Abstract
Purpose of Review
Given the rising prevalence of obstructive sleep apnea (OSA), we aimed to review the epidemiologic and pathophysiologic relationship of OSA, hypertension, and cardiovascular disease, and to summarize recent advances in the treatment of OSA.
Recent Findings
OSA is associated with an elevated risk of hypertension and cardiovascular disease. Several pathophysiologic factors contribute to the relationship between OSA and vascular risk, including neurohormonal dysregulation, endothelial dysfunction, and inflammation. While CPAP reduces blood pressure, it has not been demonstrated to reduce cardiovascular risk. The combination of CPAP and weight loss has a synergistic effect on blood pressure and several metabolic parameters. Adherence to CPAP is poor across studies, potentially contributing to the attenuation of perceived cardiovascular benefit from CPAP therapy.
Summary
A greater emphasis on adherence to CPAP and the combination of CPAP and weight loss are central to reducing cardiovascular risk among individuals with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a breathing disorder where normal ventilation is impaired during sleep due to upper airway narrowing [1•]. Initially noted by scholars centuries ago as episodes of snoring and suffocating gasps coupled with perpetual drowsiness and difficulty in arousal [2], OSA is now diagnosed by polysomnography. The severity of OSA on polysomnography is designated using the apnea-hypopnea index (AHI). Based on this metric, The Wisconsin Sleep Cohort, a longitudinal population-based study established over two decades ago, estimated the prevalence of OSA to be 9% in women and 24% in men [3]. Since the Wisconsin Sleep Cohort’s inception, there has been a dramatic relative increase in prevalence of OSA over time. Extrapolating Wisconsin Sleep Cohort estimates to the US population using data from the National Health and Nutrition Examination Survey (NHANES) adjusted for sex, age, and body mass index (BMI), the prevalence of OSA in 2007–2010 compared with 1988–1994 increased by 14–55%, depending on the subgroup analyzed [4, 5].
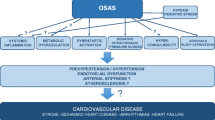
The increase in prevalence of OSA has been linked to rising rates of obesity over the past several decades [6]. Obesity, central body fat distribution, and large neck girth are well-established risk factors for OSA (Fig. 1). Additional risk factors that have been linked to OSA include older age, male sex, craniofacial and upper airway abnormalities, and nighttime nasal congestion [7,8,9]. OSA has been associated with multiple adverse cardiovascular effects including hypertension, ischemic heart disease, stroke, and arrhythmias [10,11,12,13, 14••, 15•] as well as metabolic disorders including diabetes mellitus, lipid profile derangements, and non-alcoholic fatty liver disease [15•, 16,17,18,19]. These chronic sequelae are mediated by several pathophysiologic mechanisms that are upregulated in OSA, including elevated sympathetic nervous system activity, renin-angiotensin aldosterone system activity, endothelial dysfunction, inflammation, and metabolic dysregulation. Various treatment modalities are available for OSA, comprising lifestyle modifications, medical treatments, and surgical intervention [20]. Due to its increasing prevalence and significant adverse effects, untreated OSA has an appreciable economic burden, estimated between $34 and $69 billion annually worldwide [21].
Pathophysiologic drivers of hypertension and cardiovascular disease in obstructive sleep apnea. Obesity and large neck girth are strong independent risk factors for OSA. OSA can also increase the risk of obesity due to inactivity and neurohormonal dysregulation. OSA and obesity both result in elevated sympathetic nervous system activity, renin-angiotensin system activity, endothelial dysfunction, inflammation, and metabolic dysregulation that, in turn, increase the risk of developing hypertension and cardiovascular disease
Risk Factors for Obstructive Sleep Apnea
Several anatomic and functional factors predispose patients to developing OSA. Anatomically, patients with OSA have been found to have craniofacial differences including smaller pharyngeal airways, larger volume of soft tissue surrounding their upper airway, lower placement of the hyoid bone, and larger volume of the lateral pharyngeal wall [7]. When awake, these patients are able to compensate by increasing upper airway muscle activity to maintain a patent airway [22]. During sleep, however, upper airway muscles relax and are dyssynchronous, likely contributing to airway obstruction [23]. While OSA occurs across a breadth of body sizes, many of these anatomic abnormalities occur more frequently in obesity and among individuals with large neck girths, contributing to the higher rates of OSA in obesity.
