Abstract
Purpose of Review
This review describes the effects of psychological stress on the physiology of the entire vascular system, from individual cellular components to macrovascular and microvascular responses, and highlights the importance of the vascular system in the context of current limitations in cardiac imaging for evaluation of the cardiovascular response to mental stress.
Recent Findings
The physiological responses that mediate vascular changes are based on evolutionary needs, but there is increasing evidence that the long-term consequences of psychological stress can precipitate the development and progression of cardiovascular disease (CVD). While there is an extensive body of literature describing localized physiological responses or overt cardiovascular manifestations, often framed within the organ-specific scope of cardiovascular imaging, there has not been a comprehensive description of the global vascular effects of psychological stress. Given the global nature of these processes, targeted cardiovascular imaging modalities may be insufficient. Here we approach the vascular response to mental stress systematically, describing the effects on the endothelium, vascular smooth muscle, and adventitia. We then address the mental stress effects on large vessels and the microvascular compartment, with a discussion of the role of microvascular resistance in the pathophysiology of mental stress-induced myocardial ischemia.
Summary
Vascular responses to psychological stress involve complex physiological processes that are not fully characterized by routine cardiovascular imaging assessments. Future research incorporating standardized psychological assessments targeted toward vascular mechanisms of stress responses is required to guide the development of behavioral and therapeutic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emotional stress is a universal experience that affects human behavior and physiology [1]. Stress affects every organ system and is associated with the development and progression of many disease states, including cardiovascular disease (CVD), reactive airway disease, inflammatory bowel disease, diabetes mellitus, neurological disorders, and susceptibility to infection [2]. The pairing of cardiovascular imaging with psychological triggers has elucidated the physiological mechanisms underlying cardiovascular responses to emotional stress, which include activation of the autonomic nervous system with accompanying parasympathetic withdrawal, hypothalamic-pituitary-adrenal axis signaling, and renin-angiotensin-aldosterone system activation [3]. Despite the association of psychological stress with the pathogenesis of hypertension, atherosclerotic coronary artery disease (CAD), and cerebrovascular disease, emotional stress is seldom addressed and is challenging to modify as a risk factor in the clinical care of patients with CVD. Furthermore, while emotional stress is a shared experience among all humans, only some people manifest overt pathological and deleterious cardiovascular consequences [4]. While hereditary factors, lifestyle behaviors, traditional cardiac risk factors, underlying inflammatory conditions, and aging have all been recognized as contributing factors in the development and progression of CVD, the importance of psychological stress as a cardiovascular risk factor remains under-recognized and challenging to address.
Much of the literature to date has focused on the cognitive or cardiac contributions to the effects of mental stress on cardiovascular health, omitting the contribution of the vascular system as the primary site of transducing psychological stress into CVD. Foundational studies have described the clinical manifestations of cardiovascular responses to emotional stress within the framework of existing diagnostic tests and imaging, such as silent ST depression by electrocardiographic monitoring, stress-induced left ventricular dysfunction by transthoracic echocardiography, or abnormalities in myocardial perfusion imaging [5,6,7,8,9]. Subsequent work has linked the pathophysiology of emotional stress responses to findings on cardiovascular imaging, but in order to describe potential mechanistic insights, these studies have largely remained limited in scope [10,11,12,13,14]. We propose that by starting with a birds-eye perspective of the vascular system, the reader can develop an understanding of the multi-organ system response to emotional stress and thus better interpret the limitations of currently available cardiovascular imaging modalities. We believe that this rests on two key questions addressed in this review:
-
How does psychological stress affect compartments of the vascular system?
-
What are the physiological manifestations of the vascular response to stress?
This review will describe the acute and chronic vascular effects of emotional stress and propose a role for stress-induced alterations in vascular homeostasis as a common pathway for the development of CVD.
Functional Significance of Vascular Anatomy in Response to Psychological Stress
We will focus on the arteriolar system which is the afferent system that is innervated by the autonomic nervous system and regulates blood delivery and organ perfusion, with a specific focus on vascular biologic pathways that are perturbed in psychological stress. The discussion will be organized by the anatomy of the arteriolar vessels (intima, media, and adventitia), adrenergic and inflammatory responses, and macrovascular/microvascular effects of stress.
