Abstract
Purpose of Review
Because of effective combination antiretroviral therapy, people living with HIV (PLWH) are living longer but developing chronic age-related conditions including cardiovascular disease (CVD), the leading cause of death globally. This review aims to discuss the epidemiology, mechanisms, and clinical considerations of CVD in PLWH from a global perspective.
Recent Findings
PLWH are at greater risk for CVD at chronologically younger ages than those without HIV. Potential underlying mechanisms for CVD in PLWH include systemic inflammation, comorbidities, immune-mediated, or treatment-related mechanisms. There is also risk factor variation based on geographical location, including non-traditional CVD risk factors.
Summary
CVD is prevalent in PLWH and increasing on a global scale. Further understanding the unique epidemiology, risk factors, and treatment of CVD in this population will improve the care of PLWH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: HIV-related Cardiovascular Disease as a Global Health Problem
Overview of Impact of HIV Infection on the Cardiovascular System
An estimated 37.7 million individuals are living with human immunodeficiency virus (HIV) globally, with the greatest burden of disease in sub-Saharan Africa [1, 2]. With the advent of combination antiretroviral therapy (cART), the profile of people living with HIV (PLWH) is changing and the world has seen a narrowing in the gap in life expectancy between those living with and without HIV [2]. Although the early epidemic was marked by high mortality, PLWH are now able to live a near normal lifespan [3]. The increasing effectiveness and lower toxicity of cART contributed to a global decrease in opportunistic infections and subsequently a decrease in AIDS-associated morbidity and mortality [4]. Thus, there has been a significant transition in the spectrum of HIV-related disease from opportunistic AIDS-related diseases to those related to aging including heart disease, impaired renal function, and cognitive decline [5].
PLWH are at higher risk for both atherosclerotic CVD (ASCVD) and heart failure. Compared to people without HIV, PLWH have a twofold greater risk of developing ASCVD [6••], a twofold greater risk for myocardial infarction (MI) [7], and a fourfold greater risk of sudden cardiac death compared with people without HIV [8]. Globally, the burden of ASCVD in PLWH has tripled over the last two decades, with the majority in sub-Saharan Africa and the Asia Pacific region [6••]. PLWH have accelerated vascular disease including subclinical coronary artery disease (CAD) [9] and an approximately 50% increased risk of MI as compared to uninfected controls [10]. In terms of heart failure, prior to effective cART, dilated cardiomyopathy was relatively common in the setting of poor viral suppresson. However, in the contemporary treatment era, diastolic dysfunction has emerged as the predominant pathophysiology leading to heart failure with preserved ejection fraction (HFpEF). Despite improvements in HIV care, questions still remain regarding how to optimally predict, prevent, risk stratify, and treat CVD in PLWH. The purpose of this review is to provide a comprehensive overview of the global impact, epidemiology, mechanisms, and treatment of HIV-related CVD.
Epidemiology of HIV and CVD
Currently, in higher-income countries (HIC), up to one-third of PLWH are 50 years of age or older, in contrast to roughly 10% in low to middle-income countries (LMIC) [11]. An epidemiologic model based on the AIDS Therapy Evaluation in the Netherlands (ATHENA) cohort estimated that by the year 2030, 73% of PLWH will be 50 years of age or older, and 78% of PLWH will have CVD [12]. As HIV-associated CVD becomes more prevalent and poses a higher burden on the health care system, it is imperative to understand geographical variations in disease presentation, course and CVD risk factors.
The phenotype of HIV associated CVD differs between LMIC and HIC both in terms of ASCVD and heart failure. Much of the contemporary data focused on ASCVD originated from studies performed in HICs, and it has been well-established that PLWH have a twofold increased risk for myocardial infarction compared to people without HIV [13]. Less is known about the prevalence of ASCVD in LMIC, as most studies used to estimate incident CVD originated from Europe, North America, and the Asia Pacific region [6••].
On a global scale, there are geographical differences in HIV-associated heart failure phenotypes. In HIC, diastolic dysfunction is the predominant heart failure phenotype in PLWH, in the setting of hypertension and advanced age [14]. However, in LMIC, left ventricular systolic dysfunction is relatively more prevalent compared to HIC; however, much of our current understanding of cardiomyopathy in LMIC stems from studies performed prior to widespread cART [15].
