Abstract
Purpose of Review
Positron emission tomography (PET) is a leading non-invasive modality for the diagnosis of coronary artery disease due to its diagnostic accuracy and high image quality. With the latest advances in PET systems, clinicians are able to assess for myocardial ischemia and myocardial blood flow while exposing patients to extremely low radiation doses. This review will focus on the basics of acquisition and processing of hybrid PET/CT systems from appropriate patient selection to common artifacts and pitfalls.
Recent Findings
The continued development of hybrid PET/CT technology is producing scanners with exquisite sensitivity capable of generating high-quality images while exposing patients to low radiation doses. List mode acquisition is an essential component in all modern PET/CT scanners allowing simultaneous dynamic and ECG-gated imaging without lengthening scan duration. Various PET radiotracers are currently being developed but rubidium-82 and 13N-ammonia remain the most commonly used perfusion radiotracers. The development of mini 13N-ammonia cyclotrons is a promising tool that should increase access to this radiotracer. Misregistration, attenuation from extra-cardiac activity, and patient motion are the most common causes of artifacts during perfusion imaging. Techniques to automatically realign images and correct respiratory or patient motion artifacts continue to evolve.
Summary
Despite the continuous evolution of PET imaging techniques, basic knowledge of scan parameters, acquisition techniques, and post processing tools remains essential to ensure high-quality images are produced and artifacts are recognized and corrected. Future research should focus on optimizing scanners to allow for shorter scan protocols and lower radiation exposure as well as continue developing techniques to minimize and correct for motion and misregistration artifacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myocardial perfusion imaging (MPI) is a cornerstone for the diagnosis of patients with suspected or established coronary artery disease (CAD). Single photon emission computed tomography (SPECT) has been a mainstay for the diagnosis and risk stratification for ischemic heart disease. Currently, in the era of high value-based care, there is a push towards improving diagnostic accuracy and cost-effective imaging. Cardiac positron emission tomography (PET) is now becoming a favored method for assessing myocardial ischemia owing to its high spatial resolution, high diagnostic accuracy, and ability to assess regional myocardial blood flow. With the increasing availability of certain radiotracers such as rubidium-82 (82Rb) and hybrid PET computed tomography (PET/CT) scanners, more clinicians have the ability to provide optimal diagnostic imaging accuracy. This review will focus on the basics of acquisition and processing of hybrid PET/CT systems from appropriate patient selection to common artifacts and pitfalls.
Basics of PET imaging
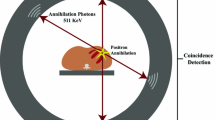
PET imaging relies on the detection of photons emitted from the decay of radiotracers injected into the body. During the decay of radiotracers, positrons are emitted which collide (annihilate) with an adjacent electron. This process results in the formation of two 511-keV photons (gamma-rays) moving at 180° from each other. The PET scanner has 360° stationary detector rings, allowing the simultaneous detection of these photons and whole heart coverage. The detectors can be configured to register only photon pairs that strike opposing detectors at approximately the same time (coincidence detection). These detector rings can have septa allowing coincidence detection in a particular plane (2D systems) or no septa with simultaneous detection of coincidence events in all directions (3D systems) [1]. During a scan, millions of coincidence events are recorded, and projections of the activity distribution are used to reconstruct images. Traditional PET systems are standalone scanners using a rotating rod for attenuation correction [2]. Current systems are a hybrid PET with computed tomography (PET/CT) scanners. The CT is typically used for photon attenuation and scatter correction of the PET data [3].
Patient Selection
In general, myocardial perfusion imaging (MPI) using PET/CT has better diagnostic accuracy compared to the more widely available SPECT. Advantages include shorter scanning time and improved efficiency, superior spatial and temporal resolution, ability to quantify myocardial blood flow, and lower radiation exposure [4•]. Until PET/CT becomes more widely available, thoughtful patient selection is required. The following groups of patients represent ideal candidates for PET MPI:
-
1.
Extreme obesity: High BMI, particularly those > 40 kg/m2, may cause attenuation artifacts on SPECT leading to a decrease in specificity to as low as 17% [5]. Harnett et al. found that PET/CT performs better in this sub-group—sensitivity and specificity of PET/CT was better compared to SPECT to detect ≥70% stenosis (sensitivity: PET 86% vs. SPECT 75%; specificity: PET 87% vs. SPECT 60%) [6].