Upper airway patency is also influenced by functional factors such as leptin. In experimental models, leptin has been found to influence sleep architecture and upper airway resistance [24]. Leptin is a peptide produced by adipose tissue that affects satiety and plays a role in metabolic regulation including glucose and fatty acid metabolism as well as insulin resistance [25]. Leptin has also been suggested as a marker of cardiovascular disease risk [26]. Patients with OSA have elevated levels of leptin, with a positive correlation observed between leptin levels and severity of OSA [24]. Furthermore, Phillips et al. found that leptin levels were higher among 32 obese men with OSA compared with 32 matched obese controls without OSA, suggesting that OSA may play a role in leptin resistance [27]. The presence of leptin resistance in individuals with obesity and OSA likely hinders losing weight and contributes to worsening of OSA.
Epidemiology of Hypertension in Obstructive Sleep Apnea
The high prevalence of hypertension in individuals with OSA is well-established [14••], and there seems to be a dose-response relationship between OSA and blood pressure [28, 29]. OSA has been found to be particularly common in resistant and refractory hypertension [30, 31]. In a large cross-sectional study (n = 2677), the odds of HTN increased by 1% for every episode of apnea per hour of sleep and up to 13% for every 10% decrease in oxygen saturation [29] In The Sleep Heart Health Study, a cohort of 6132 patients, the prevalence of hypertension was found to be 59%, 62%, and 67% in mild, moderate, and severe sleep apnea, respectively [32]. The association of obstructive sleep apnea and hypertension was no longer significant in participants aged 60 years and older [33]. In the Wisconsin Sleep Cohort, there was a linear increase in blood pressure with increasing severity of sleep apnea, independent of age, sex, and BMI [28].
Evidence suggests that hypertension is a sequela of OSA [34], although the temporal relationship between OSA and hypertension is somewhat elusive. In a longitudinal analysis of The Sleep Heart Health Study, after excluding individuals who had hypertension at baseline, O’Connor et al. found a significant association between severity of OSA and risk of developing hypertension [35]. This association was no longer significant after adjusting for BMI. Similarly, in the Victoria Sleep Cohort, a longitudinal study of 2148 Spanish subjects followed for 7.5 years, Cano-Pumarega et al. demonstrated a significant association between severity of OSA and subsequent risk of incident hypertension, which dissipated after univariate adjustment for age, as well as adjustment for sex, BMI, fitness level, and several health behaviors [36]. Nonetheless, pooled meta-analytic results across prospective studies estimate a 48% increased risk of hypertension among individuals with OSA (pooled hazard ratio 1.48, 95% confidence interval 1.04–1.91) [14••].
Epidemiology of Cardiovascular Disease in OSA
The frequent concurrence of hypertension and OSA likely drives the increased risk of cardiovascular disease observed in patients with OSA. OSA is independently associated with carotid intima-media thickness, a marker of atherosclerosis [37]. OSA is also associated with increased risk of atrial fibrillation, with a dose-response relationship observed between severity of OSA and magnitude of the risk of developing atrial fibrillation [13]. In the Wisconsin Sleep Cohort Study, more severe OSA was significantly associated with adverse cardiac remodeling, including worsening of both left and right ventricular function [38•]. Consequently, OSA has been linked with an increased risk of incident heart failure [39, 40]. Meta-analyses of longitudinal studies demonstrate an increased risk of coronary artery disease and cardiovascular mortality among individuals with moderate-to-severe OSA [10, 11, 15•]. Several studies have also shown a 2-fold increased risk of stroke among individuals with OSA compared with those without OSA [10,11,12].
Pathophysiology of Hypertension and Cardiovascular Disease in Obstructive Sleep Apnea
There are multiple interrelated pathophysiologic mechanisms that contribute to hypertension and cardiovascular disease in obstructive sleep apnea. In a human experimental model, exposure to intermittent hypoxia leads to a measurable increase in mean arterial pressure, cerebral vascular resistance, and pressor response [41]. Correspondingly, in OSA, recurrent episodes of apnea result in intermittent hypoxia which causes various autonomic, hemodynamic, and biochemical changes contributing to the pathophysiology of hypertension and cardiovascular disease.