The differences in global CVD epidemiology may be partially attributed to geographical variations in demographics and CVD risk factors (Table 1). Within sub-Saharan Africa, PLWH are younger, with a lower prevalence of traditional CVD risk factors including tobacco smoking and dyslipidemia, with the exception of hypertension which has a higher prevalence compared with PLWH from HIC [16••]. In contrast, PLWH in the Asia–Pacific region have a high prevalence of all conventional CVD risk factors such as diabetes mellitus, hyperlipidemia, tobacco use, and hypertension [17,18,19]. Understanding differences in risk factor profiles and their impact on CVD throughout geographical regions is essential to better inform future treatment strategies, and more studies are needed, especially in LMIC.
CVD Mechanisms in HIV
The pathophysiology of HIV-associated CVD is multifactorial and incompletely understood (Fig. 1). The purported mechanisms that may contribute to ASCVD in HIV include viral-mediated (e.g., immune activation), metabolic side effects of cART, and non-viral-mediated effects that may lead to chronic persistent systemic inflammation.
Viral-mediated Mechanisms of ASCVD
Although there is minimal evidence of ASCVD in PLWH before the era of cART, the few that were published are striking. Joshi et al. reported on 3 of 6 children who died of acquired immune deficiency syndrome (AIDS) and were found to have infiltration of lymphocytes and mononuclear cells in vessel walls, a precursor to atherosclerosis [20]. Additionally, other post-mortem analyses revealed major atherosclerotic plaques in young patients between 23 and 32 years of age who had been living with HIV [21]. These early reports of premature atherosclerosis in the setting of HIV/AIDS led to numerous investigations into the pathogenesisof of HIV-mediated ASCVD.
There is now strong evidence supporting the pathophysiology of untreated HIV infection leading to pro inflammatory effects causing CD4 + T-cell depletion, increased intestinal permeability, and microbial translocation [22]. CD4 + T-cell depletion in PLWH is associated with increased risk of ASCVD events such as acute MI. Additionally, HIV can directly stimulate the endothelium causing increased endothelial permeability and subsequent entrance of leukocytes into vessel walls with resultant vascular inflammation and endothelial dysfunction [23].
In the general population, there is overwhelming evidence that systemic inflammation plays an important role in the development and progression of ASCVD [24]. Levels of C-reactive protein (CRP), a proinflammatory marker, increase over time in PLWH, and those who progress to AIDS have steeper increases in the biomarker [25]. Interleukin (IL)-6, a cytokine-activating stimulation of CRP release from hepatocytes, is also elevated in PLWH and is a prognostic marker for CVD and all-cause mortality [26]. Several studies have also reported higher serum levels of proprotein convertase/kexin type 9 (PCSK9), a protease involved in cholesterol trafficking and a mediator of systemic inflammation, in PLWH compared to controls without HIV [27,28,29].
While treatment with cART reduces some inflammatory markers, many markers remain elevated even with undetectable HIV RNA levels [30]. There is evidence suggesting that while cART promotes CD4 + T-cell recovery and decreases the level of inflammation, there is still low-level persistent viral replication likely within lymphoid organs. The Strategies for Management of Antiretroviral Therapy or ”SMART” trial compared episodic cART use, based on CD4 + T-cell counts, with continuous cART and found that groups with higher inflammatory markers had a higher odds of future ASCVD [31]. A separate study combining data on over 3700 PLWH from 3 worldwide studies found that baseline levels of inflammatory markers were associated with higher future CVD risk [32].
Non-viral-mediated Mechanisms of ASCVD
Alterations in the lipid profile of PLWH are also thought to play an important role in mediating HIV-associated ASCVD. In particular, both elevated triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C) levels are frequently present in PLWH. Abnormalities of lipid metabolism were reported early in the epidemic in people with advanced AIDS prior to the widespread initiation of cART. It was noted that patients with AIDS had elevated TG levels resulting from increased hepatic output of low-density lipoprotein cholesterol (LDL) and decreased clearance through cytokine-mediated decrease in lipase activity [33, 34]. Additionally, greater levels of pro-atherogenic small dense LDL and lipoprotein(a) (Lp(a)) were found in PLWH [35]. Higher levels of HIV RNA have been associated with increased atherogenic effect of allele-specific apolipoprotein-a [36]. In a prospective study of men with HIV, significant declines in mean serum total cholesterol (TC), HDL-C, and LDL were observed for several years after contraction of HIV when compared to pre-seroconversion levels [37]. There is not a known unifying etiology to explain why HIV infection causes these changes in lipid profiles; however, alterations in cholesterol trafficking and inflammation may play roles [38].