Effects of Psychological Stress on Endothelial Function
The innermost layer of arteries is the intima which lines the vessel lumen with longitudinally oriented endothelial cells (EC) and is commonly referred to as the endothelium. The muscular layer, or media, is the next layer which contains circumferentially oriented vascular smooth muscle cells (VSMC) and extracellular matrix [15]. Normal endothelium maintains vascular tone, mediates VSMC proliferation, and regulates thrombogenesis/fibrinolysis [16,17,18]. Vascular tone, as reflected by the balance between vasodilation and vasoconstriction, is mediated by the synthesis and release of biologically active substances from EC which act on adjacent VSMC. Among the vasodilatory substances is nitric oxide (NO), previously referred to as an “endothelium-derived relaxing factor,” which is synthesized from L-arginine by endothelial nitric oxide synthase (eNOS) in response to shear stress, autonomic nervous system stimulation, or activation by acetylcholine, bradykinin, serotonin, or thrombin [19, 20]. The endothelium is involved in balancing vasodilation with vasoconstriction via endothelin [21, 22]. Endothelin is a 21-residue vasoconstrictor peptide that was first isolated from porcine aortic EC and found to belong to a family of three distinct but related genes [23, 24]. Classical endothelin, or endothelin-1 (ET-1), is a potent vasoconstrictor in vitro and in vivo and potentiates the effects on VSMC of other vasoconstrictive substances such as catecholamines, serotonin, and angiotensin II [25]. ET-1 is produced by ECs, activated macrophages, and VSMCs [22].
Psychological stress is involved in endothelial injury and cell turnover. Cynomolgus monkeys who were exposed to a new social group for 3 days were found to have increased endothelial cell damage and replication in the thoracic aorta and coronary arteries [26] demonstrating that the psychosocial stress of an altered environment/social structure is associated with endothelial injury and increased turnover. The effect of psychological stress and anger on endothelial damage is further supported by more recent work that showed an increase in circulating endothelial cell-derived microparticles (derived from the membranes of apoptotic endothelial cells) after a public speaking task [27, 28].
Beyond the integrity of endothelial cells, stress affects the physiology of the endothelium. In animal models, subacute and chronic mental stress reduce arterial eNOS mRNA expression, resulting in endothelial dysfunction [29, 30]. The effect of psychological stress on endothelium-dependent vasodilation in humans has been demonstrated in multiple vascular beds, but mechanistic studies have focused on forearm vasodilator responses as a surrogate for coronary vascular reactivity. Spieker et al. assessed the effect of a 3-min mental stress task (responding to randomly flashing color lights by pushing a corresponding button) on radial artery flow-mediated vasodilation (FMD) as measured by an A-mode ultrasound device [31]. The authors found that after the mental stress task, radial artery FMD decreased by half and this effect persisted for 45 min. The infusion of a selective ETA (ET-1) receptor antagonist prevented the stress-induced decrease in radial artery FMD, but there was no significant change in the level of ET-1 with stress as compared with baseline suggesting that this effect may be related to decreased nitric oxide availability. Thus, sudden mental stress transiently impairs endothelium-dependent flow-mediated vasodilation, and this effect can be prevented by ET-1 receptor blockade. Similar effects on endothelial-dependent brachial artery dilation have been demonstrated in response to various laboratory mental stress tasks [32, 33•].
Effects of Psychological Stress on the Adventitia and Vasa Vasorum
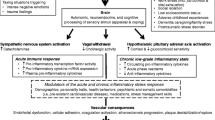
The adventitia is the outermost layer of arteries which contains connective tissue, fibroblasts, macrophages, mast cells, and a microvascular bed called the vasa vasorum which provides perfusion to the vessel [15] (Fig. 1). The adventitia is innervated by autonomic nerve fibers which have endings that are in contact with adventitial mast cells at the adventitial-medial border [34]. With regard to physiological responses to psychological stress, the adventitia is the site of inflammatory responses that are involved in vasospasm and the development of atherosclerosis [35].
Vascular compartments and mediators of vascular tone in response to psychological stress. Simplified schematic showing vessel wall layers of a typical arterial segment with associated structures. The intima is the innermost layer which contains the endothelium, with psychological stress affecting vasodilatation via nitric oxide and vasoconstriction via endothelin-1 (ET-1). The media contains vascular smooth muscle cells which are under the influence of autonomic and inflammatory signaling via sympathetic nerve fibers on the adventitia
The autonomic nervous system is involved in vasodilatory, and vasoconstrictor responses via autonomic nerve endings lie in the adventitia and release neurotransmitters that act on vascular smooth muscle within the tunica media. In most vascular beds, the primary role for sympathetic innervation is vasoconstriction (in coronary arteries, sympathetic activity can be involved in vasodilatation as well) [15]. The primary neurotransmitter released from sympathetic nerves is norepinephrine, which is involved in vasoconstriction via α-1 and α-2 adrenergic receptors [36]. Completing mentally stressful tasks is associated with an increase in venous and arterial norepinephrine concentration [37], which correlates with stress-induced increases in mean arterial pressure. A recent laser Doppler flowmetry study demonstrated greater microvascular vasoconstriction as well as increased responsiveness to norepinephrine in healthy subjects who reported high levels of daily psychosocial stress as compared with subjects who reported low levels of daily psychosocial stress [38], and similar results have been reported with peripheral arterial tonometry (PAT) [39]. These studies demonstrate the role of the adventitia in the regulation of vasomotion in response to psychological stress.