Sympathetic Nervous System Activity
During most normal sleep, there is increased parasympathetic and decreased sympathetic activity which contributes to physiologic nocturnal “dipping” in blood pressure and heart rate [42]. In OSA, several pathophysiologic mechanisms lead to a persistent increase in sympathetic drive. Negative pressure against obstruction and repetitive intermittent hypoxia are thought to cause renal, adrenal, and peripheral chemoreceptor activation. This generates an increase in circulating hormones such as catecholamines, renin, angiotensin II, and enthothelin-1 and downregulation of nitric oxide synthesis, resulting in increased sympathetic nervous system activity [43]. In 1988, Hedner et al. directly measured muscle sympathetic nerve activity in patients with OSA, demonstrating that these patients had progressively increasing sympathetic activity during apneic events [44]. Measurement of muscle sympathetic nerve activity also revealed that patients with OSA have persistently elevated sympathetic activity even when they are awake [45]. This suggests that the effects of sympathetic drive are persistent even after withdrawal of the hypoxic stimulus. Accordingly, urinary catecholamine levels are positivity correlated with the severity of OSA [46]. With regard to chemoreceptors, the carotid body, an oxygen sensing receptor, is stimulated by hypoxia, resulting in increased sympathetic activation. A persistent increase in sympathetic drive ultimately leads to an increase in vascular resistance and vascular remodeling, which also contributes to the observed increase in blood pressure [47].
Renin-Angiotensin System Activity
The sympathetic nervous system closely interacts with the kidney whereby renal sympathetic nerves activate the renin-angiotensin system, resulting in sodium retention, impaired natriuresis, and increased blood pressure. Thus, patients with OSA have higher angiotensin II and aldosterone levels compared with non-OSA controls [48]. In a small randomized double-blind placebo controlled cross-over study, intermittent hypoxia was found to increase both systolic and diastolic blood pressure; this relationship was diminished by exposure to a type 1 angiotensin II receptor blocker [49].
Endothelial Dysfunction
In an experimental model, intermittent hypoxia was shown to increase endothelial dysfunction [50•]. In a large cohort of older adults, flow-mediated dilation showed a statistically significant relationship between arterial diameter and the severity of hypoxia [51]. Compared with healthy controls, Gjørup et al. found that individuals with OSA had increased plasma levels of endothelin-1, and that mean nocturnal levels of endothelin correlated with the severity of OSA and with increased ambulatory blood pressure [52]. Ip et al. demonstrated that serum nitrites and nitrates were lower in patents with OSA [53]. Several studies have also showed an improvement in endothelial function with successful treatment of OSA [54,55,56].
Inflammation
OSA has been described as a low-grade chronic inflammatory disease [57]. Patients with OSA have higher circulating levels of inflammatory markers including high sensitivity CRP, interleukin-6, interleukin-8, TNF alpha, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 [58, 59]. While systemic inflammation has been linked to hypertension, atherosclerosis, and cardiovascular disease, its role in OSA may be confounded by obesity [60].
Metabolic Dysregulation
The Sleep Heart Health Study showed an association between OSA and impaired fasting glucose, glucose tolerance, and diabetes [61]. These associations were similar in magnitude in both obese and non-obese patients with OSA. In a meta-regression analysis of over eighteen thousand pooled subjects, patients with OSA were found to have higher levels of total cholesterol, triglycerides, and low-density lipoprotein and lower levels of high-density lipoprotein; the degree of dyslipidemia correlated with the severity of OSA [16]. Recent studies have also shown a relationship between OSA and hepatic steatosis, fibrosis, and non-alcoholic fatty liver disease [17], as well as chronic kidney disease [15, 62].
Treatment of Obstructive Sleep Apnea
Surgical intervention for OSA is uncommon, employed mainly for patients with morbid obesity requiring bariatric surgery [63, 64] and patients who do not tolerate conservative treatment [65]. Non-surgical approaches to managing OSA include a variety of behavioral measures, among them the avoidance of alcohol, sedatives, and tobacco and the adjusting sleep positioning (e.g., adding pillows, oral appliances) [66]. The mainstays of treatment, however, are medically supervised weight loss and nightly use of continuous positive airway pressure (CPAP). CPAP provides pneumatic splinting of the upper airway leading to significant improvement in the apnea-hypopnea index [65]. Lowering of the apnea-hypopnea index is significantly correlated with improvement in the symptoms of OSA, which include daytime sleepiness and disturbed cognition and mood. The associations of OSA with both physiologic disturbances and with cardiovascular risk factors have reinforced the proposal that treatment with CPAP may prevent life-threatening cardiovascular events [65, 67]. Such an effect has proven difficult to demonstrate, partly due to the complex, multidirectional relationships between OSA, hypertension, and obesity.