ART-related Effects on CVD Risk
The widespread use of cART revolutionized the care of PLWH, dramatically decreasing mortality; however, there are also significant metabolic side effects associated with cART including dyslipidemia, insulin resistance, and overt diabetes mellitus. The complex interplay of HIV infection itself, traditional cardiovascular risk factors, and cART, and the extent to which these factors contribute to progression of CVD in PLWH is an area of active investigation. Findings from the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study indicated that the incidence of MI increased with longer exposure to cART [39]. The DAD study included 3 years of follow-up and reported a significant relationship specifically between exposure to protease inhibitors and myocardial infarction [40]. This relationship was not seen with exposure to non-nucleoside reverse-transcriptase inhibitors and suggests that the previously reported finding of an excess risk with increased exposure to cART was likely due to exposure to early generations of the protease inhibitor class.
Protease inhibitors are known to increase total cholesterol and low-density lipoprotein cholesterol levels to a greater extent than non-nucleoside reverse transcriptase inhibitors; however, newer protease inhibitor formulations have fewer metabolic side effects compared to earlier generations [41]. An advantage of the non-nucleoside reverse transcriptase inhibitors is that they can increase HDL cholesterol levels significantly [42]. Notably, increases in the risk of MI associated with protease inhibitors were not fully explained by changes in lipid profile, suggesting alternative mechanisms by which protease inhibitors may cause increased CVD. Early in the HIV/AIDS pandemic, there were several reports which described alterations in glucose metabolism in patients treated with the then-approved protease inhibitors [43, 44]. These alterations in glucose metabolism and subsequent glucose intolerance were attributed to insulin resistance thought to be due to defects in triglyceride storage from protease inhibitors [45]. More recent studies showed that direct cellular mechanisms may be responsible for promoting atherogenesis [46, 47]. Notably, the studies that showed a positive association between protease inhibitors and CVD evaluated agents such as indinavir, which is no longer a first line agent. The more commonly used protease inhibitors (such as atazanavir/darunavir) have not been shown to increase CVD risk [48].
Additionally, patients initiated on cART often experience weight gain, which may exacerbate metabolic abnormalities associated with HIV and cART [49]. The gain in abdominal fat and body mass index has been associated with increased rates of incident ASCVD and diabetes mellitus in PLWH on cART [50]. As obesity is increasing among PLWH, further understanding risk factors, including specific cART regimens associated with greater weight gain, are needed, as well as the optimal approach to manage weight gain in this population.
Common Comorbidities and Increased Risk of CVD
Although HIV infection alone is an independent risk factor for CVD, the increased incidence of comorbidities commonly associated with aging, particularly in HIC, often confounds the association. A large multi-cohort study found that 18% of PLHW were already obese at the time of initial diagnosis, and 18% of those overweight when initiating cART became obese within 3 years [51]. Similarly, the prevalence of diabetes mellitus in PLWH in the USA (12–19%) closely mirrors that seen in people without HIV and adds to overall CVD risk [52]. The prevalence of hypertension among over 65,000 PLWH across the world was found to be 35% in those on cART in comparison to an estimated 30% in people without HIV [53]. Since hypertension is a significant worldwide contributor to CVD, these estimates highlight the importance of CVD surveillance and prevention programs.
Tobacco use is one of the classic CVD risk factors that has fortunately decreased over time among the general population of HIC; however, PLWH are still estimated to have a 2–3 times greater prevalence of tobacco smoking compared with uninfected people [54]. A Danish study reported a fourfold increased risk of CVD-related mortality among PLWH who were current smokers compared to never smokers with an estimated 6 years of life lost secondary to smoking in PLWH [55]. Illicit drug use among PLWH varies considerably depending on the country and study; however, PLWH generally have higher rates of both injection and non-injection drug use. Cocaine use in particular is linked to increased CVD risk, and the prevalence of use in PLWH has been estimated at 36% in one meta-analysis [56]. The approach to CVD prevention should consider the multitude of risk factors present in the PLWH population and ideally be tailored to the individual.
The Role of Cardiovascular Imaging in HIV-related Heart Disease
Echocardiography remains the cornerstone modality globally for detecting HIV-related clinical and subclinical changes in cardiac structure and function. With the advent of earlier HIV diagnosis and treatment and viral suppression with cART, the phenotype of HIV-related cardiomyopathy has shifted from that of left ventricular (LV) systolic dysfunction with chamber dilation to one characterized by diastolic dysfunction, subtler changes in LV structure, and/or subclinical changes in systolic function, as well as occult pulmonary hypertension. Subclinical changes could presage the progression to and development of symptomatic heart failure and arrhythmias.
