone. In addition to phase 1 safety probing [13,14,15,16,17,18], finerenone pharmacokinetics was thoroughly assessed. Early studies showed that finerenone, being a molecule with high polarity, has excellent tissue distribution in the kidney and heart [19]. Upon oral administration, it is completely absorbed and metabolized by a hemoprotein—a member of the cytochrome P450 family of oxidizing enzymes (CYP3A4)—in the gut wall and the liver, resulting in an absolute bioavailability of 44% [20–22]. Finerenone has a short plasma half-life, with the added advantage of not degrading into active metabolites with potential side effects [13, 14, 16, 17].
Given that finerenone is a CYP3A4 substrate, concomitant use with a CYP3A4 inhibitor increases finerenone exposure and can increase risk of finerenone-related side effects. As such, concomitant use of finerenone with strong CYP3A4 inhibitors is contraindicated. When administered in conjunction with weak or moderate CYP3A4 inhibitors (diltiazem or verapamil for example), serum potassium should be more closely monitored.
Encouraging results related to finerenone pharmacokinetics and safety motivated both mechanistic studies in animal models, as well as initially probing its potential to reduce kidney function decline and cardiovascular complications in adults with chronic kidney disease (CKD) associated with type 2 diabetes mellitus (T2DM). Collectively, results of animal model investigations and human cohort studies (FIDELIO-DKD and FIGARO-DKD—see below) led to FDA approval of finerenone for this indication [10••, 11••]. However, data from ongoing research indicates an even broader array of disease states that could benefit from finerenone.
Effects of Finerenone on Kidney Function and Blood Pressure
Studies in Animal Models
Using the Munich Wistar Frömter (MWF) rat as a model of CKD, treatment for 4 weeks with finerenone (10 mg/kg/day) lowered albuminuria by greater than 40% and significantly reduced systolic blood pressure as compared to controls. The effects of the drug were attributed to an upregulation of endothelial nitric oxide (eNOS) and an increase in NO availability [23]. In a preclinical rat disease model, labeled finerenone was found to be significantly and equally distributed in cardiac and renal tissues, encouraging further testing of its therapeutic applications [19]. Challenging deoxycorticosterone acetate-salt rats (a model of salt-sensitive hypertension) with finerenone (1 mg/kg/day) reduced proteinuria and renal fibrosis [19]. These effects were independent of changes in systemic blood pressure, as this parameter was not altered with the finerenone concentration used.
In a mouse model where kidney fibrosis was induced via unilateral ureteral obstruction or ischemia, treatment orally with finerenone (3 or 10 mg/kg/day) diminished the number of myofibroblasts and collagen deposition, reduced ischemia-induced albuminuria, but had no significant influence on blood pressure [24]. This effect was compared to empagliflozin, a sodium-glucose cotransporter-2 (SGLT-2) inhibitor. Empagliflozin (at 10 or 30 mg/kg/day) reduced ischemia-induced albuminuria, but did not reduce kidney collagen or myofibroblast density [24].
Similarly, in search for additional mechanisms of effect of finerenone, acute kidney injury was induced in mice via bilateral kidney ischemia/reperfusion. Finerenone reduced renal fibrosis and kidney dysfunction in association with augmented level of the M2-anti-inflamatory macrophages and reduced level of inflammatory M1-macrophages [25].
UNx-HS db/db mice with hyperaldosteronism are used as a model to mimic human diabetic nephropathy, considering their hypertension, hypokalemia, albuminuria, and glomerular injury. These mice were used to study the effect of finerenone on CKD promoted by a crosstalk between RAS-related C3 botulinus toxin substrate 1 (Rac1) and MRs. Administration of finerenone and the Rac1 inhibitor NSC23766 improved blood pressure and glomerular injury compared to vehicle treatment [26].
Together, studies in different experimental models point to a protective effect of finerenone on renal fibrosis, improvement of albuminuria, and a dose-dependent anti-hypertensive effect, likely owing to its indirect inhibition of multiple signaling pathways otherwise mediated by activation of MRs.
