Abstract
Purpose of Review
This paper will review myocardial infarction with non-obstructive coronary arteries (MINOCAs) briefly, and then focus on the imaging tools that should be employed when caring for patients with suspected MINOCA.
Recent Findings
Diagnostic imaging has a critical role in assessing patients with suspected or confirmed MINOCA. The extent at which these diagnostic tests are used in any given patient will depend on the clinical acumen for the underlying condition, as well as the available resources.
Summary
There are myriad conditions that can lead to MINOCA; further testing to exclude other underlying causes of myocardial injury is crucial. Cardiovascular imaging may assist in identifying the etiological cause in cases where MINOCA remains the most likely diagnosis. A systematic approach to the diagnostic assessment will help to uncover the underlying diagnosis, guide therapy, and provide the patient and their families with appropriate feedback.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background and Introduction
Coronary angiography in the clinical context of acute myocardial infarction (AMI) identifies obstructive coronary artery disease (CAD) in approximately 85–95% of patients. For these patients with significant CAD, the benefits of revascularization and cardioprotective therapies are well established and supported in clinical practice guidelines [1, 2]. However, in up to 15% of patients with clinical diagnostic features of AMI, early angiography does not reveal obstructive CAD [3,4,5,6]. A diagnosis of myocardial injury with non-obstructive CAD should prompt further investigation to determine whether there is indeed an ischemic mechanism of myocardial injury, (and therefore an AMI with no obstructive CAD (MINOCA) or whether a non-ischemic mechanism of myocardial injury is responsible for the clinical presentation (Table 1).
The American Heart Association [7••] and European Society of Cardiology [8] have published consensus documents defining MINOCA and outlining the various etiologies responsible for this syndrome as well as possible treatment regimens. The diagnosis of MINOCA requires three core criteria: (1) clinical evidence of AMI, as defined by the Universal Definition of Infarction [9]; (2) absence of any coronary lesions with ≥ 50% stenosis by coronary angiography; (3) no alternative cause for the clinical presentation. It is intuitive, therefore, that in order to arrive at a diagnosis of MINOCA, one must first review the initial presentation and confirm that the clinical features do support a diagnosis of an AMI. For this reason, further diagnostic studies are often necessary in order to exclude additional conditions that may be masquerading as MINOCA, and when MINOCA remains the working diagnosis, to discern the underlying etiology. This document will briefly review the syndrome of MINOCA, with a focus on the imaging tools that should be employed when caring for patients with suspected MINOCA.
Overview of MINOCA
MINOCA is encountered in 5–15% of AMI patients undergoing coronary angiography [3,4,5,6]. MINOCA patients are younger than patients with AMI and obstructive CAD (AMI-CAD), with a larger proportion of female patients seen in the MINOCA cohort of AMI [5]. MINOCA comprises a heterogeneous syndrome with various etiologies.
Treatment for patients with MINOCA should focus on treatment of the underlying condition, (i.e., anti-platelets and statins for patients with plaque disruption; aspirin and beta-blockers for patients with spontaneous coronary artery dissection (SCAD); calcium channel blockers for patients with epicardial coronary artery spasm; anti-anginal therapies for patients with microvascular disease and anti-platelet or anti-thrombotic regimens for patients with embolic or hypercoagulable conditions). Additionally, observational studies have demonstrated improved outcomes with the routine use of statins and angiotensin receptor antagonists/angiotensin-converting enzyme inhibitors in all MINOCA patients [10,11,12] (cardioprotective therapies). Furthermore, a study examining the benefits of routine cardioprotective therapies with beta-blockers and angiotensin receptor antagonists/angiotensin-converting enzyme inhibitors for 3500 patients with MINOCA (Randomized Evaluation of β-Blocker and ACEI/ARB Treatment in MINOCA Patients (MINOCA-BAT) trial-clinicaltrials.gov Identifier: NCT03686696) is ongoing. This study will evaluate whether these routine therapies will reduce the incidence of the composite endpoint of death, readmission for AMI, ischemic stroke, or heart failure in patients with MINOCA. The results are expected to be released in October 2025.