Notably, the adventitia is the site of transduction for neuroendocrine responses to stress via stimulation of the autonomic nervous system [40]. In response to psychological stress, corticotropin-releasing hormone (CRH) and the related peptide urocortin are released in the amygdala and hypothalamus, resulting in an increase in circulating catecholamines and activation of the sympathetic nervous system [41, 42]. Sympathetic nerve fibers in contact with adventitial perivascular mast cells at the adventitial-medial border release peptides such as the neurotransmitter substance P and calcitonin gene–related peptide to stimulate mast cell degranulation, resulting in the release of vasoactive compounds such as histamine and leukotriene [34, 43, 44]. The resulting vasodilatation and increase in microvascular permeability can be stimulated by mental stress and blocked with anti-histamines (diphenhydramine or cyproheptadine), demonstrating the role for histamine as a key mediator of stress-induced vasodilation [45, 46]. This complex neuroendocrine-vascular interaction underlies common responses to psychological stress, such as skin flushing and sweating, as well as increased gastrointestinal symptoms in patients with irritable bowel syndrome or inflammatory bowel disease [47, 48]. These biological mechanisms can have deleterious cardiovascular effects—psychological stress in mice has been shown to precipitate perivascular mast cell degranulation in the aortic root as well as plaque destabilization as evidenced by increased intra-plaque hemorrhage in areas of atherosclerosis [49], and in humans, there is an association between adventitial mast cells and atherosclerosis as well as myocardial infarction [34, 50, 51].
Inflammatory Response to Mental Stress
The sympathetic stimulation of perivascular mast cells in response to psychological stress is an example of the involvement of the immune system in stress responses. In addition to sympathetic activation, a cardinal feature that distinguishes the biologic response to cognitive triggers, as opposed to exercise triggers, is the cholinergic anti-inflammatory pathway or parasympathetic inflammatory reflex [36, 52]. This inflammatory reflex is fundamental to understanding the pathophysiology induced by cognitive and emotional stress. As a brief description, central nervous system efferent activity in the vagus nerve leads to acetylcholine release in organs of the reticuloendothelial system, including the heart, liver, spleen, and gastrointestinal tract. Acetylcholine interacts with receptors on tissue macrophages, stimulating the release of inflammatory cytokines, including interleukin-1 (IL-1), interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and nuclear factor kappa B [53,54,55]. Thus, the observed withdrawal of parasympathetic activity during mental stress results in the converse, i.e., the release of inflammatory cytokines and attendant effects on vasomotor tone and function.
As described in the discussion of endothelial responses, brachial artery FMD is reduced in response to mental stress, which is associated with a significant rise in serum cortisol levels. Inhibition of downstream cortisol metabolism with metyrapone has been shown to abrogate this effect of stress on FMD, suggesting an inflammatory mechanism to stress-induced endothelial dysfunction [53, 55]. After a standardized speaking task, healthy male physicians were found to have elevated plasma cortisol as well as an increase in IL-1β, IL-2, and soluble intracellular adhesion molecule (ICAM-1) [56]. Other studies have demonstrated the involvement of TNF-α in animal models of unpredictable chronic mild stress, with impaired endothelial-dependent smooth muscle relaxation that was reversed with infliximab, a TNF-α inhibitor [57, 58]. The systemic inflammatory response to stress is reviewed in detail elsewhere, and the psychological stress-induced inflammatory changes in vascular biology remain poorly understood [54, 59, 60].
Macrovascular Effects
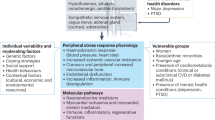
Psychological stress exerts subclinical effects on the vascular system that reflect a summation of the pathophysiological processes if vasomotor regulation, adrenergic stimulation, and inflammation precede adverse clinical outcomes [61] (Fig. 2). The hemodynamic response to emotional or physical stress includes an increase in heart rate and blood pressure, which reflects augmented cardiac output and stroke volume with dynamic changes in systemic vascular resistance [62]. This response is evolutionary—in response to physical stress, augmentation in cardiac output, and peripheral vasodilatation which generally matches increases in energy demands [63]. However, the physiologic response to psychological stress is much more complex and can generate a mismatch, with augmented cardiac output in the face of peripheral vascular resistance, promoting the pathological vascular effects of acute and long-term psychological stress [64]. Using impedance cardiography, Sherwood and colleagues described changes in cardiac output, total peripheral vascular resistance, and heart rate in response to exercise and psychological stress (shock avoidance) [63]. While exercise and psychological stress both caused an increase in heart rate and estimated cardiac output, there was no significant change in oxygen consumption during psychological stress. Furthermore, vascular resistance was found to decrease in response exercise, but there were two phenotypes of response to psychological stress. Those subjects with a large increase in cardiac output during shock avoidance showed a decrease in vascular resistance, whereas those with a minimal change in cardiac output showed a slight increase in vascular resistance. The subjects with a decrease in vascular resistance with psychological stress were postulated to have an anticipatory “fight or flight” response that would precede physical exertion, but the latter group represents a maladaptive response [65]. Our group described changes in left ventricular function and vascular resistance in a radionuclide scintigraphy study where subjects with CAD underwent mental arithmetic and anger recall tasks [66]. In subjects with a fall in left ventricular ejection fraction, there was a decrease in cardiac output and increase in peripheral vascular resistance, whereas in those without a change in left ventricular ejection fraction, there was an increase in cardiac output and a less prominent change in peripheral vascular resistance. Similar results were reported from the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study [67]. [68] The response characterized by a decrease in cardiac output, decrease in left ventricular ejection fraction, and increase in systemic vascular resistance may represent a pathologic response (Fig. 2).