The Impact of Treatment for Obstructive Sleep Apnea on Blood Pressure
In patients with normal blood pressure or moderate hypertension, treatment of OSA with CPAP produces a very modest decline in blood pressure. Hu et al., for example, found both systolic and diastolic blood pressure fell by 2–3 mmHg after CPAP was administered to patients with hypertension of mixed severity [68]. Twenty-four-hour ambulatory monitoring in other studies demonstrated a similar modest decline in systolic blood pressure, diastolic blood pressure, and blood pressure variability when CPAP was initiated [34, 69,70,71]. The small decrements in blood pressure in moderately hypertensive patients have shifted attention to the potential benefits of CPAP for OSA patients with severe hypertension [72]. The anti-hypertensive effect of CPAP in such patients is indeed slightly greater, with systolic and diastolic blood pressure typically declining about 6–7 mmHg and 4–5 mmHg, respectively [70, 73]. In randomized trials, CPAP alone did not reduce the incidence of new onset hypertension [74].
The Impact of Treatment for Obstructive Sleep Apnea on Cardiovascular Risk
A few observational studies have supported speculation that the association of OSA with hypertension and obesity—both cardiovascular risk factors—means that treatment with CPAP may prevent cardiovascular events [75, 76]. This hypothesis remains unconfirmed. The Sleep Apnea Cardiovascular Endpoints (SAVE) study randomly assigned over 2500 patients with OSA and a history of cardiovascular disease to usual care plus CPAP versus usual care alone [77••]. While CPAP improved daytime sleepiness and health-related quality of life, it did not reduce the number of serious cardiovascular events during a mean follow-up of 3.7 years [77••]. Similarly negative findings have been replicated across multiple large, randomized, multicenter studies [74, 78,79,80,81, 82••]. Poor patient adherence across studies complicates the interpretation of these findings.
The Impact of Adherence on the Relationship Between CPAP and Cardiovascular Risk
Approximately 30% of patients placed on CPAP never actually initiate treatment [83]. Among those patients who do initiate CPAP, 25% abandon it within 1 year. The SAVE study demonstrated that cardiovascular events were reduced for the small number of patients using CPAP more than 4 h per night [77••]. To more definitively elucidate the relationship between CPAP treatment and cardiovascular outcomes, future studies will need to systematically assess adherence (e.g., using in-center polysomnography and other objective measures of adherence), requiring a minimum of 4 h per night of CPAP use.
Weight Loss as a Treatment for Obstructive Sleep Apnea
Weight gain, like hypertension, is closely linked to OSA [72, 84]. In a longitudinal study of normal-weight subjects, 10% weight gain was associated with a sixfold increased risk of OSA [85]. Conversely, 10% weight loss produced a 26% decline in the apnea-hypopnea index. As with hypertension, the association between obesity and OSA is mutually reinforcing. For example, obesity is associated with chronic subclinical inflammation which has been hypothesized to predispose individuals to OSA, but OSA is, itself, a pro-inflammatory stimulus [86]. Similarly, obesity is both a risk factor for OSA—reflecting architectural changes to the airway, impaired breathing mechanics—and a consequence of OSA—perhaps due to inactivity and altered neurohumoral status [87]. Nonetheless, a meta-analysis of over 3000 OSA patients reported that treatment with CPAP was associated with a mean weight gain of 0.4 kg [87]. The authors speculate that a by-product of improved slow-wave sleep may be a higher level of weight gain-inducing anabolic hormones. Acknowledging the relationship of OSA and obesity, The American Thoracic Society guidelines encourage a calorie restriction for all patients with OSA and a body mass index greater than 25 kg/m2. The committee notes, however, a “very low certainty” that symptoms or cardiovascular risk will be altered by diet alone [88•].
The complexity of the relationship between OSA, hypertension, and obesity is highlighted by a randomized controlled trial by Chirinos et al. that compared the anti-hypertensive effects of CPAP and weight loss (by diet and exercise), both alone and in combination [89]. Systolic blood pressure declined by 14.1 mmHg in the dual intervention group. Since this reduction exceeded the summed effect of a regimen limited to either weight loss (6.8 mmHg) or CPAP (3.0 mmHg) alone, the results suggested weight loss and CPAP synergistically contribute to blood pressure control. Chirinos et al. also found a reduction in insulin resistance and triglyceride levels associated with combined weight loss and CPAP. As in other studies, greater adherence to CPAP was associated with greater reduction in blood pressure.