-
2.
Female patients: Taqueti et al. astutely noted that PET/CT offers incremental advantages in women. These include better diagnostic accuracy in the presence of breast and adipose tissue on the chest wall as well as in the presence of smaller left ventricular cavities, decreased radiation exposure using PET/CT which is particularly relevant in women of child-bearing age, and the ability to quantify coronary flow reserve on PET/CT given that microvascular dysfunction is twice as prevalent in women compared to men [7].
-
3.
Young patients: Reducing radiation exposure to as low as reasonably achievable is important in young patients to decrease downstream cancer risk. A PET/CT study leads to ~3.6 mSv exposure per study which is significantly lower compared to SPECT (~10-15 mSv per study). Interestingly, all PET laboratories are able to keep radiation exposure below the ASNC goal of 9 mSv per study, whereas only 2.6% SPECT laboratories are able to achieve this [8]. As such, it is reasonable to consider MPI using PET/CT in young patients when information about exercise capacity is not vital.
-
4.
Suspicion for significant CAD/balanced ischemia: The PACIFIC study investigators found that in patients with stable chest pain and suspected CAD, PET/CT had a higher sensitivity to detect hemodynamically significant stenosis compared to SPECT (87% vs. 57%) [9]. This suggests that in patients with known or suspected CAD who are unable to exercise, PET/CT may be the modality of choice.
-
Tip 1: Consider PET MPI in obese patients, female patients with possible microvascular dysfunction, and patients with suspected CAD who are unable to exercise.
Patient Preparation
Prior to MPI, fasting (except for water) is recommended for at least 6 h [10••]. For patients undergoing MPI using adenosine or regadenoson, caffeine must be avoided for ≥ 12 h and theophylline containing medications must be avoided for ≥ 48 h as these are adenosine receptor antagonists [10••]. Patients with established CAD or those taking anti-anginal medications should continue taking these medications prior to testing. In instances where patients are undergoing testing to establish a diagnosis of obstructive CAD, withholding of anti-anginal medication should be considered to optimize the test’s sensitivity [11]. In the rare instances that dobutamine is used, beta-blockers must be held for 5 half-lives where the clinician determines it is safe to do so [12].
There are several stress agents used in PET, all having differing risk profiles (Table 1). Prior to proceeding with the test, it is important to ensure the absence of contraindications. For adenosine and regadenoson, the contraindications include (a) bronchospastic lung disease with active wheezing, (b) 2nd or 3rd-degree heart block without a pacemaker, (c) SBP <90 mm Hg, (d) SBP > 200 mm Hg or DBP > 110 mm Hg, (e) dipyridamole use within 48 h, (f) known hypersensitivity to the pharmacologic agent, and (g) unstable angina, ongoing acute coronary syndrome (ACS), or less than 2-4 days from an acute MI [13]. As there are reports that regadenoson lowers the seizure threshold, adenosine is preferred in that situation [14]. Dobutamine is rarely used as a stress agent in PET. Its contraindications include (a) hemodynamically significant LV outflow tract obstruction, (b) unstable or complicated ACS, (c) serious cardiac arrhythmias (VT, complete AV block), and (d) severe systemic hypertension [8].
-
Tip 2: Regadenoson is the most widely used vasodilator in PET perfusion imaging; however, adenosine is preferred in patients with a history of seizure disorders. Consider dobutamine in patients with recent caffeine intake or to provoke ischemia in patients with anomalous coronary arteries.
-
Tip 3: Anti-anginal medications should be discontinued prior to stress testing if the goal is to establish a diagnosis of CAD. In patients with established CAD, medications can be continued if the aim of study is to assess response to therapy.
Patient Positioning
Patients should be in the supine position with the arms resting above their heads outside the camera’s field of view. An overhead bar can be used as a handhold for arm support. In cases where patients are unable to raise their arms above their head, images can be obtained with arms resting by their side. Care must be taken to ensure that the position of the arms does not change between the transmission and emission scans. A similar patient position needs to be maintained for both the rest and stress images.
-
Tip 4: Ensure patient comfort to help them maintain the same position throughout the test to reduce the occurrence of artifacts.