A recent large cross-sectional study compared echo findings in 1185 middle-aged American men living with and without HIV with similar risk factors for HIV acquisition in the current cART era who were enrolled in the Multicenter AIDS Cohort Study (MACS) [57]. The majority of the men living with HIV were virally suppressed among whom prevalence of LV systolic dysfunction was low at 2.4%, similar to that seen in the HIV seronegative men. Positive HIV serostatus was related to larger LV mass, left atrial, and right ventricular (RV) sizes; lower RV systolic function; and higher prevalence of diastolic dysfunction [57]. HIV disease severity factors were not related to the echo findings. These findings support that of a number of other studies in HIC that also reported the higher prevalence of diastolic dysfunction among PLWH [61,62,63,64,65]. Subclinical differences in LV function as detected by LV global longitudinal strain (GLS) have also been reported. A retrospective analysis of clinical echocardiograms from 253 PLWH with LVEF > 50% observed abnormal LV GLS in over three quarters of individuals [66]. Abnormal GLS was significantly correlated with CD4 + T-cell count with borderline relationship with HIV viral load [66]. Studies from LMIC, however, suggest different findings. In a study among 394 young South African individuals living with HIV, half of whom were oncART, but had fewer cardiac risk factors than participants in American studies; there were no differences in cardiac structure and function compared to HIV-negative controls [58]. Other studies of PLWH in Africa also report low prevalence (1–7% compared to 22–37% in HIC) of diastolic dysfunction with no difference by HIV serostatus [59, 60]. These results may be attributable to the younger ages of African PLWH compared to PLWH in HIC and the lower prevalences of traditional cardiac risk factors.
Echocardiography can also detect pulmonary hypertension, for which PLWH may be at increased susceptibility. A recent study of 13,028 participants from the Veterans Aging Cohort Study (VACS) reported a higher incidence rate of pulmonary hypertension among veterans with HIV compared to HIV-seronegative veterans (28.6 vs. 23.4 per 1000 person-years, p = 0.0004; hazard ratio 1.25, 95% confidence interval 1.12–1.40, p < 0.0001) [61]. The higher risk remained significant after multivariable adjustment for non-HIV factors predisposing to pulmonary hypertension, thus supporting an independent HIV-association. Higher HIV viral loads and lower CD4 counts were associated with increased pulmonary hypertension risk.
Cardiovascular magnetic resonance imaging (CMR) can also provide insight into HIV-associated subclinical myocardial disease, particularly diffuse fibrosis and macroscopic scar using late gadolinium enhancement (LGE). Several studies in HIC have reported the independent association between HIV serostatus and increased extracellular volume fraction (ECV) or native myocardial T1 values reflecting diffuse fibrosis and/or focal LGE scar, generally involving nonischemic midwall or subepicardial patterns. Elevated T1 times among PLWH were associated with increased risk for a composite of major adverse cardiovascular events [65]. There is a suggestion that CMR findings in PLWH may differ in LMIC. A recent study compared CMR findings in a middle-aged cohort of South African individuals, of whom 134 were predominantly virally-suppressed PLWH and 95 were HIV seronegative and matched by age, sex, and hypertension status [61]. The observed prevalence of CMR LGE was higher among both PLWH and uninfected controls compared to data from HIC and did not differ by HIV serostatus. However, CMR ECV was higher among PLWH, as seen in HIC. The association between HIV serostatus and myocardial fibrosis was stronger among women and younger participants despite the absence of other traditional cardiac risk factors. These findings support differences in cardiac phenotypic profiles between PLWH in LMIC compared to HIC that warrant further investigation. Several studies have also reported the higher incidence of myocardial steatosis using MR spectroscopy, which may contribute to increased risk for diastolic dysfunction and heart failure [62, 64, 66]. Increased left atrial volume has also been described which may be a marker for both diastolic dysfunction and increased risk for atrial arrhythmia [67].
In terms of ASCVD, cardiac computed tomography (CT) and coronary CT angiography (CCTA) have been used to characterize subclinical CAD in PLWH and have provided important insights into HIV-related coronary atherosclerosis [68]. An analysis of the MACS showed that men living with HIV had an increased prevalence of non-calcified and mixed coronary plaques compared to uninfected men using CCTA, even after adjusting for CVD risk factors [69]. However, only a borderline association remained between HIV status and the degree of coronary artery stenosis after adjustment for risk factors. The MACS study also evaluated coronary artery calcification (CAC), an important marker of coronary atherosclerosis, and reported that after adjustment for age, race, and study location, men living with HIV had a greater prevalence of CAC than controls and they were at significantly higher risk for development of CAC on serial imaging over a mean follow-up of 5 years [69, 70]. Additional investigation within the MACS demonstrated that progression of coronary artery stenosis was associated with cART adherence and viremia [71]. These studies focused on subclinical CAD in the MACS cohort have provided important insights on the extent and characteristics of CAD in PLWH. The findings of prevalent subclinical CAD suggest the importance of modifying traditional CVD risk factors in PLWH.