Several studies have demonstrated that surgical weight loss often reduces OSA severity and results in remission of hypertension [90, 91]. In a prospective, multicenter before-and-after study of 132 patients with OSA who underwent laparoscopic Roux-en-Y gastric bypass surgery, Peromaa-Haavisto et al. found that OSA resolved in 45% of patients and improved in another 33% of the patients [91]. However, this magnitude of reduction in OSA prevalence after bariatric surgery is not consistent across studies. A systematic review and meta-analysis by Wong et al. evaluated participant-level data from two RCTs comparing bariatric surgery with intensive lifestyle modifications for weight loss [92]. The analyses demonstrated a greater reduction in severity of sleep apnea and body weight in participants who underwent bariatric surgery compared with those who underwent lifestyle modifications. Nonetheless, 97.5% of participants who underwent surgical weight loss and 100% of participants who underwent lifestyle modifications had persistent OSA at 1 year following the intervention. There is a paucity of evidence specifically evaluating if there is a synergistic or complimentary effect of surgical weight loss on OSA, hypertension, and cardiovascular risk.
Conclusions
The prevalence of OSA has been increasing in recent decades in the setting of rising obesity rates. There is a high incidence and prevalence of hypertension in OSA, promoting vascular injury and ultimately leading to elevated cardiovascular risk in individuals with OSA. Several pathophysiologic mechanisms contribute to the increased risk of hypertension and cardiovascular disease among individuals with OSA, including upregulation of neurohormonal pathways, endothelial dysfunction, and inflammation. The metabolic effects of obesity in OSA likely also contribute to these mechanisms, representing a multifaceted, mutually reinforcing relationship across these disease states. CPAP alone has not been demonstrated to reduce the risk of hypertension and cardiovascular disease in individuals with OSA, only to modestly lower blood pressure. However, generally poor adherence to CPAP likely contributes to its lack of perceived efficacy. The combination of CPAP and weight loss has the greatest promise for cardiovascular risk reduction on OSA. Future research is needed to identify ways to improve patient adherence to CPAP and to clarify the relationship between CPAP and cardiovascular risk in the setting of adequate adherence. Newer therapies should focus on reducing nocturnal hypoxia while minimizing patient burden.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. https://doi.org/10.5664/jcsm.6506 Clinical practice guideline on the diagnosis of obstructive sleep apnea, favoring the use of polysomnography for diagnosis.
Kryger MH. Sleep apnea. From the needles of Dionysius to continuous positive airway pressure. Arch Intern Med. 1983;143(12):2301–3.
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. https://doi.org/10.1056/NEJM199304293281704.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. https://doi.org/10.1093/aje/kws342.
Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7(5):920–9. https://doi.org/10.3978/j.issn.2072-1439.2015.04.52.
Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–9. https://doi.org/10.1152/japplphysiol.00587.2005.
Neelapu BC, Kharbanda OP, Sardana HK, Balachandran R, Sardana V, Kapoor P, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79–90. https://doi.org/10.1016/j.smrv.2016.01.007.
Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–6. https://doi.org/10.1001/jama.291.16.2013.
Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2018;22(1):241–9. https://doi.org/10.1007/s11325-017-1482-9.
Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis. 2013;229(2):489–95. https://doi.org/10.1016/j.atherosclerosis.2013.04.026.
Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–8. https://doi.org/10.1161/CIRCOUTCOMES.111.964783.
Xie C, Zhu R, Tian Y, Wang K. Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: a meta-analysis. BMJ Open. 2017;7(12):e013983. https://doi.org/10.1136/bmjopen-2016-013983.
Zhao E, Chen S, Du Y, Zhang Y. Association between sleep apnea hypopnea syndrome and the risk of atrial fibrillation: a meta-analysis of cohort study. Biomed Res Int. 2018;2018:5215868. https://doi.org/10.1155/2018/5215868.
•• Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. https://doi.org/10.7189/jogh.08.010405 A systematic review and meta-analysis demonstrating the cross-sectional increased prevalence of hypertension in OSA as well as the prospectively increased risk of new onset hypertension in OSA.