Studies have shown that the prognosis of patients with MINOCA is generally more favorable than patients with AMI-CAD [3, 5, 6]. In the ACTION Registry-GWTG which examined 322,523 patients with AMI, in-hospital mortality was nearly 3 times higher for patients with AMI-CAD as compared to patients with MINOCA. On the other hand, other studies evaluating longer term follow-up have not reported more favorable outcomes in patients with MINOCA. The Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) registry (a cohort of predominantly young, patients with MINOCA with a 2:1 female to male enrollment) demonstrated similar 1-year outcomes in MINOCA patients as compared to patients with AMI-CAD [4]. Additionally, data from the KAMIR registry reported similar 2-year rates of death and/or myocardial infarction for patients with MINOCA as compared to those with AMI-CAD [11]. Finally, as compared with a population of patients without AMI or angiographic CAD, MINOCA patients are more likely to suffer from cardiovascular events in follow-up [6]. These discrepant findings likely reflect differences in study design among the various reports including differences in the populations evaluated, duration of follow-up, and comparator groups. The multicenter ACTION Registry-GWTG focused on in-hospital events, while the latter studies reported outcomes with longer term follow-up. This implies that the adverse clinical outcomes in patients with MINOCA are not exclusively limited to the post-MI period and will likely depend on the underlying etiology for MINOCA.
Clinical Presentation and Differential Diagnosis
The definition of MINOCA is based on an assumption that the totality of findings supports an AMI. Therefore, if coronary angiography demonstrates non-obstructive CAD or the absence of CAD, it is important to revisit the clinical presentation before entertaining a working diagnosis of MINOCA. An elevation in cardiac biomarkers (most often cardiac troponin I) that is > 99th percentile for the upper limit of normal is referred to as myocardial injury [9]. Acute myocardial injury characteristically displays a rise and/or fall in these enzymes. Patients with acute myocardial injury can be divided into two subgroups: (1) myocardial injury due to a non-ischemic mechanism and (2) myocardial injury due to an ischemic mechanism—which must be accompanied by signs or symptoms of ischemia in order to qualify as an AMI [9] (Table 1). The term MINOCA should be reserved for patients with characteristic signs and symptoms of AMI in whom there is a presumed ischemic mechanism of myocardial injury and no obstructive CAD [7••, 9]. While the formal definition of an AMI does not always require symptoms of acute ischemia [9], a diagnosis of MINOCA is best supported by a patient presenting with ischemic symptoms including chest discomfort, epigastric pain, shortness of breath, or shoulder/jaw pain. Additionally, the presence of other acute conditions, (i.e., sepsis, acute on chronic kidney injury, acute on chronic heart failure) in the absence of symptoms of ischemia, generally argues against a diagnosis of MINOCA.
The Role of Imaging in Assessing a Patient with MINOCA
Diagnostic testing is critical in the evaluation of patients with suspected or confirmed MINOCA. There is an array of diagnostic imaging modalities available to the clinician to aid in diagnosing MINOCA (Fig. 1) and, when MINOCA is present, to identify the underlying etiology (Table 2). The extent at which these diagnostic tests are used in any given patient will depend on the clinical acumen for the underlying condition, as well as the available resources.
Cardiac Catheterization
A working diagnosis of MINOCA begins in the catheterization laboratory at the time of coronary angiography. Before taking the patient off the table, one should always review the angiogram to ensure that there are no “missed obstructions” (i.e., a flush occlusion or a severe stenosis of a small/distal vessel), because if there is obstructive CAD, consideration should be given to revascularization, and the patient would no longer carry a diagnosis of MINOCA (Video 1). Additionally, intracoronary imaging, when available, should be considered. Studies have shown that close to 40% of patients with MINOCA have evidence of plaque disruption (plaque erosion, plaque rupture, or calcific nodules) on intracoronary imaging [13,14,15]. In the proper clinical context, one might also consider provocative testing for epicardial or microvascular spasm, although provocative spasm testing, when employed, is typically done at a time remote from the acute event.
Optical Coherence Tomography
Optical coherence tomography (OCT) provides high-resolution images (10–15 μm) of the arterial lumen and the layers of the arterial wall and therefore can provide a more detailed assessment of intravascular pathology. In patients with MINOCA, OCT is useful in assessing the integrity and thickness of the fibrous cap and identifying intracoronary thrombus. It can identify plaque erosion, which is not seen on IVUS [16]. For this reason, it is the preferred imaging device to assess for plaque disruption (Figs. 2, and 3). In one study [15] examining 38 patients with MINOCA undergoing OCT, plaque disruption was noted in 9 (24%) patients (8 with plaque rupture, 1 with calcific nodule, and 1 with both plaque rupture and calcific nodule). Additionally, 7 (18%) of patients had coronary thrombus, and 4 (11%) of patients had plaque erosion. None of the patients with angiographically normal coronary arteries had plaque disruption. OCT is also an excellent tool when assessing for the presence and extent of SCAD. It is important to note however that coronary angiography remains the first-line diagnostic tool for patients presenting with acute coronary syndrome due to suspected SCAD, while intracoronary imaging should be reserved for cases when there is uncertainty on coronary angiography, especially for cases due to intramural hematoma without intimal tear. Due to the small but real risk of propagating the dissection or causing guide-catheter-induced dissection, appropriate patient selection and meticulous technique is important when considering the use of intracoronary imaging in suspected SCAD patients [17].