Clinical Management Considerations
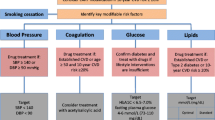
The clinical management of CVD in PLWH typically mirrors recommendations for those without HIV, however there are important additional considerations for this patient population. As in the general population, risk factors in PLWH such as age, diabetes, hypertension, tobacco use, and hyperlipidemia contribute to increased cardiovascular risk. PLWH carry excess risk for CVD beyond these factors, but the relatively recent transformation of HIV into a chronic disease has resulted in limited long-term data on CVD incidence [72, 73]. Thus, data in the current era tends to rely on observational, prospective studies using surrogate markers for CVD. A 2019 statement by the AHA proposed multiplication of a patient’s atherosclerotic CVD risk by 1.5–2 times if risk-enhancing factors, including prolonged duration of HIV viremia, delay in cART initiation, nadir, or current CD4 count < 350 cells/mm3, cART failure or nonadherence, metabolic syndrome, abnormalities in fat distribution, hepatic steatosis, and HCV co-infection, are present in PLWH [74]. Patients who lack those specific risk factors, receive prompt treatment for HIV, and achieve viral suppression may not have significantly elevated CVD risk; however, further studies are needed to confirm this. The most commonly used risk estimator in the USA is the ASCVD 10-year pooled cohort equations; however, it is not specific to HIV [75, 76]. In contrast, the DAD prospective multinational HIV cohort study identified risk factors among PLWH that are associated with increased risk, (including CD4 count, cumulative exposure to protease inhibitors and nucleoside/nucleotide reverse transcriptase inhibitors, and abacavir use), and utilized this data to create a 5-year CVD risk equation [39, 77]. Similarly, the European AIDS Clinical Society has published guidelines for the management of PLWH, including CVD risk factors [78].
Published ASCVD calculators and national guidelines largely serve to identify high risk PLWH earlier and begin lipid-lowering therapies in addition to intensive lifestyle modifications, when appropriate. An important consideration for PLWH is the interaction of statins with certain cART medications. Patients taking cytochrome P450 inhibitors, such as ritonavir and cobicistat, are at particularly high risk for drug interactions. In general, simvastatin and lovastatin have the highest cytochrome P450 metabolism of all the statins and should be avoided in PLWH, while pravastatin and pitavastatin are least likely to cause pharmacologic interaction with commonly prescribed medications [79].
In contrast to HIC, HIV infection is not specifically listed in guidelines or national strategies regarding CVD screening and management in most LMIC [80]. Risk reduction for PLWH in LIC, notably sub-Saharan Africa, is primarily focused on effective screening and early identification of HIV with basic blood pressure and glucose testing, where available. Imaging techology needed to assist with diagnosis of CAD, heart failure, and other forms of CVD is not commonly available. Facility-based and community-based integrated care models work to combine HIV treatment and CVD risk reduction and have shown feasibility in sub-Saharan Africa [81]. However, further studies are needed to assess the long-term success of these approaches in reducing CVD outcomes.
At present, there are few proven strategies to prevent CVD in PLWH, but certain therapies aimed at lowering this risk are currently being studied. Statin therapy used to lower cholesterol in the general population may also be effective at lowering the CVD risk in PLWH. The ongoing REPRIEVE trial is a large, ongoing, multi-center randomized controlled trial evaluating the effect of pitavastatin compared to placebo to reduce the risk of CVD in stable PLWH on chronic cART [82]. Given the growing evidence supporting the role of increased systemic inflammation on risk of CVD in PLWH, several novel interventions have been investigated to reduce CVD risk. An anti-inflammatory treatment approach was investigated in randomized clinical trials using agents such as methotrexate and colchicine in PLWH but did not show improvement in endothelial function, an index of cardiovascular risk, measured non-invasively [83, 84]. Another clinical trial found that treatment with canakinumab, a monoclonal antibody binding IL‐1β, lowered systemic markers of inflammation and aortic inflammation measured by FDG‐PET in PLWH [85]. Recently, PCSK9 inhibitor therapy, a monoclonal antibody that improves LDL cholesterol trafficking, was tested in PLWH and shown to improve coronary endothelial function after 6 weeks of treatment [86]. Furthermore, long-term therapeutic intervention trials are needed to evaluate CVD outcomes using both traditional and novel interventions in PLWH.