Hemodynamic effects of psychological stress. The macrovascular and microvascular responses to physical and psychological stress. Physical stress predominantly involves an increase in cardiac output but a decrease in systemic and coronary vascular resistance. In psychological stress, there is variability in the physiologic response, leading to two phenotypes: (1) increase in cardiac output accompanied by a decrease in vascular resistance (appropriate response) and (2) decrease or no change in cardiac output with an increase in systemic vascular resistance (pathologic response). The former reflects an anticipatory “fight or flight” response where psychological stress elicits physiologic preparation for a physical response. The latter may represent a maladaptive stress response and, in patients with mental stress-induced myocardial ischemia, may be accompanied by increased coronary microvascular resistance and afterload
The physiological response to stress is influenced by the interplay of race, gender, co-existing medical conditions, and psychosocial stress, which may predispose individuals to the development of overt CVD. In a study of mildly hypertensive men and women, African American men were found to have greater peripheral resistance during public speaking than Caucasian men, and women exhibited lower vascular resistance at rest and with stress [69]. In general, men respond to psychological stressors with increased diastolic blood pressure and higher total peripheral resistance, while women have a predominant increase in heart rate [69]. In a study of healthy volunteers who were assessed for depressive symptoms, the subjects with higher levels of depressive symptoms had greater increases in systemic vascular resistance in response to a mirror tracing task [70]. Patients with rheumatoid arthritis (RA) have been shown to have greater endothelial dysfunction and increases in systemic vascular resistance during mental stress in the setting of active inflammation as compared with RA patients without active inflammation [71]. Recurrent or prolonged psychological stress has been implicated in the pathogenesis and progression of hypertension as a result of stress-induced vasoconstriction and eventual vascular hypertrophy/remodeling [72]. Indeed, higher blood pressure responses to mental stress tasks were shown to predict earlier onset of hypertension in a 13-year follow-up of the CARDIA study [73].
How do long-term macrovascular effects of stress lead to the development of CVD? Studies demonstrate that the adverse macrovascular effects of psychological stress involve acute hemodynamic changes leading to long-term deleterious effects [74]. As an example, there has been considerable work on the effects of psychological stress on carotid artery intima-media (CIMT), an early marker of atherosclerosis, which can be measured by sonographic assessment. Several studies have demonstrated greater CIMT in patients with chronic anxiety, depression, or psychosocial stress [75,76,77,78]. Patients with higher resting blood pressure and predominantly hypertensive responses to psychological stress during a verbal speaking task or mirror image tracing were shown to have a greater increase in CIMT over 5 years of follow-up [79]. Increases in pulse pressure in response to mental stress had the strongest association with increases in CIMT, which may reflect the effect of increased stroke volume combined with reduced vascular compliance in response to stress. The Kuopio Ischemic Heart Disease study demonstrated that Finnish men with high systolic and diastolic blood pressure reactivity to mental stress tasks had both a greater burden of CIMT and a greater increase in CIMT from baseline to 4 years of follow-up, with significant increases in CIMT for each ~ 7 mmHg rise in systolic blood pressure during mental stress [80, 81]. In the Whitehall II study 10,308 civil servants in London, England, were recruited to assess the effects of psychosocial risk factors on the development of CVD. Over a range of multiple stressors, people of lower socioeconomic status and a delayed post-stress blood pressure recovery underwent sonographic exams 3 years later and were found to have higher CIMT compared with either subjects of higher socioeconomic status or those with adequate post-stress blood pressure recovery [82].