Clinical Studies
As displayed in Table 1, several clinical trials have examined the safety and efficacy of finerenone—alone or in combination with other drugs—in patients with kidney disease with or without diabetes or heart failure. Among the first trials to explore the safety and tolerability of different doses of finerenone in adults was in patients with HFrEF and CKD (ARTS trial; NCT01345656). This double-blinded, randomized, multi-center study enrolled 457 participants with either mild (estimated glomerular filtration rate (eGFR) 60–90 mL/min/1.73 m2) or moderate (eGFR 30–60 mL/min/1.73 m2) CKD, with comorbid HFrEF already on guideline directed heart failure medical therapy. Dosages from 2.5 to 10 mg daily of finerenone were explored, and individuals with mild CKD were compared to a placebo arm, while those with moderate CKD were compared to an active comparator, spironolactone. Safety and tolerability were assessed vs. placebo in the mild CKD group, and then the primary endpoint of change in serum potassium over 4 weeks was assessed vs. spironolactone in the moderate CKD group. Incidence of adverse events due to finerenone was low, including hyperkalemia, with the highest proportion of such events in the study actually being related to spironolactone [27].
Another Phase 2 study focused on patients with T2DM and diabetic nephropathy (ARTS-DN; NCT01874431). This double-blinded, randomized, multi-center study involving 823 participants with urine albumin to creatinine ratio (UACR) greater than 30 mg/g and eGFR of 30–90 mL/min/1.73 m2 examined different dosages of finerenone (1.25–20 mg daily) for 90 days. The measured primary outcome was UACR at day 90 as compared to baseline. Addition of finerenone (doses ranging from 7.5 to 20 mg daily) to either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker improved UACR, as compared to placebo [20]. Probing a similar inquiry in 96 Japanese patients with T2DM and diabetic nephropathy using similar dosages and duration treatment, the ARTS-DN Japan study (NCT01968668) concluded that all dosages of finerenone tested were safe, but only 20 mg daily significantly reduced albuminuria. The authors ascribed this divergence from the results of the international ARTS-DN study to the small sample size of the Japanese trial.
Next came the early finerenone in reducing kidney failure and disease progression in diabetic kidney disease (FIDELIO-DKD) trial, which was a multi-center, double-blinded, randomized, phase 3 study involving 978 sites and 5734 participants. Finerenone was administered at a starting dose of either 10 or 20 mg daily for subjects with an eGFR between 25 and 60 mL/min/1.73 m2 or between 60 and 90 mL/min/1.73 m2, respectively, until study completion. The primary composite outcomes (measured with a median follow-up of 2.6 years) were progression to end-stage renal disease (ESRD), a persistently greater than 40% reduction in eGFR from baseline, or death from renal causes. It was found that finerenone reduced the above measures of CKD progression, as well as composite cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure), compared to placebo [10••, 28•, 29•]. While incidence of hyperkalemia was significantly higher in the finerenone arm, overall, the frequency of adverse events was similar as compared to placebo. Furthermore, a subsequent sub-analysis and pharmacokinetic model of the data from the trial showed that incidence of hyperkalemia could be reduced to similar to placebo using certain dose titration techniques [30].
A sub-analysis of the FIDELIO-DKD trial examined the influence of concurrent use of SGLT-2 inhibitors (SGLT-2i) on the treatment effects of finerenone. Of 5674 patients, 259 were on an SGLT-2i at baseline, which was continued for the trial. Reduction in UACR with finerenone was found with or without baseline SGLT-2i use. Finerenone still significantly reduced the kidney and key secondary cardiovascular outcomes versus placebo, with no clear difference in the results by SGLT-2i use at baseline. Interestingly though, there was lower incidence of hyperkalemia in the concurrent finerenone SGLT-2i group vs. finerenone alone [31].
Effects of Finerenone on the Cardiovascular System
Studies in Animal Models
A study using deoxycorticosterone acetate/salt rats (a model of hypertension) compared the effects of finerenone to the steroidal MRA eplerenone in protecting against heart and/or kidney damage. Compared at equinatriuretic doses, finerenone treatment more significantly diminished cardiac hypertrophy and proteinuria than eplerenone. In a rat model of coronary artery ligation-induced heart failure, only finerenone (1 mg/kg daily) improved left ventricular function [19]. The effects of finerenone (2.5 mg/kg daily) on cardiac complications of renal failure were studied in a mouse model of CKD induced by subtotal nephrectomy with consequent cardiac dysfunction. Finerenone improved cardiac function as assessed by echocardiography and invasive hemodynamics, as well as decreased cardiac fibrosis [32].