Dosage
Many factors determine the appropriate dose of radiotracer for each patient. Firstly, patients’ size and habitus are an important consideration with large patients benefiting from higher dosage. Second, the type of PET/CT scanners can influence the amount of dose required due to the difference in sensitivities of each system. For instance, 3D PET/CT scanners offer four to six times higher sensitivity for the detection of photons compared to 2D scanners [15]. For instance, the standard dose for 3D scanners is in the range of 20-40 mCi of rubidium-82 (82Rb) compared to 40-60 mCi for 2D scanners for each rest and stress injection [16]. It is important to note however that this increased sensitivity may lead to detector saturation if a rapid bolus of radiotracer is injected and therefore a low-dose, slow infusion protocol is preferred [17]. On the other hand, the most recent 3D digital PET/CT scanners have even higher sensitivity allowing doses as low as 10mCi of 82Rb for each rest and stress protocol [18].
It is important for staff members to avoid standing near the patient or generator during injection of the radiotracer. Wearing of lead aprons is not helpful as it may increase staff exposure to radiation from the interaction of the 511-keV photon with the lead.
-
Tip 5: The most advanced digital 3D PET/CT scanners have the highest sensitivity allowing sub-1 mSv studies (total for rest and stress).
Radiopharmaceutical Agents
Although several myocardial perfusion PET tracers are available, we will review the most widely used and the only two FDA-approved agents in the USA, 82Rb and nitrogen-13-ammonia (13N-ammonia) (Table 2).
Rubidium-82 Protocol
82Rb is the most widely used radiotracer for PET myocardial perfusion imaging in the USA. It is a potassium analog that is produced by commercially available generators through the decay of strontium-82. It has similar kinetic properties to thallium-201 and has a physical half-life of 75 s which allows short scanning protocols (25-30 min total time) and improves throughput through the PET lab. The radiation dosimetry from 82Rb varies in adults but is roughly 1 mSv for 20 mCi injections [19]. With the advances in PET instrumentation, current digital 3D scanners allow rest and stress protocols to be completed using a total dose of 20-40 mCi of 82Rb, resulting in significantly lower radiation exposure. A typical 82Rb rest-stress protocol is depicted in Fig. 1.
There are two commonly used infusion protocols for 82Rb: constant-activity-rate and constant-flow-rate (bolus) administration. This is particularly important for the quantification of myocardial blood flow, to limit variability in measurement and improve test-retest repeatability of imaging. These two protocols were compared recently and the constant-activity-rate infusion demonstrated more consistent and repeatable tracer injection profiles [20].
13N-Ammonia Protocol
13N-ammonia is a cyclotron-produced radiotracer with a half-life of ~ 10 min. After infusion, 13N-ammonia disappears from the circulation into the cells and is trapped after its conversion to glutamine. Typically, 10-20 mCi are injected for rest and stress images. The radiation dosimetry for a 20-mCi dose of 13N-ammonia is 1.5 mSv in adults [19]. Due to its longer half-life, 30-50 min are needed to allow sufficient radio-active decay prior to obtaining stress images [21]. A typical 13N-ammonia protocol is shown in Fig. 1. Despite the excellent image quality provided, its use is limited due to the need for an on-site cyclotron.
A few recent advances in this field include the experimental use of a mini-cyclotron system with demonstrable excellent image quality and low tracer-related radiation exposure [22]. The small number of patients, lack of comparison with images from a traditional cyclotron, and the absence of data on myocardial blood flow are notable limitations of the study. This however is a welcome proof of concept study with promising results. Additionally, a low-dose rest, high-dose stress protocol has been recently trialed in a small cohort of patients with excellent results potentially shortening the conventional protocols [23]. For this protocol to be successful however, it is preferable to use the most sensitive PET/CT scanners to avoid noisy and low-quality images from the low-dose rest injection.
Attenuation Correction
Annihilation photons traveling through tissue can be absorbed or scattered reducing the likelihood that they will strike a detector; this is known as attenuation. Cardiac PET imaging should only be performed with attenuation correction due to coincidence effect amplifying the effect of attenuation.
Standalone PET scanners without CT or MR capabilities typically employ rotating rod sources or rings of germanium-68/gallium-68 or cesium-137 to acquire a transmission scan for attenuation correction purposes. This adds at least 60 s to the overall imaging time which may be acceptable for cardiac imaging but is a significant disadvantage for whole-body imaging.