Microvascular Effects
In the cardiovascular system, the impact of psychological stress on the microvascular vessels may be particularly important in the pathophysiology of mental stress-induced myocardial ischemia (MSIMI). The microvascular bed involves arterioles and capillaries less than 400 μm in diameter (Fig. 2), and in the coronary circulation, more than 80% of resistance to blood flow is regulated by the microvascular vessels. Of note, arterioles exist in every mammalian organ system and include the majority of the vascular endothelium (by surface area) [83]. The phenomenon of MSIMI involves the interaction between emotional or psychological stress and abnormal myocardial perfusion, which is associated with an elevated heart rate and blood pressure, endothelial dysfunction, and altered coronary blood flow as measured non-invasively [31, 84]. Autoregulation of myocardial blood flow and changes in microvascular resistance occur via the sympathetic nervous system, which directly innervates the coronary arterioles via α-adrenergic receptors and the myocardium via β-adrenergic receptors [85]. These are under the influence of catecholamines, such as norepinephrine and epinephrine, which may rise and cause vasoconstriction and adverse cardiovascular events in response to mental stress [86, 87]. The neurohormonal activation that occurs with mental stress may also include neurotransmitters such as serotonin, as selective serotonin reuptake inhibitors have been shown to blunt the cardiovascular hemodynamic response to mental stress [88, 89].
Studies of MSIMI first demonstrated that angiographically normal epicardial coronary arteries exhibit vasodilatation and increased coronary blood flow in response to mental stress. In contrast, the presence of atherosclerosis alters the endothelial response resulting in local vasoconstriction in segments with luminal disease or epicardial stenosis [90]. However, the decrease in coronary blood flow that occurs in the setting of mental stress is out of proportion to the degree of dynamic vasoconstriction of the epicardial vessel, suggesting that mental stress also affects resistance in the coronary microcirculation [90,91,92]. Physiological studies with coronary Doppler flow assessment have demonstrated higher microvascular resistance in response to mental stress in patients with non-obstructive CAD as compared with those with normal coronary arteries [91]. Myocardial perfusion imaging studies have shown that mental stress creates heterogeneity in myocardial blood flow in patients with known CAD [36, 92]. Furthermore, patients with CAD and MSIMI have been shown to have diminished coronary blood flow on myocardial perfusion imaging in territories without epicardial stenosis, indicating that elevated microcirculatory resistance may be present [92].
In unpublished work by our group, we have found that there is a dynamic increase in endothelial-dependent coronary microvascular resistance in response to mental stress in patients with non-obstructive CAD. The microvascular response to mental stress appears to be a global effect, rather than being limited to the coronary circulation, as increases in peripheral arterial microcirculatory resistance as measured by peripheral arterial tonometry (EndoPAT, Itamar Medical, Israel) correlate with rate pressure product adjusted decreases in coronary blood flow and perfusion defects during mental stress [5].
The effects of stress beyond the coronary circulation are significant. Psychological stress alters the function of the microcirculation throughout the body. In patients with systemic sclerosis and documented microvascular involvement who are prone to distal limb ischemia from severe endothelial dysfunction, mental stress stimulates a significant increase in ET-1 production [93]. Mental stress stimulates inflammatory responses, mast cell degranulation, and release of vasoactive substances in rat mesenteric vessels, leading to increased microvascular permeability [94] and reduced gut motility. The effects of mental stress on the cerebral microcirculation can be profound. In rats chronic social stress has been shown to decrease density of microvascular vessels in the hippocampus, and psychological stress reduces neo-revascularization after ischemic injury [95, 96]. In humans, greater blood pressure reactivity in response to mental stress is associated with increased evidence of silent cerebrovascular disease (small cerebral infarcts or infarct like lesions) [97], but the role of the microcirculation has not yet been determined. A meta-analysis of markers of microvascular dysfunction (endothelial biomarkers, albuminuria, skin/muscle microcirculation, retinal arteriolar diameter, or cerebral small vessel disease) showed that there is an association with an increased risk of developing late life depression [98].
Conclusions
Over the past four decades, there has been an increasing awareness and understanding of the effects of emotional stress on cardiovascular health [4, 99••, 100]. Both acute and chronic stress have been shown to have adverse cardiovascular effects ranging from elevated blood pressure, increased rates of myocardial infarction, cerebrovascular disease, atherosclerosis, and possibly cardiovascular death [101]. The mechanistic basis for these clinical syndromes involves the global effect of psychological stress on the vascular system, with resulting alterations in vascular homeostasis, hemodynamic changes, inflammation, and altered organ perfusion.