Future Directions and Knowledge Gaps
As HIV treatment and management have evolved over the decades, great strides have been made towards recognition of the epidemiology and underlying pathophysiology of associated CVD, including but not limited to ASCVD, systolic and diastolic heart failure, and pulmonary hypertension. The tradeoff of improved longevity in this population is the greater prevalence of age-related and metabolic diseases as has been shown by increasing rates of diabetes, hypertension, obesity, and hyperlipidemia. The development of highly effective cART regimens and tailoring of certain drug classes to minimize harmful side effects remain active fields of investigation. Earlier diagnosis of the subclinical cardiovascular sequelae that afflicts this population is more common, particularly in HIC, with the help of specialized non-invasive imaging studies as such echocardiography and CT and CMR when indicated. However, despite the disproportionate burden of the HIV epidemic concentrated in LMIC, data on epidemiology of CVD in this population is lacking and remains desperately needed.
The recent era of prolonged HIV survival and near-normal life expectancy leaves obvious gaps in information regarding long-term health outcomes. There are currently over 70 ongoing studies including registered clinical trials focused on preventing and treating CVD in PLWH. One of the larger pending studies is the HIV/HEART Aging study, a prospective multicenter observational study currently ongoing in Germany to assess 15-year CVD outcomes (NCT04330287). In addition to further investigation into the underlying mechanisms contributing to CVD in PLWH, future research should focus on developing accurate prediction models and effective prevention and management strategies in both HIC and LMIC.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
CONFRONTING INEQUALITIES: Lessons for pandemic responses from 40 years of AIDS [Internet]. GLOBAL AIDS UPDATE 2021; Available from: https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf
Murray CJL, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70.
Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE. 2013;8: e81355.
Michaels SH, Clark R, Kissinger P. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;339:405–6.
Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–97.
•• Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation. 2018;138:1100–12. Review analyzing the rate and risk of CVD in PLWH.
Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–73.
Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–6.
Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–67.
Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30.
Nou E, Lo J, Hadigan C, Grinspoon SK. Pathophysiology and management of cardiovascular disease in patients with HIV. Lancet Diabetes Endocrinol. 2016;4:598–610.
Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–8.
Grinspoon SK, Grunfeld C, Kotler DP, Currier JS, Lundgren JD, Dubé MP, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation. 2008;118:198–210.
Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–6.
Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–74.
•• So-Armah K, Benjamin LA, Bloomfield GS, Feinstein MJ, Hsue P, Njuguna B, et al. HIV and cardiovascular disease. Lancet HIV. 2020;7:e279–93. Review highlighting the global variation in risk factors and future research needs in HIV associated CVD.
Bijker R, Kumarasamy N, Kiertiburanakul S, Pujari S, Lam W, Chaiwarith R, et al. Cardiovascular disease incidence projections in the TREAT Asia HIV Observational Database (TAHOD). Antivir Ther. 2019;24:271–9.
Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61:511–23.
Bijker R, Choi JY, Ditangco R, Kiertiburanakul S, Lee MP, Siwamogsatham S, et al. Cardiovascular Disease and Cardiovascular Disease Risk in HIV-Positive Populations in the Asian Region. Open AIDS J. 2017;11:52–66.
Joshi VV, Pawel B, Connor E, Sharer L, Oleske JM, Morrison S, et al. Arteriopathy in children with acquired immune deficiency syndrome. Pediatr Pathol. 1987;7:261–75.
Paton P, Tabib A, Loire R, Tete R. Coronary artery lesions and human immunodeficiency virus infection. Res Virol. 1993;144:225–31.
Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71.
Kline ER, Sutliff RL. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med. 2008;56:752–69.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70.
Neuhaus J, Jacobs DR, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95.
Kohli P, Ganz P, Ma Y, Scherzer R, Hur S, Weigel B, et al. HIV and hepatitis C-coinfected patients have lower low-density lipoprotein cholesterol despite higher proprotein convertase subtilisin kexin 9 (PCSK9): an apparent “PCSK9-lipid paradox.” J Am Heart Assoc. 2016;5: e002683.
Boccara F, Ghislain M, Meyer L, Goujard C, Le May C, Vigouroux C, et al. Impact of protease inhibitors on circulating PCSK9 levels in HIV-infected antiretroviral-naive patients from an ongoing prospective cohort. AIDS. 2017;31:2367–76.
