Abstract
Implantable cardioverter-defibrillators (ICDs) are a powerful tool in preventing sudden cardiac death due to ventricular arrhythmias in ischemic cardiomyopathy. ICD indications and timing in acute coronary syndromes are unclear.
Purpose of Review
We reviewed several trials that delineated the indications for a cardiac defibrillator in patients with coronary artery disease.
Recent Findings
The role of cardiac defibrillators in secondary prevention has been well established by AVID, CIDS, and CASH trials. AVID showed reduction in both all-cause mortality and arrhythmic death while the two smaller trials showed only improvement in arrhythmic death. Similarly, trials like MADIT, CABG Patch, MUSTT, MADIT-II, DINAMIT, and the IRIS trial have fine-tuned the indications for ICD in primary prevention of sudden cardiac death. Benefits of an ICD were most pronounced in those with reduced ejection fraction and 40 days or more since myocardial infarction or in those who were not immediately post revascularization. The recent VEST trial aimed to study wearable cardioverter-defibrillators (WCDs) in patients who did not have an indication for an implantable defibrillator. The arrhythmic deaths (1.6% vs. 2.4%) were not reduced by the WCD.
Summary
Based on consistent reduction in arrhythmic death in all primary and secondary prevention trials, defibrillators are effective in carefully selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sudden cardiac death (SCD) is defined as the unexpected death from a cardiac cause within a short period of time, specifically less than an hour from the onset of symptoms, in a person without any prior condition that would appear fatal [1]. This is in contrast with sudden cardiac arrest (SCA) which refers to the onset of life-threatening cardiac abnormalities that, if not promptly reversed, progress to SCD [2]. The incidence of SCD in the USA was 347,322 during the year 2016 (based on the extrapolation of data from Resuscitation Outcomes Consortium Registry) [3]. Ventricular arrhythmias (VAs) related to the coronary artery disease (CAD) are the underlying etiology in the majority of cases [4]: nearly half of the patients with CAD die due to sustained ventricular tachycardia (VT) or ventricular fibrillation (VF) [5]. Implantable cardiac defibrillators (ICDs) have proven to be a powerful tool for treatment of unstable VAs and, by the same token, in prevention of SCD in high-risk patients. This article reviews the role of cardiac defibrillators in prevention of SCD after an acute coronary syndrome (ACS).
Prevention of Sudden Cardiac Death
SCA can be aborted by termination of life-threatening arrhythmias by either synchronized cardioversion or defibrillation. It is important to understand that ICD terminates an episode of VA without altering the underlying mechanisms leading to the arrhythmia. Beyond defibrillators, management of VAs also involves the use of antiarrhythmic drugs and invasive catheter ablation therapies.
Drugs such as beta-blockers, amiodarone, lidocaine, dofetilide, and sotalol have been used successfully for prevention and treatment of these arrhythmias [5]. Yet, no mortality benefit has been achieved by these drugs as mentioned in the following text. Class Ib agents (flecainide and encainide) have been shown to lead to a higher rate of arrhythmic death [6] when used after MI and therefore, should be avoided. Catheter ablation has been shown to reduce the need for defibrillator therapy [7, 8] and mortality [8], when used in carefully selected patients.
Defibrillator therapy can abort an episode of ventricular tachycardia and hence, as outlined in the current guidelines, remains the mainstay for prevention of SCD in selected patients [9].
Current Guidelines for Cardiac Defibrillators in SCD Prevention After ACS
The effective termination of VAs by ICDs has led to effective prevention of SCDs in patients with established ischemic cardiomyopathy. Currently, guidelines support their use in patients with significant LV dysfunction that persists 40 days after an acute myocardial infarction, or 3 months after revascularization. Within those time frames, even though the incidence of VAs may be significant, the role of ICDs is less clear.
There are two types of defibrillators: implantable cardioverter-defibrillators (ICDs) and wearable cardioverter-defibrillators (WCDs). Based on current guidelines [9], the following are the indications for ICD placement after an ACS:
- 1.