• Strausz S, Havulinna AS, Tuomi T, Bachour A, Groop L, Makitie A, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open. 2018;8(10):e022752. https://doi.org/10.1136/bmjopen-2018-022752 A population-based cohort study of 36,963 individuals in Finland followed up to 25 years, demonstrating that OSA is an independent risk factor for the development of coronary artery disease, diabetes, and diabetic chronic kidney disease.
Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10(5):475–89. https://doi.org/10.5664/jcsm.3690.
Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):841–51. https://doi.org/10.1007/s11325-018-1625-7.
Nagayoshi M, Punjabi NM, Selvin E, Pankow JS, Shahar E, Iso H, et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med. 2016;25:156–61. https://doi.org/10.1016/j.sleep.2016.05.009.
Strand LB, Carnethon M, Biggs ML, Djousse L, Kaplan RC, Siscovick DS, et al. Sleep disturbances and glucose metabolism in older adults: the cardiovascular health study. Diabetes Care. 2015;38(11):2050–8. https://doi.org/10.2337/dc15-0137.
Deacon NL, Jen R, Li Y, Malhotra A. Treatment of obstructive sleep apnea. Prospects for personalized combined modality therapy. Ann Am Thorac Soc. 2016;13(1):101–8. https://doi.org/10.1513/AnnalsATS.201508-537FR.
Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. https://doi.org/10.1016/j.wjorl.2015.08.001.
Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest. 1992;89(5):1571–9. https://doi.org/10.1172/JCI115751.
Oliven R, Cohen G, Dotan Y, Somri M, Schwartz AR, Oliven A. Alteration in upper airway dilator muscle coactivation during sleep: comparison of patients with obstructive sleep apnea and healthy subjects. J Appl Physiol. 2018;124(2):421–9. https://doi.org/10.1152/japplphysiol.01067.2016.
Imayama I, Prasad B. Role of leptin in obstructive sleep apnea. Ann Am Thorac Soc. 2017;14(11):1607–21. https://doi.org/10.1513/AnnalsATS.201702-181FR.
Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–43. https://doi.org/10.1038/415339a.
Chai SB, Sun F, Nie XL, Wang J. Leptin and coronary heart disease: a systematic review and meta-analysis. Atherosclerosis. 2014;233(1):3–10. https://doi.org/10.1016/j.atherosclerosis.2013.11.069.
Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–7. https://doi.org/10.1152/ajpheart.2000.279.1.H234.
Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–52.
Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–82. https://doi.org/10.1136/bmj.320.7233.479.
Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–7. https://doi.org/10.1161/HYPERTENSIONAHA.111.179788.
Martinez-Garcia MA, Navarro-Soriano C, Torres G, Barbe F, Caballero-Eraso C, Lloberes P, et al. Beyond resistant hypertension. Hypertension. 2018;72(3):618–24. https://doi.org/10.1161/HYPERTENSIONAHA.118.11170.
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–36. https://doi.org/10.1001/jama.283.14.1829.
Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–21. https://doi.org/10.1161/01.CIR.0000154540.62381.CF.
Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000a;342(19):1378–84. https://doi.org/10.1056/NEJM200005113421901.
O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–64. https://doi.org/10.1164/rccm.200712-1809OC.
Cano-Pumarega I, Duran-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martinez-Null C, et al. Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med. 2011;184(11):1299–304. https://doi.org/10.1164/rccm.201101-0130OC.
Zhou M, Guo B, Wang Y, Yan D, Lin C, Shi Z. The association between obstructive sleep apnea and carotid intima-media thickness: a systematic review and meta-analysis. Angiology. 2017;68(7):575–83. https://doi.org/10.1177/0003319716665985.
• Korcarz CE, Peppard PE, Young TB, Chapman CB, Hla KM, Barnet JH, et al. Effects of obstructive sleep apnea and obesity on cardiac remodeling: the Wisconsin Sleep Cohort Study. Sleep. 2016;39(6):1187–95. https://doi.org/10.5665/sleep.5828 A prospective study of individuals with OSA, demonstrating that higher apnea-hypopnea index is associated with reduced left and right ventricular function.
Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. https://doi.org/10.1161/CIRCULATIONAHA.109.901801.
Javaheri S, Blackwell T, Ancoli-Israel S, Ensrud KE, Stone KL, Redline S, et al. Sleep-disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193(5):561–8. https://doi.org/10.1164/rccm.201503-0536OC.