Despite the tremendous efforts to characterize these effects particularly through the use of cardiac imaging tools, there remain critical knowledge gaps. Previous work to define the effects of psychological stress on vascular function has largely been associative, focusing on one organ system, and as we have described, stress affects blood vessels and entire arterial systems (large vessels and the microvasculature) and has a global effect throughout the body. For example, focusing on one causal agent such as nitric oxide may omit the effects of psychological stress on inflammation, catecholamines, or mast cell function, which are all likely concurrently involved in the regulation of vasomotor tone. Additionally, diagnostic imaging strategies to describe the effects of mental stress on CVD rely on technologies developed for cardiology clinical care (myocardial perfusion imaging and echocardiography). Often, these modalities provide a single snapshot, such as left ventricular ejection fraction, which represents only a cross section at one moment rather than the continuous effects on vascular function of a lifetime of stress exposure and may not be representative of the complex and dynamic physiology involved. Another shortcoming of diagnostic imaging is that the microvascular compartment harbors the majority of the endothelium; however, accurately characterizing psychological stress-induced changes in microvascular function with diagnostic imaging has remained elusive.
Routine clinical practice often fails to systematically assess the impact of mental stress on disease onset and progression and of the myriad tools that have been developed for psychological evaluation, none have been adopted into standard clinical assessments of CVD. Thus, there are no targeted medications to ameliorate the adverse vascular effects of psychological stress, and behavioral interventions are underutilized in clinical practice despite supporting data from randomized clinical trials. Future research programs should focus on the development of clinically practical psychological assessments that can be incorporated into the routine assessment of vascular disease, as well as the incorporation of psychosocial stress burden as a risk factor for CVD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–9.
McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–101.
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409.
Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–62.
Hammadah M, Kim JH, Al Mheid I, et al. Coronary and peripheral vasomotor responses to mental stress. J Am Heart Assoc. 2018;7(10):e008532.
Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24(8):690–703.
Bairey CN, Krantz DS, Rozanski A. Mental stress as an acute trigger of ischemic left ventricular dysfunction and blood pressure elevation in coronary artery disease. Am J Cardiol. 1990;66(16):28G–31G.
Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22(2):440–8.
Arrighi JA, Burg M, Cohen IS, Soufer R. Simultaneous assessment of myocardial perfusion and function during mental stress in patients with chronic coronary artery disease. J Nucl Cardiol. 2003;10(3):267–74.
Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96(10):3390–5.
Campisi R, Czernin J, Schöder H, Sayre JW, Schelbert HR. L-arginine normalizes coronary vasomotion in long-term smokers. Circulation. 1999;99(4):491–7.
Boyle SH, Matson WR, Velazquez EJ, Samad Z, Williams RB Jr, Sharma S, et al. Metabolomics analysis reveals insights into biochemical mechanisms of mental stress-induced left ventricular dysfunction. Metabolomics. 2015;11(3):571–82.
Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61(7):714–22.
Andrews TC, Parker JD, Jacobs S, Friedman R, Cummings N, MacCallum G, et al. Effects of therapy with nifedipine GITS or atenolol on mental stress-induced ischemic left ventricular dysfunction. J Am Coll Cardiol. 1998;32(6):1680–6.
Hilenski LL, Griendling KK. Vascular smooth muscle. In Creager MA, Beckman JA, Loscalzo J (eds). Vascular Medicine: A Companion to Braunwald's Heart Disease, 3rd Ed. 2013, 32–63. Elsevier, San Diego, CA.
Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol. 2001;12(4):383–9.
Creager MA, Lüscher TF, prepared with the assistance of, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108(12):1527–32.
Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–32.
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–12.
Broten TP, et al. Role of endothelium-derived relaxing factor in parasympathetic coronary vasodilation. Am J Phys. 1992;262(5 Pt 2):H1579–84.
Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6(4):S188–91.
Drexler HG. Responsiveness of B-cll cells to growth factors. Blood. 1988;72(4):1435–6.
Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86(8):2863–7.
Winkles JA, Alberts GF, Brogi E, Libby P. Endothelin-1 and endothelin receptor mRNA expression in normal and atherosclerotic human arteries. Biochem Biophys Res Commun. 1993;191(3):1081–8.
Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, et al. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation. 2001;104(10):1114–8.
Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, et al. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68(5):1270–9.
Spicer J, Shimbo D, Johnston N, Harlapur M, Purdie-Vaughns V, Cook J, et al. Prevention of stress-provoked endothelial injury by values affirmation: a proof of principle study. Ann Behav Med. 2016;50(3):471–9.
Shimbo D, Rosenberg LB, Chaplin W, Zhao S, Goldensohn ER, Cholankeril M, et al. Endothelial cell activation, reduced endothelial cell reparative capacity, and impaired endothelial-dependent vasodilation after anger provocation. Int J Cardiol. 2013;167(3):1064–5.
Custodis F, Gertz K, Balkaya M, Prinz V, Mathar I, Stamm C, et al. Heart rate contributes to the vascular effects of chronic mental stress: effects on endothelial function and ischemic brain injury in mice. Stroke. 2011;42(6):1742–9.