Leucker TM, Weiss RG, Schär M, Bonanno G, Mathews L, Jones SR, et al. Coronary endothelial dysfunction is associated with elevated serum PCSK9 levels in people with HIV independent of low-density lipoprotein cholesterol. J Am Heart Assoc. 2018;7: e009996.
Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–67.
Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96.
Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS ONE. 2016;11: e0155100.
Grunfeld C, Kotler DP, Shigenaga JK, Doerrler W, Tierney A, Wang J, et al. Circulating interferon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1991;90:154–62.
Shor-Posner G, Basit A, Lu Y, Cabrejos C, Chang J, Fletcher M, et al. Hypocholesterolemia is associated with immune dysfunction in early human immunodeficiency virus-1 infection. Am J Med. 1993;94:515–9.
Feingold KR, Krauss RM, Pang M, Doerrler W, Jensen P, Grunfeld C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab. 1993;76:1423–7.
Enkhmaa B, Anuurad E, Zhang W, Abbuthalha A, Li X-D, Dotterweich W, et al. HIV disease activity as a modulator of lipoprotein(a) and allele-specific apolipoprotein(a) levels. Arterioscler Thromb Vasc Biol. 2013;33:387–92.
Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–82.
Sarkar S, Haberlen S, Whelton S, E Schneider E, Kingsley L, Palella F, et al. Greater IL-6, D-dimer, and ICAM-1 levels are associated with lower small HDL particle concentration in the multicenter AIDS cohort study. Open Forum Infect Dis. 2019;6:ofz474.
Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003.
DAD Study Group, Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte A d’Arminio, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35.
Kajogoo VD, Gorret Atim M, Amare D, Geleta M, Muchie Y, Tesfahunei HA, et al. HIV protease inhibitors and insulin sensitivity: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12: 635089.
van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral- therapy-naive patients infected with HIV-1. PLoS Med. 2004;1: e19.
Visnegarwala F, Krause KL, Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947.
Dubé MP, Johnson DL, Currier JS, Leedom JM. Protease inhibitor-associated hyperglycaemia. Lancet. 1997;350:713–4.
Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–3.
Zhou H, Pandak WM, Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol. 2005;68:690–700.
Allred KF, Smart EJ, Wilson ME. Estrogen receptor-alpha mediates gender differences in atherosclerosis induced by HIV protease inhibitors. J Biol Chem. 2006;281:1419–25.
Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–30.
Lakey W, Yang L-Y, Yancy W, Chow S-C, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013;29:435–40.
Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS ONE. 2010;5: e10106.
Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32:50–8.
Sarkar S, Brown TT. Diabetes in People with HIV. Curr Diab Rep. 2021;21:13.
Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11:530–40.
Raposeiras-Roubín S, Abu-Assi E, Iñiguez-Romo A. Tobacco, illicit drugs use and risk of cardiovascular disease in patients living with HIV. Curr Opin HIV AIDS. 2017;12:523–7.
Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56:727–34.
Nduka CU, Uthman OA. Drug abuse in people living with HIV in the era of highly active antiretroviral therapy: a systematic review and meta-analysis. J Addict Res Ther [Internet]. 2015 [cited 2022 Apr 10];06. Available from: https://www.omicsonline.org/open-access/drug-abuse-in-people-living-with-hiv-in-the-era-of-highly-active-antiretroviral-therapy-a-systematic-review-and-metaanalysis-2155-6105-1000255.php?aid=66245
Doria de Vasconcellos H, Post WS, Ervin A-M, Haberlen SA, Budoff M, Malvestutto C, et al. Associations between HIV serostatus and cardiac structure and function evaluated by 2-dimensional echocardiography in the multicenter AIDS cohort study. J Am Heart Assoc. 2021;10:e019709.
Roozen GVT, Meel R, Peper J, Venter WDF, Barth RE, Grobbee DE, et al. Electrocardiographic and echocardiographic abnormalities in urban African people living with HIV in South Africa. PLoS ONE. 2021;16: e0244742.
Woldu B, Temu TM, Kirui N, Christopher B, Ndege S, Post WS, et al. Diastolic dysfunction in people with HIV without known cardiovascular risk factors in Western Kenya. Open Heart. 2022;9: e001814.
Mahtab S, Lawrenson J, Jamieson-Luff N, Asafu-Agyei NA, Meiring A, Lemmer-Hunsinger C, et al. Echocardiographic findings in a cohort of perinatally HIV-infected adolescents compared with uninfected peers from the Cape Town adolescent antiretroviral cohort. J Am Soc Echocardiogr. 2020;33:604–11.