Secondary prevention
Patients who survived SCA secondary to VAs and do not have a reversible etiology (class I, level A)
Patients with non-sustained VAs, history of prior myocardial infarction (MI), left ventricular ejection fraction (LVEF) < 40%, and inducible VAs during an electrophysiological study (class I, level B)
- 2.
Primary prevention
Patients with prior MI, LVEF < 35% despite optimal medical therapy, NYHA class II–III, and more than 40 days since last MI or 90 days since most recent revascularization (class I, level A)
Patients with prior MI, LVEF < 30% despite optimal medical therapy, NYHA class I, and more than 40 days since last MI or 90 days since most recent revascularization (class I, level A)
The following are the indications for WCD, based on the most recent guidelines [9], after an episode of ACS:
WCDs may be appropriate as a bridging therapy in situations associated with an increased risk of SCA in which ICDs have been shown to reduce arrhythmic death, but not overall mortality such as within 40 days of MI (class IIb; level of evidence C).
Below, we will summarize the evidence supporting the role of defibrillator therapy in primary and secondary prevention of SCD.
Secondary Prevention of Sudden Cardiac Death After ACS
This refers to the prevention of SCD in those patients who have survived a prior cardiac arrest or sustained VT. ICD placement in patients with cardiac conditions associated with a high risk of sudden death and unexplained syncope, which is likely to be secondary to VAs, is also considered to fall under the category of secondary prevention. Below we discuss some of the most important clinical trials that shaped the current guidelines for secondary prevention of SCD. Findings of these trials are summarized in Table 1.
The AVID (Antiarrhythmics Versus Implantable Defibrillator) trial was the largest trial comparing ICD against drug therapy (amiodarone and sotalol) [10]. The study enrolled 1016 patients with either SCA caused by VT or VF or sustained VT leading to syncope or severe hemodynamic compromise in patients with a LVEF less than 40%. After a mean follow-up of 18.2 months, the study was terminated early because of a significant reduction in mortality in the ICD group. This study showed a 31% relative risk reduction in the primary outcome of overall mortality at 3 years (p < 0.02) primarily due to prevention of death due to arrhythmia [11••].
Similar to the AVID trial, CIDS (Canadian Implantable Defibrillator Study) trial enrolled patients who either survived cardiac arrest or had VF or sustained VT leading to hemodynamic compromise in those with a LVEF less than 40% [12••]. Those with unmonitored syncope and inducible VT in the setting of reduced LVEF were also included. A total of 659 patients were enrolled and randomized to receive either ICD or amiodarone drug therapy. The trial showed a relative risk reduction of 20% in all-cause mortality; however, this did not reach statistical significance (p = 0.142).
Similarly, the CASH (Cardiac Arrest Study Hamburg) trial was another prospective, randomized controlled trial which enrolled survivors of SCA secondary to VT or VF [13]. A total of 288 patients were randomized to either ICD placement or medical therapy (amiodarone and metoprolol). After a minimum follow-up of 2 years, the trial showed a 23% relative risk reduction in the overall mortality benefitting the ICD group; however, again this benefit did not reach statistical significance (p = 0.08).
All three of these secondary prevention trials, mentioned above, were conducted with similar enrollment criteria and showed similar results; i.e., ICD therapy was superior to antiarrhythmic drugs (AAD) alone. Of note, smaller trials (CIDS and CASH trials) did not reach statistical significance but showed a trend in favor of the device therapy. This benefit was confirmed in a meta-analysis of all 3 trials (AVID, CIDS, and CASH) showing a statistically significant reduction in total mortality [14]. Consequently, ICD therapy is listed as a class I indication with level of evidence A for patients who survive a cardiac arrest caused by VT or VF or sustained stable VT which cannot be attributed to a transient or reversible cause according to ACC/AHA guidelines. Examples of reversible causes include acute myocardial infarction, electrolyte abnormalities, and arrhythmia secondary to drugs. Needless to state, clinical judgment is fundamental in identification of these transient or reversible causes of SCA in the setting of acute or recent myocardial infarction. Minor abnormalities of electrolytes and mild elevations of cardiac enzymes may lead to a diagnostic dilemma as they may be cause or the effect of a cardiac arrest.