Chung IM, Kim YM, Yoo MH, Shin MK, Kim CK, Suh SH. Immobilization stress induces endothelial dysfunction by oxidative stress via the activation of the angiotensin II/its type I receptor pathway. Atherosclerosis. 2010;213(1):109–14.
Spieker LE, Hürlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105(24):2817–20.
Sherwood A, Johnson K, Blumenthal JA, Hinderliter AL. Endothelial function and hemodynamic responses during mental stress. Psychosom Med. 1999;61(3):365–70.
Ghiadoni L, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–8 This study demonstrated that healthy men exposed to psychological stress with a public speaking task rapidly develop endothelial dysfunction as measured by reduced brachial artery flow-mediated dilation, and this persists for up to four hours.
Laine P, Naukkarinen A, Heikkila L, Penttila A, Kovanen PT. Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation. 2000;101(14):1665–9.
Alevizos M, Karagkouni A, Panagiotidou S, Vasiadi M, Theoharides TC. Stress triggers coronary mast cells leading to cardiac events. Ann Allergy Asthma Immunol. 2014;112(4):309–16.
Soufer R, Jain H, Yoon AJ. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr Cardiol Rep. 2009;11(2):133–40.
Freyschuss U, et al. Cardiovascular and sympathoadrenal responses to mental stress: influence of beta-blockade. Am J Phys. 1988;255(6 Pt 2):H1443–51.
Greaney JL, Surachman A, Saunders EFH, Alexander LM, Almeida DM. Greater daily psychosocial stress exposure is associated with increased norepinephrine-induced vasoconstriction in young adults. J Am Heart Assoc. 2020;9(9):e015697.
Alkhoder A, et al. Abstract 17559: Norepinephrine levels are associated with the magnitude of vasoconstriction during mental stress. Circulation. 2015;132(suppl_3):A17559-A17559.
Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin N Am. 2001;30(3):695–728 vii-viii.
Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147(10):4578–88.
Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN, et al. Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides. 2004;25(10):1711–21.
Kalsner S, Richards R. Coronary arteries of cardiac patients are hyperreactive and contain stores of amines: a mechanism for coronary spasm. Science. 1984;223(4643):1435–7.
Allen S, Dashwood M, Morrison K, Yacoub M. Differential leukotriene constrictor responses in human atherosclerotic coronary arteries. Circulation. 1998;97(24):2406–13.
Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130(3):224–31.
Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, et al. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288(3):1349–56.
Arck PC, Slominski A, Theoharides TC, Peters EMJ, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126(8):1697–704.
Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–9.
Lagraauw HM, Wezel A, van der Velden D, Kuiper J, Bot I. Stress-induced mast cell activation contributes to atherosclerotic plaque destabilization. Sci Rep. 2019;9(1):2134.
Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99(3):361–9.
Bot I, de Jager SCA, Bot M, van Heiningen SH, de Groot P, Veldhuizen RW, et al. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ Res. 2010;106(1):89–92.
Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96.
Steptoe A, et al. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond). 2001;101(2):185–92.
Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52(1):1–23.
Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, Bigiani A, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25(5):500–6.
Heinz A, Hermann D, Smolka MN, Rieks M, Gräf KJ, Pöhlau D, et al. Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: implications for coronary heart disease. Psychopharmacology. 2003;165(2):111–7.
Demirtas T, et al. The link between unpredictable chronic mild stress model for depression and vascular inflammation? Inflammation. 2014;37(5):1432–8.
d'Audiffret AC, et al. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol (1985). 2010;108(5):1041–51.
Gu HF, Tang CK, Yang YZ. Psychological stress, immune response, and atherosclerosis. Atherosclerosis. 2012;223(1):69–77.
Huang CJ, et al. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013;4:314.
Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–32.
Astrand PO, et al. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–74.
Sherwood A, Allen MT, Obrist PA, Langer AW. Evaluation of beta-adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiology. 1986;23(1):89–104.
Obrist PA, Light KC. Comments on “carotid dP/dt as a psychophysiological index of sympathetic myocardial effects: some considerations”. Psychophysiology. 1980;17(5):495–8.
Sherwood A, Turner JR. Hemodynamic responses during psychological stress: implications for studying disease processes. Int J Behav Med. 1995;2(3):193–218.
Jain D, Shaker SM, Burg M, Wackers FJT, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31(6):1314–22.
Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference group for the psychophysiological investigations of myocardial ischemia (PIMI) study. Circulation. 1996;94(11):2768–77.
Sherwood A, May CW, Siegel WC, Blumenthal JA. Ethnic differences in hemodynamic responses to stress in hypertensive men and women. Am J Hypertens. 1995;8(6):552–7.