Foster GE, Brugniaux JV, Pialoux V, Duggan CT, Hanly PJ, Ahmed SB, et al. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J Physiol. 2009;587(Pt 13):3287–99. https://doi.org/10.1113/jphysiol.2009.171553.
Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–58. https://doi.org/10.1016/j.jacc.2016.11.069.
Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26(1):15–9. https://doi.org/10.1093/sleep/26.1.15.
Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;6(4):S529–S31.
Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–904. https://doi.org/10.1172/JCI118235.
Lam JC, Yan CS, Lai AY, Tam S, Fong DY, Lam B, et al. Determinants of daytime blood pressure in relation to obstructive sleep apnea in men. Lung. 2009;187(5):291–8. https://doi.org/10.1007/s00408-009-9161-7.
Phillips CL, O’Driscoll DM. Hypertension and obstructive sleep apnea. Nat Sci Sleep. 2013;5:43–52. https://doi.org/10.2147/NSS.S34841.
Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol. 2016;13(4):333–43. https://doi.org/10.11909/j.issn.1671-5411.2016.03.020.
Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension. 2010;56(3):369–77. https://doi.org/10.1161/HYPERTENSIONAHA.110.152108.
• Khalyfa A, Zhang C, Khalyfa AA, Foster GE, Beaudin AE, Andrade J, et al. Effect on intermittent hypoxia on plasma exosomal micro RNA signature and endothelial function in healthy adults. Sleep. 2016;39(12):2077–90. https://doi.org/10.5665/sleep.6302 An in vitro study in ten men demonstrating an association between intermittent hypoxia and serologic markers of endothelial dysfunction.
Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169(3):354–60. https://doi.org/10.1164/rccm.200306-756OC.
Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens. 2007;20(1):44–52. https://doi.org/10.1016/j.amjhyper.2006.05.021.
Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162(6):2166–71. https://doi.org/10.1164/ajrccm.162.6.2002126.
Munoz-Hernandez R, Vallejo-Vaz AJ, Sanchez Armengol A, Moreno-Luna R, Caballero-Eraso C, Macher HC, et al. Obstructive sleep apnoea syndrome, endothelial function and markers of endothelialization. Changes after CPAP. PLoS One. 2015;10(3):e0122091. https://doi.org/10.1371/journal.pone.0122091.
Ohike Y, Kozaki K, Iijima K, Eto M, Kojima T, Ohga E, et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure--possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ J. 2005;69(2):221–6.
Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169(3):348–53. https://doi.org/10.1164/rccm.200306-767OC.
Kheirandish-Gozal L, Gozal D. Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci. 2019;20(3). https://doi.org/10.3390/ijms20030459.
Testelmans D, Tamisier R, Barone-Rochette G, Baguet JP, Roux-Lombard P, Pepin JL, et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62(2):210–6. https://doi.org/10.1016/j.cyto.2013.02.021.
Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–12. https://doi.org/10.5664/jcsm.3070.
Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007;30(8):991–6. https://doi.org/10.1093/sleep/30.8.991.
Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31(5):1001–6. https://doi.org/10.2337/dc07-2003.
Hwu DW, Lin KD, Lin KC, Lee YJ, Chang YH. The association of obstructive sleep apnea and renal outcomes-a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):313. https://doi.org/10.1186/s12882-017-0731-2.
Dixon JB, Schachter LM, O’Brien PE, Jones K, Grima M, Lambert G, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308(11):1142–9. https://doi.org/10.1001/2012.jama.11580.
Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23(3):414–23. https://doi.org/10.1007/s11695-012-0862-2.
Spicuzza L, Caruso D, Di Maria G. Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis. 2015;6(5):273–85. https://doi.org/10.1177/2040622315590318.
de Vries GE, Wijkstra PJ, Houwerzijl EJ, Kerstjens HAM, Hoekema A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:55–68. https://doi.org/10.1016/j.smrv.2017.10.004.
Phillips CL, McEwen BJ, Morel-Kopp MC, Yee BJ, Sullivan DR, Ward CM, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax. 2012;67(7):639–44. https://doi.org/10.1136/thoraxjnl-2011-200874.
Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2015;17(3):215–22. https://doi.org/10.1111/jch.12472.
Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–23. https://doi.org/10.1161/HYPERTENSIONAHA.106.085175.
Iftikhar IH, Valentine CW, Bittencourt LR, Cohen DL, Fedson AC, Gislason T et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014;32(12):2341–2350; discussion 50. https://doi.org/10.1097/HJH.0000000000000372.