Modern PET scanners are hybrid PET/CT systems which utilize CT scans for attenuation correction. The use of CT has several advantages: (a) The transmission scan typically takes less than 10 s which helps patient comfort and lab throughput, (b) it eliminates the need for periodic replacement of the rotating line source, and (c) it provides complimentary anatomical information to the functional PET data. Breathing protocols are not currently established but the CT is often acquired at either end-expiration or during shallow breathing to minimize the chance of misalignment. Respiratory gating techniques continue to evolve and can be used to compensate for respiratory motion [24, 25]. Transmission scans are typically performed at the beginning of the study and thus care should be taken for potential misregistration due to upward creep of the diaphragm from visceral fat pressure [26]. Another transmission scan performed after stress imaging is recommended to minimize misregistration artifacts.
-
Tip 6: Ideally, both a rest and a stress transmission scan should be performed as patients may move during the scan particularly after the injection of the stress agent.
Acquisition and Reconstruction
As discussed in the “Introduction” section, during scan acquisition, millions of coincidence events are recorded, and projections of the activity distribution are used to reconstruct images. Static images are created by summing the acquired data of the uptake scan from 2-5 min post injection. This provides images of relative tracer redistribution that can be used for perfusion assessment.
Due to the high number of counts, PET can provide images of sufficient quality to determine left ventricular function. Electrocardiographic-gated images are thus used to obtain information about regional and global ventricular function. Data is sorted into 8 or 16 gates of the cardiac cycle using an ECG trigger. Arrhythmias such as atrial fibrillation or PVC’s can lead to erroneous-gated information and the monitoring of accepted and rejected beats is vital to ensure accurate information.
Dynamic imaging provides an evolving picture of the radiotracer as it circulates the heart over time and eventually clears from the blood into the tissues. Imaging starts prior to the bolus injection and continues for 3-5 min. This is essential for assessment of the tracer’s kinetic properties allowing myocardial blood flow quantification and analysis.
Available now with all scanners is list mode acquisition which enables simultaneous dynamic and ECG-gated acquisitions. This permits the reconstruction of static, gated, and dynamic images all from one injection without lengthening the duration of the scan. The one drawback of this mode is it requires larger storage space for the datasets (0.5 Gigabytes per study).
-
Tip 7: The current recommended acquisition mode is list mode, which allows static, gated, and dynamic images to be reconstructed from one injection.
Potential Pitfalls
The most significant source of artifact in cardiac PET is misregistration of the transmission and emission scans. Misregistration of the transmission and emission scans can create false-positive defects which typically occur in the anterior or anterolateral wall segments (Fig. 2) [26, 27]. Previous reports indicate that nearly 20% of all PET scans have detectable misregistration artifacts and with 40% of all studies having a change in diagnosis after misregistration correction [26, 28•]. This can occur due to patient motion or diaphragmatic displacement particularly after the injection of the stress agent.
Example of a misregistration artifact. Top panel: The stress perfusion images reveal a mild perfusion defect in the anterolateral myocardial segments. On inspection of the fusion images, it is clear that the transmission and emission scans are misaligned. Bottom panel: After reconstruction with proper registration, the rest and stress images show completely normal myocardial perfusion
It is essential that the fusion images be reviewed prior to image interpretation to detect any misalignment and allow adjustments to be made. Most commercial PET/CT scanners include software tools to correct for transmission-emission misalignment [29]. While slightly time-consuming, there is no substitute to manually shifting the emission images to achieve precise co-registration and reconstruct the emission images from shifted data.
Substantial patient motion is a significant source of artifact and may not be readily appreciated on PET imaging as opposed to SPECT which utilize rotating projections and sinograms. Instead, readers need to carefully inspect the data for any evidence of loss of image fidelity. Occasionally motion can produce two defects that are 180° apart but if substantial motion occurs, the images will appear uniformly blurred (Fig. 3). Dynamic imaging may be beneficial to combat this artifact as it enables the rejection of the compromised frames and allows frames of acceptable quality to be summed for final image analysis. Ultimately, it is best to ensure patients remain still and awake during the duration of the scan.
-
Tip 8: Always review fusion images prior to scan interpretation to ensure no misregistration occurs between the transmission and emission scans.
The insertion of intravenous lines for PET/CT imaging to facilitate tracer and stress agent injection is now routine practice. Care must be taken to ensure proper function of the intravenous line prior to the radiopharmaceutical injection. Infiltration of the dose can result in lower count density and poorer image quality. The use of a chemotherapy port for intravenous access should be avoided if possible as it may act as a reservoir for the radiotracer resulting in low count statistics and poor image quality. Additionally, if the port is in close proximity to the heart, it may result in a hot focus, compromising the evaluation of the adjacent myocardium.