McAdoo WG, Weinberger MH, Miller JZ, Fineberg NS, Grim CE. Race and gender influence hemodynamic responses to psychological and physical stimuli. J Hypertens. 1990;8(10):961–7.
Baldwin R, Jeffries S, Jackson A, Sutcliffe C, Thacker N, Scott M, et al. Neurological findings in late-onset depressive disorder: comparison of individuals with and without depression. Br J Psychiatry. 2005;186:308–13.
Veldhuijzen van Zanten JJ, Kitas GD. Inflammation, carotid intima-media thickness and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. 2008;10(1):102.
Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139(9):761–76.
Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110(1):74–8.
McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44.
Paterniti S, Zureik M, Ducimetière P, Touboul PJ, Fève JM, Alpérovitch A. Sustained anxiety and 4-year progression of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(1):136–41.
Haas DC, Davidson KW, Schwartz DJ, Rieckmann N, Roman MJ, Pickering TG, et al. Depressive symptoms are independently predictive of carotid atherosclerosis. Am J Cardiol. 2005;95(4):547–50.
Lynch J, Kaplan GA, Salonen R, Cohen RD, Salonen JT. Socioeconomic status and carotid atherosclerosis. Circulation. 1995;92(7):1786–92.
Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997;15(1):49–55.
Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Lassila HC, Wolfson SK. Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke. 1998;29(8):1525–30.
Kamarck TW, Everson SA, Kaplan GA, Manuck SB, Jennings JR, Salonen R, et al. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men: findings from the Kuopio ischemic heart disease study. Circulation. 1997;96(11):3842–8.
Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110(15):2198–203.
Steptoe A, Donald AE, O’Donnell K, Marmot M, Deanfield JE. Delayed blood pressure recovery after psychological stress is associated with carotid intima-media thickness: Whitehall psychobiology study. Arterioscler Thromb Vasc Biol. 2006;26(11):2547–51.
Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985). 2008;105(1):370–2.
Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–12.
Soufer R, Burg MM. The heart-brain interaction during emotionally provoked myocardial ischemia: implications of cortical hyperactivation in CAD and gender interactions. Cleve Clin J Med. 2007;74(Suppl 1):S59–62.
Lampert R, Jain D, Burg MM, Batsford WP, McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000;101(2):158–64.
Soufer R, Fernandez AB, Meadows J, Collins D, Burg MM. Body mass index and risk for mental stress induced ischemia in coronary artery disease. Mol Med. 2016;22:286–91.
Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92(8):2102–8.
Golding M, Kotlyar M, Carson SW, Hoyler S, Lazarus C, Davidson C, et al. Effects of paroxetine on cardiovascular response to mental stress in subjects with a history of coronary artery disease and no psychiatric diagnoses. Psychopharmacology. 2005;182(3):321–6.
Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ Jr, Ganz P, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325(22):1551–6.
Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO III. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76(3):125–30.
Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, et al. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356(9226):310–1.
Fontana F, Bernardi P, Lanfranchi G, Conti E, Spampinato S, di Toro R, et al. Endothelin-1 response to mental stress in early ischemic lesions of the extremities due to systemic sclerosis. Peptides. 2005;26(12):2487–90.
Wilson LM, Baldwin AL. Effects of environmental stress on the architecture and permeability of the rat mesenteric microvasculature. Microcirculation. 1998;5(4):299–308.
Czeh B, et al. Quantitative changes in hippocampal microvasculature of chronically stressed rats: no effect of fluoxetine treatment. Hippocampus. 2010;20(1):174–85.
Maingrette F, Dussault S, Dhahri W, Desjarlais M, Mathieu R, Turgeon J, et al. Psychological stress impairs ischemia-induced neovascularization: protective effect of fluoxetine. Atherosclerosis. 2015;241(2):569–78.
Waldstein SR, Siegel EL, Lefkowitz D, Maier KJ, Pelletier Brown JR, Obuchowski AM, et al. Stress-induced blood pressure reactivity and silent cerebrovascular disease. Stroke. 2004;35(6):1294–8.
van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(7):729–39.
Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–217 Comprehensive review regarding the cardiovascular effects of acute and chronic stress including an overview of psychosocial factors, vascular biology, the role of the autonomic nervous system, and clinical syndromes.
Das S, O'Keefe JH. Behavioral cardiology: recognizing and addressing the profound impact of psychosocial stress on cardiovascular health. Curr Atheroscler Rep. 2006;8(2):111–8.
Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–46.
Funding
This work was supported by NIH NHLBI R01 HL059619 and R01 HL071116, awarded to RS and MB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Shah, S.M., Meadows, J.L., Burg, M.M. et al. Effects of Psychological Stress on Vascular Physiology: Beyond the Current Imaging Signal. Curr Cardiol Rep 22, 156 (2020). https://doi.org/10.1007/s11886-020-01406-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-020-01406-x