Primary Prevention of Sudden Cardiac Death in Established Ischemic Cardiomyopathy
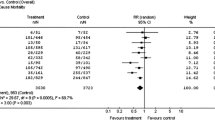
Once ICDs were proven to be safe and efficient for secondary prevention of SCA, their use was explored in lower risk populations. Patients who had structural heart disease but no evidence of sustained VT or VF were known to be at risk for SCA and henceforth, were likely to benefit from ICDs. Initial studies for primary prevention were focused on patients with known coronary artery disease. A discussion of the trials studying effectiveness follows. The findings of these trials are summarized in Table 2 and Fig. 1.
MADIT (Multicenter Automatic Defibrillator Implantation Trial) was the first large-scale trial to study the effectiveness of an ICD in primary prevention of SCD [15]. Electrophysiological study yielding induction of sustained VT or VF and not suppressed with procainamide infusion in patients with known coronary artery disease, prior MI, and left ventricular ejection fraction < 35% was a prerequisite for enrollment of this trial. A total of 196 patients were randomized to receive either an ICD or an AAD (mostly amiodarone). At 2 years, there was a relative risk reduction of 54% in the primary outcome of overall mortality which was statistically significant (p = 0.009).
MADIT-II (Multicenter Automatic Defibrillator Implantation Trial II) was the first trial to assess the survival benefit of an ICD in patients with prior MI without the prerequisite electrophysiological testing [16]. A total of 1232 patients, with reduced LVEF (< 30%) and MI that occurred a month or more prior to enrollment, were randomized in a 3:2 ratio to either ICD or medical therapy alone. After an average follow-up of 20 months, reduction in the primary outcome of total mortality attributable to device therapy was 31%. This suggests a number needed to treat (NNT) of 18 patients. A post hoc analysis of the 202 deaths in this trial determined that the reduction in total mortality in the ICD group was entirely due to reduction in SCD [17]. Both MADIT and MADIT-II trials were crucial in establishing the role of an ICD in primary prevention of SCD. MUSTT (Multicenter Unsustained Tachycardia Trial) also showed a benefit of ICD in a similar population [18]; findings of this trial are summarized in Table 2.
The entire population enrolled in MADIT and MADIT-II and majority (84%) in MUSTT trial were enrolled well after the index ACS or MI that led to ischemic cardiomyopathy. Patients within 3 weeks after MI were excluded in MADIT, 4 weeks were excluded in MADIT-II, and 86% of enrollees in MUSTT were > 1 month post most recent myocardial infarction.
The effectiveness of ICD in SCD prevention goes beyond ischemic cardiomyopathy with reduced ejection fraction and extends to non-ischemic cardiomyopathy patients with reduced ejection fraction as well. Its effectiveness has also prevailed over time, despite improvements in medical therapy. Most recently, it was proven in the Swedish study SWEDE-HF [19] that utilized a prospective heart failure registry dating back to the year 2000. For this analysis, they looked at patients who met guidelines for primary prevention ICD implantation, i.e., EF ≤ 40%, heart failure duration of ≥3 months, and NYHA Class ≥ II. Of the 16,702 ICD eligible patients, only 1599 (10%) had an ICD implantation. They matched 1305 of the patient with an implanted ICD to 1305 patients in the registry without an ICD. Of the ICD indicated patients, 32% received a CRT-D. Enrollment completed in 2016 and the average follow-up was 2.6 years; of note, 50% of the patients were enrolled after 2012. The result without matching (just comparing ICD and no ICD patients), ICDs were associated with a 25% risk reduction in all-cause mortality at 5 years; after matching, ICDs were associated with a 12% risk reduction in all-cause mortality at 5 years.
Another recent study, EU-CERT-ICD (European Comparative Effectiveness Research to Assess the Use of Primary Prophylactic Implantable Cardioverter Defibrillators) [20], was a prospective study funded by a grant from the European Union, which included 44 sites in 15 European countries. Patients were enrolled if they had heart failure, EF ≤ 35%, and a class I indication for ICDs (excluding CRT patient). Patients who received an ICD (N = 1526) were compared with patients who did not receive an ICD (N = 731); patient and clinicians made the decision whether to receive the ICD (it was not randomized). Enrollment started in May 2014 and the average follow-up was 2.4 years. ICDs were associated with a 31% risk reduction in all-cause mortality at 5 years (p = 0.0016) and 83% reduction in sudden cardiac death (p < 0.0001).