-
Tip 9: Ask patients not to bend their arms during tracer injection. Avoid using ports for tracer injection.
Artifacts from Extra-Cardiac Activity
Artifacts due to metallic components are a recognized limitation of CT-based attenuation correction. The CT-AC scaling algorithm does not account for the strong photoelectric absorption of metal leading to overcorrection of PET images. Pacemaker leads do not appear to cause artifact in PET/CT images. On the other hand, the radio-opaque shock coils on ICDs artifactually increase the apparent myocardial uptake in the adjacent areas [30]. Several metal artifact reduction techniques have been proposed to reduce the influence of metallic objects from the CT attenuation scan. A detailed technical analysis is beyond the scope of this review and is discussed in great detail elsewhere [31].
Artifacts from extra-cardiac radiotracer activity near the myocardium may occur in PET imaging. Activity in the gastrointestinal tract may lead to spillover of activity resulting in elevated counts or cause a ramp filter artifact resulting in a reduction in counts in the adjacent myocardium. It is important to note that these artifacts are more commonly encountered with SPECT rather than PET imaging.
-
Tip 10: ICD shock coils, but not pacemaker wires, may result in an apparent increase of tracer uptake in neighboring myocardial regions.
Conclusions
As PET continues to become the favored method for assessing myocardial ischemia in patients with suspected or established coronary artery disease (CAD), clinicians and technologists involved with PET imaging should be familiar with the basic scan parameters, image acquisition protocols, and post processing tools. Not only will this help in identifying and resolving artifacts but it will ensure high-quality images are produced and erroneous results are avoided.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gundlich B, Musmann P, Weber S, Nix O, Semmler W. From 2D PET to 3D PET: issues of data representation and image reconstruction. Z Med Phys. 2006;16(1):31–46.
Al-Mallah MH, Sitek A, Moore SC, Di Carli M, Dorbala S. Assessment of myocardial perfusion and function with PET and PET/CT. J Nucl Cardiol. 2010;17(3):498–513.
Koepfli P, Hany TF, Wyss CA, Namdar M, Burger C, Konstantinidis AV, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med. 2004;45(4):537–42.
• Patel KK, Badarin FA, Chan PS, Spertus JA, Courter S, Kennedy KF, et al. Randomized comparison of clinical effectiveness of pharmacologic SPECT and PET MPI in symptomatic CAD patients. JACC Cardiovasc Imaging. 2019;12(9):1821–31. This randomized trial showed that downstream invasive testing rates with PET MPI were more consistent with high-risk features than those with SPECT MPI.
Higgins AR, Jaber W. SPECT and PET MPI: the future has arrived but it is unevenly distributed. J Nucl Cardiol. 2020;27(2):417–8.
Harnett DT, Hazra S, Maze R, Mc Ardle BA, Alenazy A, Simard T, et al. Clinical performance of Rb-82 myocardial perfusion PET and Tc-99m-based SPECT in patients with extreme obesity. J Nucl Cardiol : Off Publ Am Soc Nucl Cardiol. 2019;26(1):275–83.
Taqueti VR, Dorbala S. The role of positron emission tomography in the evaluation of myocardial ischemia in women. J Nucl Cardiol. 2016;23(5):1008–15.
Desiderio MC, Lundbye JB, Baker WL, Farrell MB, Jerome SD, Heller GV. Current status of patient radiation exposure of cardiac positron emission tomography and single-photon emission computed tomographic myocardial perfusion imaging. Circ Cardiovasc Imaging. 2018;11(12):e007565.
Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2017;2(10):1100–7.
•• Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187–226. The document provides imaging guidelines, protocols, and technical aspects for physicians and technologists practicing nuclear cardiology.
Zoghbi GJ, Dorfman TA, Iskandrian AE. The effects of medications on myocardial perfusion. J Am Coll Cardiol. 2008;52(6):401–16.
Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, et al. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33(1):1–41.e8.
Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol. 2016;23(3):606–39.
Agarwal V, DePuey EG. Regadenoson and seizures: a real clinical concern. J Nucl Cardiol. 2014;21(5):869–70.
Knesaurek K, Machac J, Krynyckyi BR, Almeida OD. Comparison of 2-dimensional and 3-dimensional 82Rb myocardial perfusion PET imaging. J Nucl Med. 2003;44(8):1350–6.