A study using neonatal rat cardiac fibroblasts probed mechanisms of the effect of finerenone. Finerenone significantly inhibited aldosterone-induced upregulation of connective tissue growth factor and of the matrix cross-linking enzyme, lysyl oxidase, both of which are known contributors to a fibrotic extracellular matrix. Additionally, treatment over 5 months with finerenone of transgenic mice with cardiac-specific overexpression of Rac1 (which results in increased left ventricular end-diastolic and end-systolic volumes compared to control mice) prevented left ventricular dilatation. Finerenone also prevented left atrial dilatation and left atrial fibrosis, compared to vehicle-treated transgenic mice [33].
A 2021 study used hypertensive, N(ω)-nitro-l-arginine methyl ester-treated, renin-transgenic (mRen2)27 rats treated orally with either vehicle or finerenone (1 and 3 mg/kg daily), empagliflozin (3 and 10 mg/kg daily), or a combination of these respective low doses. Low-dose combination increased survival by twofold compared to placebo. Administration of finerenone or the low-dose combination decreased systolic blood pressure and protected from cardiac and kidney fibrosis and vasculopathy after 5 weeks of treatment, with low-dose drug combination being more efficient [34]. Using MWF rats, a rat model of CKD with intrinsic mesenteric arterial stiffness, finerenone (10 mg/kg daily), significantly reduced intrinsic arterial stiffness in conjunction with reduced plasma levels of matrix-remodeling proteins such as matrix metalloproteinase-2 and matrix metalloproteinase-4, as well as reduced the level of superoxide anions and increased nitric oxide compared to control-treated animals [35]. Likewise, also using the MWF rat CKD model, finerenone (10 mg/kg daily) reduced systolic blood pressure and albuminuria [23].
In another study, finerenone’s effect was examined both in cultured cells and in vivo in a mouse model of vascular injury. Finerenone dose-dependently inhibited the stimulatory effect of aldosterone on proliferation of cultured vascular smooth muscle cells. In an endothelial cell culture, it also inhibited aldosterone-induced apoptosis. Accordingly, in a mouse model of wire-induced injury of the femoral artery, 10 days of treatment with finerenone inhibited intimal and medial cell proliferation. At day 21, there was a noticeable reduction in the development of neointimal hyperplasia as compared to vehicle-treated mice [36].
Finerenone also improves heart function and exercise capacity in post-menopause model mice. The study was motivated by reports of increased incidence of heart failure in post-menopausal women. One month of treatment with finerenone (1 mg/kg daily) in 7-month-old mice that underwent ovariectomy, compared to controls, reduced weight gain, blood pressure, blood glucose, and insulin resistance [37].
Together, preclinical animal models of different cardiovascular disease conditions demonstrate a protective effect of finerenone, whether on cardiac hypertrophy, atrial fibrosis, vasculopathy, systolic blood pressure, arteriosclerosis, or neointimal hyperplasia post vascular injury.
Clinical Studies
A trial published in 2016 explored the safety and efficacy of finerenone in 1066 adults with worsening HFrEF, and either comorbid T2DM, moderate CKD (GFR of 30–60 mL/min/1.73 m2), or both (ARTS-HF; NCT01807221). This double-blinded, randomized, multi-center trial enrolled participants requiring emergency presentation to the hospital and IV diuretic treatment for acute decompensated heart failure not due to acute ischemia. Participants were randomized to starting doses of finerenone ranging from 2.5–15 mg daily, or eplerenone 25 mg every other day initially and increased to daily on day 30 and to 50 mg on day 60. Primary outcome was relative decrease at 90 days in N-terminal prohormone B-type natriuretic peptide (NT pro-BNP, a marker of cardiac stress in heart failure) of more than 30% from baseline. Secondary outcomes included death due to any cause, adverse cardiac events (including hospitalizations and emergency presentation for heart failure), rate of change of NT pro-BNP, and heart failure quality of life questionnaires. While the percentage of patients achieving the primary endpoint was statistically similar between the eplerenone and the finerenone arms, the incidence of composite mortality and adverse cardiac events was lower in most finerenone arms [38]. There was a parallel small replication of this trial done exclusively in Japan (ARTS-HF Japan; NCT01955694) with 72 participants. No statistically significant results were obtained, but there was a signal towards larger decrease in NT pro-BNP with higher doses of finerenone, and similarly low rates of adverse events throughout all arms [39].