Primary Prevention of Sudden Cardiac Death After ACS
The challenge of preventing SCD shortly after an MI or ACS remained. Unlike previous studies, DINAMIT (Defibrillator in Acute Myocardial Infarction trial) evaluated the benefit of ICD early after a MI [21]. A total of 674 patients, with a reduced ejection fraction (EF < 35%), MI (6–40 days prior to enrollment), and depressed heart rate variability (standard deviation of normal to normal RR interval ≤ 70 ms) or increased 24-h average heart rate (mean RR interval ≤ 750 ms), were enrolled. Patients were randomized in 1:1 ratio between an ICD group and a conventional medical therapy group. After an average follow-up of 30 months, deaths from any cause (primary outcome) were similar in the two groups. The prespecified secondary outcome of death from an arrhythmic cause was significantly reduced in the ICD group (N = 12 vs. 29). However, there was no overall survival benefit due to an increase in non-arrhythmogenic death in the ICD group as compared with AAD. Similar results were seen in the IRIS trial [22]; hence, the current guidelines recommend delaying the implantation of an ICD in this patient population for at least 40 days after acute myocardial infarction. Both DINAMIT and IRIS supported the contention that ICDs effectively prevent VAs early after MI but do not improve overall survival because they somehow increase non-arrhythmogenic mortality.
The CABG (Coronary Artery Bypass Graft) Patch trial assessed the prophylactic placement of epicardial cardioverter-defibrillators at the time of revascularization [23]. A total of 900 patients with reduced LVEF (< 36%) were randomized to receive either an epicardial defibrillator or medical therapy at the time of coronary artery bypass surgery. After an average follow-up of 32 months, no significant difference was found between the two groups in terms of total mortality or arrhythmic death [24]. The arrhythmic mortality at 42 months was 6.9% in the control group and 4% in the ICD group (p 0.057). ICD therapy reduced arrhythmic death by 45% without a significant effect on non-arrhythmic death, but since, 71% of total mortality was due to non-arrhythmic causes, this did not lead to a significant difference in total mortality of both groups. It is important to mention that there was an increased rate of infection in the ICD group, like deep sternal wounds (2.7% 0.4%) and wound or catheter site infection (12.3% vs. 5.9%). Notice the use of non-conventional ICD implants like epicardial, probably would explain such findings. Thus, there is likely no benefit of a defibrillator placement soon after revascularization, and hence, guidelines recommend waiting of 90 days after revascularization prior to implanting an ICD.
A recent trial that challenges this waiting time of 90 days after revascularization in the setting of ACS was the DAPA (Defibrillator After Primary Angioplasty) [25]. It was a Medtronic-sponsored, prospective randomized study investigating ICD implantation between 30 and 60 days after angioplasty for STEMI and was conducted at 15 sites in the Netherlands and Poland (N = 262). Patients were enrolled if they had at least on risk factors like ventricular fibrillation, EF < 30%, Killip class 2 or higher, or TIMI flow less than 3 after primary PCI. The primary outcome was long-term mortality benefit. The ICD implantation group was associated with a 42% risk reduction in all-cause mortality 10 years after implant (p = 0.02). This study suggests that early prophylactic ICD implantation may be considered for high-risk patients, despite improvement of their LVEF. However, this needs to be confirmed with larger studies.