El-Tallawi KC, Aljizeeri A, Nabi F, Al-Mallah MH. Myocardial perfusion imaging using positron emission tomography. Methodist Debakey Cardiovasc J. 2020;16(2):114–21.
Nakazato R, Berman DS, Alexanderson E, Slomka P. Myocardial perfusion imaging with PET. Imaging Med. 2013;5(1):35–46.
Kaster T, Mylonas I, Renaud JM, Wells GA, Beanlands RS. deKemp RA. Accuracy of low-dose rubidium-82 myocardial perfusion imaging for detection of coronary artery disease using 3D PET and normal database interpretation. J Nucl Cardiol. 2012;19(6):1135–45.
Mattsson S, Johansson L, Leide Svegborn S, Liniecki J, Noßke D, Riklund K, et al. Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015;44(2 Suppl):7–321.
Klein R, Ocneanu A, Renaud JM, Ziadi MC, Beanlands RSB. deKemp RA. Consistent tracer administration profile improves test–retest repeatability of myocardial blood flow quantification with 82Rb dynamic PET imaging. J Nucl Cardiol. 2018;25(3):929–41.
Rosas EA, Slomka PJ, García-Rojas L, Calleja R, Jácome R, Jiménez-Santos M, et al. Functional impact of coronary stenosis observed on coronary computed tomography angiography: comparison with 13N-ammonia PET. Arch Med Res. 2010;41(8):642–8.
Pieper J, Patel VN, Escolero S, Nelson JR, Poitrasson-Rivière A, Shreves CK, et al. Initial clinical experience of N13-ammonia myocardial perfusion PET/CT using a compact superconducting production system. J Nucl Cardiol. 2019.
Fukushima K, Kaimoto Y, Matsuo Y, Nakao R, Matsumura H, Kanaya K, et al. Low dose and short time imaging protocol of rest-stress 13N ammonia PET with time of flight technique. J Nucl Med. 2018;59(supplement 1):1513.
Nehmeh SA, Erdi YE. Respiratory motion in positron emission tomography/computed tomography: a review. Semin Nucl Med. 2008;38(3):167–76.
Kesner AL, Schleyer PJ, Büther F, Walter MA, Schäfers KP, Koo PJ. On transcending the impasse of respiratory motion correction applications in routine clinical imaging-a consideration of a fully automated data driven motion control framework. EJNMMI Phys. 2014;1(1):8.
Loghin C, Sdringola S, Gould KL. Common artifacts in PET myocardial perfusion images due to attenuation-emission misregistration: clinical significance, causes, and solutions. J Nucl Med. 2004;45(6):1029–39.
Martinez-Möller A, Souvatzoglou M, Navab N, Schwaiger M, Nekolla SG. Artifacts from misaligned CT in cardiac perfusion PET/CT studies: frequency, effects, and potential solutions. J Nucl Med. 2007;48(2):188–93.
• Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007;48(7):1112–21. This study showed that misregistration of the transmission CT and PET emission images can create false-positive results in up to 40% of patients that normalize with realignment of the images.
Nordberg P, Declerck J, Brady M. Pre-reconstruction rigid body registration for positron emission tomography: an initial validation against ground truth. Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:5612–5.
DiFilippo FP, Brunken RC. Do implanted pacemaker leads and ICD leads cause metal-related artifact in cardiac PET/CT? J Nucl Med. 2005;46(3):436–43.
Abdoli M, Dierckx RA, Zaidi H. Metal artifact reduction strategies for improved attenuation correction in hybrid PET/CT imaging. Med Phys. 2012;39(6):3343–60.
Author information
Authors and Affiliations
Contributions
This was an invited review to Mouaz H. Al-Mallah. Literature search and drafting of the manuscript were performed by all coauthors: Talal Alnabelsi, Akanksha Thakkar, Ahmed Ahmed, Yushi Han, and Mouaz H. Al-Mallah. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Al-Mallah receives support from the Houston Methodist Research Institute and Siemens. The other authors have no relevant financial or non-financial interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Alnabelsi, T., Thakkar, A., Ahmed, A.I. et al. PET/CT Myocardial Perfusion Imaging Acquisition and Processing: Ten Tips and Tricks to Help You Succeed. Curr Cardiol Rep 23, 39 (2021). https://doi.org/10.1007/s11886-021-01476-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01476-5