Pengo MF, Ratneswaran C, Berry M, Kent BD, Kohler M, Rossi GP, et al. Effect of continuous positive airway pressure on blood pressure variability in patients with obstructive sleep apnea. J Clin Hypertens (Greenwich). 2016;18(11):1180–4. https://doi.org/10.1111/jch.12845.
Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26(5):281–7. https://doi.org/10.1038/jhh.2011.47.
Pedrosa RP, Drager LF, de Paula LKG, Amaro ACS, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144(5):1487–94. https://doi.org/10.1378/chest.13-0085.
Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–8. https://doi.org/10.1001/jama.2012.4366.
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. https://doi.org/10.1016/S0140-6736(05)71141-7.
Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2017;21(1):181–9. https://doi.org/10.1007/s11325-016-1393-1.
•• McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–31. https://doi.org/10.1056/NEJMoa1606599 A randomized controlled trial of 2717 adults with moderate-to-severe OSA and a prior history of cardiovascular disease, which demonstrated that CPAP plus usual care did reduce the risk of cardiovascular events compared with usual care alone.
Parra O, Sanchez-Armengol A, Bonnin M, Arboix A, Campos-Rodriguez F, Perez-Ronchel J, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37(5):1128–36. https://doi.org/10.1183/09031936.00034410.
Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol. 2017;120(4):693–9. https://doi.org/10.1016/j.amjcard.2017.05.042.
Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–20. https://doi.org/10.1164/rccm.201601-0088OC.
da Silva PF, Zhang L. Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: a meta-analysis of randomized trials. Sleep Med. 2019;54:28–34. https://doi.org/10.1016/j.sleep.2018.09.030.
•• Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156–66. https://doi.org/10.1001/jama.2017.7967 A systematic review and meta-analysis of randomized controlled trials comparing the effectiveness of CPAP compared with controls with regard to the development of cardiovascular events and death. The meta-analysis demonstrated that CPAP did not reduce the risk of adverse cardiovascular outcomes or death compared with controls.
Lettieri CJ, Williams SG, Collen JF, Wickwire EM. Treatment of obstructive sleep apnea: achieving adherence to positive airway pressure treatment and dealing with complications. Sleep Med Clin. 2017;12(4):551–64. https://doi.org/10.1016/j.jsmc.2017.07.005.
Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896–7.
Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000b;284(23):3015–21. https://doi.org/10.1001/jama.284.23.3015.
Pasha S, Kumar S, Chatterjee AB, Krishnaswamy G. An obstructive sleep apnea primer: what the practicing allergist needs to know. Ann Allergy Asthma Immunol. 2017;118(3):259–68. https://doi.org/10.1016/j.anai.2016.07.033.
Drager LF, Diegues-Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23(3):249–54. https://doi.org/10.1038/ajh.2009.246.
• Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. The role of weight management in the treatment of adult obstructive sleep apnea. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(6):e70–87. https://doi.org/10.1164/rccm.201807-1326ST A clinical practice guidline from the American Thoracic Society supporting weight loss interventions, particularly using comprehensive lifestyle modifications, as an integral part of the treatment of individuals with OSA.
Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–75. https://doi.org/10.1056/NEJMoa1306187.
Cohen JB, Cohen DL. Cardiovascular and renal effects of weight reduction in obesity and the metabolic syndrome. Curr Hypertens Rep. 2015;17(5):34. https://doi.org/10.1007/s11906-015-0544-2.
Peromaa-Haavisto P, Tuomilehto H, Kössi J, Virtanen J, Luostarinen M, Pihlajamäki J, et al. Obstructive sleep apnea: the effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med. 2017;35:85–90. https://doi.org/10.1016/j.sleep.2016.12.017.
Wong A, Barnes HN, Joosten SA, Landry SA, Dabscheck E, Mansfield DR, et al. The effect of surgical weight loss on obstructive sleep apnoea: a systematic review and meta-analysis. Sleep Med Rev. 2018;42:85–99.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Liann Abu Salman, Rachel Shulman, and Jordana B. Cohen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension
Rights and permissions
About this article
Cite this article
Salman, L.A., Shulman, R. & Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr Cardiol Rep 22, 6 (2020). https://doi.org/10.1007/s11886-020-1257-y
Published:
DOI: https://doi.org/10.1007/s11886-020-1257-y