Another recent trial studying primary prevention of SCD after ACS is the Vest Prevention of Early Sudden Death Trial (VEST), which assessed the effectiveness of the WCD in primary prevention of SCD [26••]. However, the current guidelines predate this publication. This study was aimed to assess the effectiveness of a WCD during the period after a MI and prior to when an ICD is indicated, based on current guidelines. A total of 2348 patients, with a reduced ejection fraction (EF < 35%) and an acute MI were randomized in 2:1 ratio to receive a WCD or to receive conventional therapy. After a mean follow-up of 84 days, no difference in the primary outcome, comprising rate of arrhythmic death, between the device and control groups was seen (25 (1.6%) in therapy vs. 19 (2.4%) in the control group (p = 0.18)). The total mortality was 48 (3.1%) in the device group vs. 38 (4.9%) in the control group (p = 0.04). A total of 29 participants received shocks of which 21 were appropriate. Of the 21 appropriate shocks, 6 patients died. There were study design limitations. First, the primary outcome was changed from all-cause mortality to arrhythmic mortality after enrollment began, as arrhythmic death is more difficult to adjudicate. The cause of death was adjudicated by an independent panel that was unaware of group assignments and did not review data from the WCD at time of death. This could result in a misclassification of the etiology of sudden cardiac death and could explain why total mortality was reduced significantly but arrhythmic death only showed a trend towards reduction. The high rate of crossover (19%, never used the WCD) and suboptimal compliance with wear time of 14 h a day (median), which was lower than WEARIT-II registry data 22.5 h a day (median) [27], may have compromised the intention-to-treat analysis. Investigators reported that 3 out of 4 patients who died in the WCD group did not wear their WCD at the time of death. Compliance may be the biggest limitation for this WCD. Overall, the study design has major limitations and the current use of the WCD is still controversial.
Whether these results of these trials will lead to any changes in current guidelines is yet to be seen.
Subcutaneous ICD
Subcutaneous ICD (S-ICD) is an alluring alternative for patients who have a high risk of infection or end-stage renal failure and no pacing requirement. S-ICD has major advantages of lesser adverse events, which are usually associated with a transvenous system such as systemic infection, pneumothorax, lead displacement, and safer system removal. However, its limitations are increased rate of T wave over sensing leading to inappropriate shocks which appears to decrease with preprocedure screening. Also, limited backup pacing and lack of antitachycardia pacing are other limitations [28]. At present, these devices do not have any randomized controlled trial data to support them [29]. All of our current understanding of these devices is based on investigational device exemption (IDE) trial and postmarket (EFFORTLESS) registry [30]. Based on the registry date, S-ICDs can terminate 90% and 98% of the VT/VF episodes with 1 and 5 shocks respectively.
Conclusion and Future Directions
The effectiveness of an ICD is well recognized and, therefore, remains the cornerstone of SCD prevention. The abovementioned clinical trials have helped us understand why and when to use an ICD successfully. However, there still are several questions that are left to be answered. For example, patients who are at risk for SCD but were not well represented in the clinical trials, such as those over the age of 80 or those with end-stage renal disease, and female patients. Further research is also needed in cases where patients are at risk for SCD but are not eligible to receive an ICD, such as those with recent MI or those immediately post revascularization.
A closer look at the recently published VEST trial gives us some insight into this population. The population studied in the VEST trial was similar to the DINAMIT trial including the mean left ventricular ejection fraction (LVEF 28.2%). However, this was higher than that of the population studied in MADIT-II trial (EF 23%). Based on these observations, one may believe that the populations studied in prior studies were sicker than the population studied in the VEST trial. However, the similar results of DINAMIT, IRIS, and VEST trials strongly suggest cardiac defibrillators (implantable or wearable) do not change outcomes early after a MI, despite a reduction in rate of arrhythmic death. This likely is due to the inherent disadvantage of a defibrillator; i.e., it is unable to alter the underlying substrate, which may manifest with an alternate mode of death in the most vulnerable period, immediately after an MI. Also, similar to CABG Patch trial, the majority (83.6%) of patients enrolled in the VEST trial underwent revascularization as opposed to the DINAMIT trial in which only about 36.1% underwent angioplasty; the rest underwent thrombolysis or no therapy. These results are consistent with minimal benefit of a cardioverter-defibrillator early after revascularization; whether this is due to subsequent improvements in LVEF or a reduction in ischemia is not entirely clear. The only exception is the recent DAPA trial which used a different inclusion criteria, where higher risk patients (STEMI with high-risk features) for sudden cardiac death showed benefits with earlier implantation of ICD, despite improvement of LVEF in 46% of the population. However, this needs to be confirmed with larger studies.