Given that nearly 46% of the 5674 patients in FIDELIO-DKD (described in depth above in the effects on kidney function section) had some form of cardiovascular disease to begin with, several sub-analyses have been performed to examine cardiovascular outcomes. For example, one examined the concurrent effects of glucagon-like peptide-1 (GLP-1) receptor agonist on the treatment effect of finerenone. Of the 5674 patients analyzed, 6.9% were receiving GLP-1 agonists at baseline. The previously described beneficial effects of finerenone, including reduction UACR and the primary cardiovascular endpoint (time to cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure), were not significantly different with concurrent use of GLP-1 agonists [40]. Another subgroup analysis further probed possible effects of finerenone on reduced onset of atrial fibrillation or flutter (AFF) evoked by cardiac remodeling and kidney disease. Of the 5674 patients in the study, 8.1% had a history of AFF at baseline. This did not impact the effect of finerenone on kidney and cardiovascular outcomes. There was a significant reduction in new onset of AFF during the study period between the treatment and placebo arms (hazard ratio (HR): 0.71; 95% confidence interval (CI): 0.53–0.94; p = 0.016). [29].
A large phase 3 study of finerenone involving 7352 patients with T2DM, CKD, and albuminuria (FIGARO-DKD; NCT02545049) examined treatment with finerenone (10 mg or 20 mg daily) compared to placebo, in addition to maximally tolerated renin–angiotensin system blockade on major adverse cardiovascular events. Participants had T2DM, as well as CKD and albuminuria as defined as either an eGFR of 25–90 mL/min/1.73 m2 and UACR of 30–300 mg/g, or an eGFR of greater than 60 mL/min/1.73 m2 and a UACR of 300–5000 mg/g. The primary outcome was a composite of deaths from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. A relevant secondary outcome was a composite of progression to ESRD, a sustained decrease of GFR of at least 40% from baseline, or death from renal causes. There was a significant decrease in the primary composite (cardiovascular) outcome in the finerenone arm vs. placebo, and though there was a signal towards a decrease in the secondary kidney outcome for the finerenone arm as compared to placebo, this was not statistically significant. There was a statistically higher rate of trial discontinuation in the finerenone arm due to hyperkalemia (1.2% vs. 0.4%), but no significant difference in adverse events in the treatment and placebo arms [11••].
In a subsequent sub-analysis, the same FIGARO-DKD trial data was used to further examine the incidence of heart failure in patients with CKD and T2DM. The finerenone arm showed multiple statistically significantly better outcomes, with an 18% lower risk of cardiovascular death, a 29% lower risk of first heart failure hospitalization, and a 30% lower rate of total heart failure hospitalizations. Among participants in FIAGRO-DKD, 7.8% had a history of heart failure at baseline, and new onset of heart failure in the finerenone arm was 1.9% vs. 2.8% in the placebo arm (HR, 0.68; 95% CI, 0.50–0.93]; P = 0.0162) [28].
Data available from the numerous finerenone studies have become prime for combined data analysis. Combined analysis of FIDELIO-DKD and FIGARO-DKD demonstrated that finerenone reduced the risk of adverse cardiovascular and kidney outcomes vs. placebo across the spectrum of CKD in patients with T2DM. Based on data derived from 13,026 patients with a median follow-up of 3 years, hyperkalemia leading to treatment discontinuation occurred in 1.7% of patients receiving finerenone, compared to 0.6% in placebo case, but there was no significant difference in adverse events. However, the composite cardiovascular outcome was significantly decreased in finerenone-treated patients vs. placebo (12.7% vs. 14.4%; HR, 0.86; 95% CI, 0.78–0.95; P = 0.0018), and the composite kidney outcome was significantly lower as well (5.5% vs. 7.1%; HR, 0.77; 95% CI, 0.67–0.88; P = 0.0002) [41].