Duncan MS, Alcorn CW, Freiberg MS, So-Armah K, Patterson OV, DuVall SL, et al. Association between HIV and incident pulmonary hypertension in US Veterans: a retrospective cohort study. Lancet Healthy Longev. 2021;2:e417–25.
Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–22.
Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. 2016;9: e004430.
Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. 2015;212:1544–51.
de Leuw P, Arendt CT, Haberl AE, Froadinadl D, Kann G, Wolf T, et al. Myocardial fibrosis and inflammation by CMR predict cardiovascular outcome in people living with HIV. JACC Cardiovasc Imaging. 2021;14:1548–57.
Neilan TG, Nguyen K-L, Zaha VG, Chew KW, Morrison L, Ntusi NAB, et al. Myocardial steatosis among antiretroviral therapy-treated people with human immunodeficiency virus participating in the REPRIEVE trial. J Infect Dis. 2020;222:S63–9.
Wu KC, Haberlen SA, Plankey MW, Palella FJ, Piggott DA, Kirk GD, et al. Human immunodeficiency viral infection and differences in interstitial ventricular fibrosis and left atrial size. Eur Heart J Cardiovasc Imaging. 2021;22:888–95.
Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53.
Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. American College of Physicians; 2014;160:458–67.
Kingsley LA, Deal J, Jacobson L, Budoff M, Witt M, Palella F, et al. Incidence and progression of coronary artery calcium in HIV-infected and HIV-uninfected men. AIDS. 2015;29:2427–34.
Post W, Haberlen HS, Witt MD, Zhang Long, Jacobson LP, Brown TT, et al. Suboptimal HIV suppression is associated with progression of coronary artery stenosis: the multicenter AIDS cohort study (MACS) longitudinal coronary CT angiography study. AIDS (London, England).
Feinstein MJ. Cardiovascular disease risk assessment in HIV: navigating data-sparse zones. Heart. 2016;102:1157–8.
Hsue PY, Waters DD. Time to recognize HIV infection as a major cardiovascular risk factor. Circulation. 2018;138:1113–5.
Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140:e98-124.
Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137:2203–14.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47.
Friis-Møller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the data-collection on adverse effects of anti-HIV drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23:214–23.
Ryom L, Cotter A, De Miguel R, Béguelin C, Podlekareva D, Arribas JR, et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21:617–24.
Myerson M, Malvestutto C, Aberg JA. Management of lipid disorders in patients living with HIV. J Clin Pharmacol. 2015;55:957–74.
Okello S, Amir A, Bloomfield GS, Kentoffio K, Lugobe HM, Reynolds Z, et al. Prevention of cardiovascular disease among people living with HIV in sub-Saharan Africa. Prog Cardiovasc Dis. 2020;63:149–59.
Ojo T, Lester L, Iwelunmor J, Gyamfi J, Obiezu-Umeh C, Onakomaiya D, et al. Feasibility of integrated, multilevel care for cardiovascular diseases (CVD) and HIV in low- and middle-income countries (LMICs): A scoping review. PLoS ONE. 2019;14: e0212296.
Douglas PS, Umbleja T, Bloomfield GS, Fichtenbaum CJ, Zanni MV, Overton ET, et al. Cardiovascular risk and health among people with human immunodeficiency Virus (HIV) eligible for primary prevention: insights from the REPRIEVE Trial. Clin Infect Dis. 2021;73:2009–22.
Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, et al. Safety and impact of low-dose methotrexate on endothelial function and inflammation in individuals with treated human immunodeficiency virus: AIDS Clinical Trials Group Study A5314. Clin Infect Dis. 2019;68:1877–86.
Hays AG, Schär M, Barditch-Crovo P, Bagchi S, Bonanno G, Meyer J, et al. A randomized, placebo-controlled, double-blinded clinical trial of colchicine to improve vascular health in people living with HIV. AIDS. 2021;35:1041–50.
Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, et al. IL-1β inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol. 2018;72:2809–11.
Leucker TM, Gerstenblith G, Schär M, Brown TT, Jones SR, Afework Y, et al. Evolocumab, a PCSK9-monoclonal antibody, rapidly reverses coronary artery endothelial dysfunction in people living with HIV and people with dyslipidemia. J Am Heart Assoc. 2020;9: e016263.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Global Cardiovascular Health
Rights and permissions
About this article
Cite this article
Wagle, A., Goerlich, E., Post, W.S. et al. HIV and Global Cardiovascular Health. Curr Cardiol Rep 24, 1149–1157 (2022). https://doi.org/10.1007/s11886-022-01741-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01741-1