Based on consistent reduction in arrhythmic death in all abovementioned trials, we conclude that cardiac defibrillators are effective when used in appropriately selected patients. The future of SCD prevention probably lies beyond the limited arsenal of device therapy. Understanding cellular mechanisms and anatomic disturbances will hold the key to further progress and advancement in this field.
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–51.
ACC/AHA/HRS. 2006 key data elements and definitions for electrophysiological studies and procedures. Circulation. 2006;114(23):2534–70.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
Chelly J, Mongardon N, Dumas F, Varenne O, Spaulding C, Vignaux O, et al. Benefit of an early and systematic imaging procedure after cardiac arrest: insights from the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) registry. Resuscitation. 2012;83(12):1444–50.
Bhar-Amato J, Davies W, Agarwal S. Ventricular arrhythmia after acute myocardial infarction: ‘The Perfect Storm’. Arrhythmia Electrophysiol Rev. 2017;6(3):134–9.
Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–8.
Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375(9708):31–40.
Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375(2):111–21.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138(13):e272–391.
Antiarrhythmics versus Implantable Defibrillators I. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576–83.
•• Causes of death in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J Am Coll Cardiol. 1999;34(5):1552–9. Landmark article showing evidence to use implantable defibrillators for sudden cardiac death.
•• Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101(11):1297–302. Landmark article showing evidence to use implantable defibrillators for sudden cardiac death.
Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102(7):748–54.
Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg . Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21(24):2071–8.
Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–40.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83.
Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML, et al. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol. 2004;43(8):1459–65.
Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341(25):1882–90.
Schrage B, Uijl A, Benson L, Westermann D, Stahlberg M, Stolfo D, et al. Association between use of primary prevention implantable cardioverter-defibrillators and mortality in patients with heart failure: a prospective propensity-score matched analysis from the Swedish Heart Failure Registry. Circulation. 2019.
Bauer A, Klemm M, Rizas KD, Hamm W, von Stulpnagel L, Dommasch M, et al. Prediction of mortality benefit based on periodic repolarisation dynamics in patients undergoing prophylactic implantation of a defibrillator: a prospective, controlled, multicentre cohort study. Lancet. 2019.
Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–8.
Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361(15):1427–36.
Bigger JT Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337(22):1569–75.
Bigger JT Jr, Whang W, Rottman JN, Kleiger RE, Gottlieb CD, Namerow PB, et al. Mechanisms of death in the CABG Patch trial: a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation. 1999;99(11):1416–21.
Haanschoten, D. (2019). Long-term outcome of the Defibrillator After Primary Angioplasty (DAPA) trial. European Society of Cardiology Congress, Paris, France.
•• Olgin JE, Pletcher MJ, Vittinghoff E, Wranicz J, Malik R, Morin DP, et al. Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med. 2018;379(13):1205–15. This article shows new evidence on wearable cardiac-defibrillator for prevention of sudden cardiac death, which may impact the current guideline and its use.
Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation. 2015;132(17):1613–9.
Patel KH, Lambiase PD. The subcutaneous ICD-current evidence and challenges. Cardiovasc Diagn Ther. 2014;4(6):449–59.
Chieng D, Paul V, Denman R. Current device therapies for sudden cardiac death prevention - the ICD, subcutaneous ICD and wearable ICD. Heart Lung Circ. 2019;28(1):65–75.
Burke MC, Gold MR, Knight BP, Barr CS, Theuns D, Boersma LVA, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65(16):1605–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Faheemullah Beg, Miguel Valderrabano, and Paul Schurmann declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Management of Acute Coronary Syndromes
Rights and permissions
About this article
Cite this article
Beg, F., Valderrabano, M. & Schurmann, P. Device Therapy for Sudden Cardiac Death Prophylaxis After Acute Coronary Syndrome: When and Why?. Curr Cardiol Rep 22, 4 (2020). https://doi.org/10.1007/s11886-020-1255-0
Published:
DOI: https://doi.org/10.1007/s11886-020-1255-0