A 2022 systematic review and meta-analysis on five published finerenone-related clinical trials affirmed finerenone as beneficial therapy for reducing CKD markers of disease as well as cardiovascular events in CKD patients [42]. Finerenone has antiproteinuric effects, with UACR being significantly lower across 5 trials (n = 6,732) vs. placebo. Furthermore, finerenone has a favorable effect on decrease in GFR, with 3 trials (n = 6,852) showing patients treated with finerenone are significantly less likely than placebo-treated patients to develop a durable decrease of greater than 40% in GFR, and 2 trials (n = 6519) show patients treated with finerenone are significantly less likely to progress to end-stage renal disease. Lastly, 4 trials (n = 6,992) demonstrate CKD patients treated with finerenone are significantly less likely to experience adverse cardiovascular events than the placebo group. While risk of hyperkalemia was higher with finerenone than placebo across 5 trials (n = 6,583), differences in adverse events across 5 trials (n = 7,019) were not statistically significant.
Future Directions
While the cardiac and renal benefits of finerenone are seen in several studies and different modes of analyses, results are mostly based on populations undergoing standard-of-care treatment. It would be interesting to test the effect of finerenone as monotherapy vs. add-on treatment at the onset of CKD, or in patients with T2DM alone, or with cardiovascular disease irrespective of CKD. Indeed, such a trial is ongoing, which is aimed at assessing the effects of finerenone on cardiovascular morbidity and mortality in individuals with heart failure with preserved ejection fraction (HFpEF) with or without CKD (FINEARTS-HF; NCT04435626). The degree of efficacy of finerenone in different races, ages, or sexes also warrants further investigation. For example, as noted earlier, finerenone improved heart function and exercise capacity in post-menopausal model mice [37].
Drug-drug interaction is a common concern in designing therapies. Examining and modeling possible other drug interactions with finerenone is of high relevance as well. A 2022 study developed a pharmacokinetic model for finerenone and its behavior in cytochrome P450 3A4 (CYP3A4)-mediated drug‐drug interactions. Different levels of possible drug interactions were predicted with compounds known to modulate CYP3A4 [43].
Encouraged by results with finerenone, other non-steroidal mineralocorticoid receptor blockers are being examined, including for conditions not significantly improved by finerenone, such as hypertension. For instance, esaxerenone is now approved in Japan for treatment of hypertensive patients with or without diabetes or kidney dysfunction, and a phase 3 trial is ongoing to examine the utility of this drug in diabetic nephropathy [44, 45].
Ultimately, uptake by clinical practitioners in the USA will be shaped by cost and availability of finerenone compared to the steroidal mineralocorticoid receptor antagonists such as spironolactone and eplerenone. Spironolactone and eplerenone are widely available in generic, inexpensive formulations which have demonstrated similar (but not identical) cardiovascular and renal benefits. The current average wholesale price of finerenone is between US$600 and 700 per month. An argument may be made that patients will need to attempt a trial of spironolactone or eplerenone, or have a history of hyperkalemia, before taking finerenone.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–61.
Gueret A, Harouki N, Favre J, Galmiche G, Nicol L, Henry JP, et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension. 2016;67(4):717–23.
Martinez-Martinez E, Buonafine M, Boukhalfa I, Ibarrola J, Fernandez-Celis A, Kolkhof P, et al. Aldosterone target NGAL (neutrophil gelatinase-associated lipocalin) is involved in cardiac remodeling after myocardial infarction through NFkappaB pathway. Hypertension. 2017;70(6):1148–56.
Barrera-Chimal J, Bonnard B, Jaisser F. Roles of Mineralocorticoid receptors in cardiovascular and cardiorenal diseases. Annu Rev Physiol. 2022;84:585–610.
Rico-Mesa JS, White A, Ahmadian-Tehrani A, Anderson AS. Mineralocorticoid receptor antagonists: a comprehensive review of finerenone. Curr Cardiol Rep. 2020;22(11):140.
Pitt B, Pedro Ferreira J, Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: current experience and future perspectives. Eur Heart J Cardiovasc Pharmacother. 2017;3(1):48–57.
Parthasarathy HK, Menard J, White WB, Young WF Jr, Williams GH, Williams B, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29(5):980–90.
Sueta D, Yamamoto E, Tsujita K. Mineralocorticoid receptor blockers: novel selective nonsteroidal mineralocorticoid receptor antagonists. Curr Hypertens Rep. 2020;22(3):21.
Georgianos PI, Agarwal R. Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep. 2021;6(9):2281–91.
•• Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes — FIDELIO-DKD (10). In patients with CKD and T2DM, treatment with finerenone resulted in lower risks of CKD progression and cardiovascular events than placebo. In concert with Figaro-DKD, this trial led to the FDA approval of finerenone for reduction of kidney function decline and cardiovascular complications in adults with CKD associated with T2DM.
•• Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–63. Cardiovascular events with finerenone in kidney disease and type 2 diabetes — FIGARO-DKD (11). Among patients with T2DM and stage 2 to 4 CKD with moderately elevated albuminuria or stage 1 or 2 CKD with severely elevated albuminuria, finerenone therapy improved cardiovascular outcomes as compared with placebo.
Haller H, Bertram A, Stahl K, Menne J. Finerenone: a new mineralocorticoid receptor antagonist without hyperkalemia: an opportunity in patients with CKD? Curr Hypertens Rep. 2016;18(5):41.
Gerisch M, Heinig R, Engelen A, Lang D, Kolkhof P, Radtke M, et al. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab Dispos. 2018;46(11):1546–55.
Heinig R, Gerisch M, Engelen A, Nagelschmitz J, Loewen S. Pharmacokinetics of the novel, selective, non-steroidal mineralocorticoid receptor antagonist finerenone in healthy volunteers: results from an absolute bioavailability study and drug-drug interaction studies in vitro and in vivo. Eur J Drug Metab Pharmacokinet. 2018;43(6):715–27.
Heinig R, Kimmeskamp-Kirschbaum N, Halabi A, Lentini S. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94–8862) in individuals with renal impairment. Clin Pharmacol Drug Dev. 2016;5(6):488–501.
Heinig R, Lambelet M, Nagelschmitz J, Alatrach A, Halabi A. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenonE (BAY 94–8862) in individuals with mild or moderate hepatic impairment. Eur J Drug Metab Pharmacokinet. 2019;44(5):619–28.
Heinig R, Gerisch M, Bairlein M, Nagelschmitz J, Loewen S. Results from drug-drug interaction studies in vitro and in vivo investigating the effect of finerenone on the pharmacokinetics of comedications. Eur J Drug Metab Pharmacokinet. 2020;45(4):433–44.
Lentini S, Heinig R, Kimmeskamp-Kirschbaum N, Wensing G. Pharmacokinetics, safety and tolerability of the novel, selective mineralocorticoid receptor antagonist finerenone - results from first-in-man and relative bioavailability studies. Fundam Clin Pharmacol. 2016;30(2):172–84.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94.
Snelder N, Heinig R, Drenth HJ, Joseph A, Kolkhof P, Lippert J, et al. Population pharmacokinetic and exposure-response analysis of finerenone: insights based on phase IIb data and simulations to support dose selection for pivotal trials in type 2 diabetes with chronic kidney disease. Clin Pharmacokinet. 2020;59(3):359–70.
Bakris GL, Nowack C. Finerenone for albuminuria in patients with diabetic nephropathy–reply. JAMA. 2016;315(3):306.
Gonzalez-Blazquez R, Somoza B, Gil-Ortega M, Martin Ramos M, Ramiro-Cortijo D, Vega-Martin E, et al. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol. 2018;9:1131.
Droebner K, Pavkovic M, Grundmann M, Hartmann E, Goea L, Nordlohne J, et al. Direct blood pressure-independent anti-fibrotic effects by the selective nonsteroidal mineralocorticoid receptor antagonist finerenone in progressive models of kidney fibrosis. Am J Nephrol. 2021;52(7):588–601.
Barrera-Chimal J, Estrela GR, Lechner SM, Giraud S, El Moghrabi S, Kaaki S, et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93(6):1344–55.
Hirohama D, Nishimoto M, Ayuzawa N, Kawarazaki W, Fujii W, Oba S, et al. Activation of Rac1-mineralocorticoid receptor pathway contributes to renal injury in salt-loaded db/db mice. Hypertension. 2021;78(1):82–93.
Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–63.
• Filippatos G, Anker SD, Agarwal R, Ruilope LM, Rossing P, Bakris GL, et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: analyses from the FIGARO-DKD trial. Circulation. 2022;145(6):437–47. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes (28). This FIGARO-DKD reanalysis demonstrated that finerenone reduced new-onset heart failure and improved other heart failure outcomes in patients with CKD and T2DM, irrespective of prior history of heart failure. This study was the first of its kind to explore the role of finerenone in treating heart failure, similar to other MRAs, which are standard of care in reduced ejection fraction.
• Filippatos G, Bakris GL, Pitt B, Agarwal R, Rossing P, Ruilope LM, et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J Am Coll Cardiol. 2021;78(2):142–52. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes (29). In patients with CKD and T2D, finerenone reduced the risk of new-onset atrial fibrillation or flutter (AFF). The risk of kidney or cardiovascular events was reduced irrespective of history of AFF at baseline. This study was important as it was the first of its kind to explore a potential risk reductive effect of finerenone for this condition and would be the first time an MRA is used for this purpose.
Goulooze SC, Snelder N, Seelmann A, Horvat-Broecker A, Brinker M, Joseph A, et al. Finerenone dose-exposure-serum potassium response analysis of FIDELIO-DKD phase III: the role of dosing, titration, and inclusion criteria. Clin Pharmacokinet. 2022;61(3):451–62.
Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, et al. Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep. 2022;7(1):36–45.
Bonnard B, Pieronne-Deperrois M, Djerada Z, Elmoghrabi S, Kolkhof P, Ouvrard-Pascaud A, et al. Mineralocorticoid receptor antagonism improves diastolic dysfunction in chronic kidney disease in mice. J Mol Cell Cardiol. 2018;121:124–33.
Lavall D, Jacobs N, Mahfoud F, Kolkhof P, Bohm M, Laufs U. The non-steroidal mineralocorticoid receptor antagonist finerenone prevents cardiac fibrotic remodeling. Biochem Pharmacol. 2019;168:173–83.
Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol. 2021;52(8):642–52.
Gil-Ortega M, Vega-Martin E, Martin-Ramos M, Gonzalez-Blazquez R, Pulido-Olmo H, Ruiz-Hurtado G, et al. Finerenone reduces intrinsic arterial stiffness in Munich Wistar Fromter rats, a genetic model of chronic kidney disease. Am J Nephrol. 2020;51(4):294–303.
Dutzmann J, Musmann RJ, Haertle M, Daniel JM, Sonnenschein K, Schafer A, et al. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS ONE. 2017;12(9): e0184888.
Pieronne-Deperrois M, Gueret A, Djerada Z, Crochemore C, Harouki N, Henry JP, et al. Mineralocorticoid receptor blockade with finerenone improves heart function and exercise capacity in ovariectomized mice. ESC Heart Fail. 2021;8(3):1933–43.
Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105–14.
Sato N, Ajioka M, Yamada T, Kato M, Myoishi M, Yamada T, et al. A randomized controlled study of finerenone vs. eplerenone in Japanese patients with worsening chronic heart failure and diabetes and/or chronic kidney disease. Circ J. 2016;80(5):1113–22.
Rossing P, Agarwal R, Anker SD, Filippatos G, Pitt B, Ruilope LM, et al. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: a subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes Metab. 2022;24(1):125–34.
Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–84.
Zhang MZ, Bao W, Zheng QY, Wang YH, Sun LY. Efficacy and safety of finerenone in chronic kidney disease: a systematic review and meta-analysis of randomized clinical trials. Front Pharmacol. 2022;13: 819327.
Wendl T, Frechen S, Gerisch M, Heinig R, Eissing T. Physiologically-based pharmacokinetic modeling to predict CYP3A4-mediated drug-drug interactions of finerenone. CPT Pharmacometrics Syst Pharmacol. 2022;11(2):199–211.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44(5):489–97.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15(12):1715–27.
Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jonathan D. Ravid has no disclosures to report. Luke J. Laffin reports grants to his institution from AstraZeneca and Mineralys Therapeutics; Royalties or licenses from Belvoir Media Group and Elsevier; Consulting fees from Medtronic; Payment or honoraria from Cardiometabolic Health Congress; Participation on a Data Safety Monitoring board or Advisory board for Crispr Therapeutics and Eli Lilly Pharmaceuticals; and Stock or stock options for LucidAct Health and Gordy Health.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravid, J.D., Laffin, L.J. Effects of Finerenone, a Novel Nonsteroidal Mineralocorticoid Receptor Antagonist, on Cardiovascular Disease, Chronic Kidney Disease, and Blood Pressure. Curr Cardiol Rep 24, 1251–1259 (2022). https://doi.org/10.1007/s11886-022-01750-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01750-0