a, b A 66-year-old male with no past medical history presenting with chest pain and shortness of breath, ST elevation in lateral and inferior leads, and elevated cardiac biomarkers. Angiogram non-obstructive lesion in mid-LAD (red arrow). OCT images demonstrating a thrombus overlying an intact fibrous cap in mid-LAD, consistent with plaque erosion (yellow arrow)
Intravascular Ultrasound Imaging
IVUS uses sound waves to assess lesion and vessel wall characteristics including plaque burden, plaque composition, and lumen size. IVUS has limited spatial resolution (200–250 μm) and therefore is not ideally suited to assess fibrous cap thickness, or plaque erosion, but it can identify plaque rupture (Fig. 4). Most studies of intravascular imaging in MINOCA have employed IVUS imaging and have demonstrated that roughly one-third of MINOCA patients have plaque rupture [13, 14]. In a study [14] of 42 patients with MINOCA undergoing IVUS imaging, 16 (38%) had evidence of plaque disruption with 12 (29%) demonstrating plaque rupture and 4 (10%) having plaque ulceration. Plaque disruption has been noted in angiographically normal appearing arterial segments; however, in one series of patients, plaque disruption was not evident when there was no angiographic CAD in any of the coronary arteries (i.e., normal coronary arteries) [18]. IVUS is also useful in identifying patients with SCAD when the diagnosis is not apparent on angiography (Fig. 5) [19].
A 75-year-old male with past medical history of hypertension presenting with chest pain, nonspecific ST, T changes, and elevated troponin. a Coronary angiogram revealed non-obstructive coronary artery disease, with evidence of reduction of contrast density in the mid-LAD (red arrow). b IVUS demonstrated plaque rupture in the mid-LAD (arrow)
A 42-year-old male without past medical history presenting with chest pain, mild T wave abnormalities in the anterior wall, and elevated cardiac biomarkers. a Angiogram demonstrating non-obstructive lesion in the mid-LAD (red arrow). b IVUS imaging demonstrating classical findings of SCAD with true lumen (T) and false lumen (white arrow) (F)
Provocative Spasm Testing
Provocative spasm testing for assessment of epicardial or microvascular spasm is typically performed using intracoronary acetylcholine, although some centers are using intravenous or intracoronary ergonovine as an alternative agent. Provocative spasm testing should be considered in patients with MINOCA and no other underlying etiology. It is ideally performed at a time remote from the infarct (> 6 weeks). In one series of 80 patients with MINOCA and no other clear etiology referred for provocative spasm testing, 37 (46%) had abnormal results. Among these patients, 2/3 of them had epicardial spasm and the remaining 1/3 had microvascular spasm. In the literature, the dosing regimens of intracoronary acetylcholine used for provocation testing are quite varied, with some using only a single dose, while others use incremental doses starting at 2 μg to up to 200 μg. Due to initial reports of serious adverse events with provocative spasm testing [20], provocative spasm testing is infrequently employed in the USA, with only select centers of excellence routinely performing such tests. More contemporary studies, however, have supported safety of provocative spasm testing, with low rates of serious complications [21].
Echocardiography
The echocardiogram remains one of the most important diagnostic studies used to assess patients with myocardial injury and a working diagnosis of MINOCA. If an echo was not done prior to the coronary angiogram, then it should be performed after angiography to further aide in the diagnostic cause of the patient’s presentation. Review of the echocardiogram should include a careful assessment of regional wall motion abnormalities. In cases of limited echocardiographic images, intravenous contrast may further aide in identifying subtle abnormalities. If there are regional wall motion abnormalities in a characteristic anatomical coronary distribution, then it supports a diagnosis of MINOCA. On the other hand, the echocardiogram may also be useful in supporting other non-ischemic mechanisms of myocardial injury. The presence of wall motion abnormalities inconsistent with the coronary distribution such as diffuse concentric involvement of the mid to distal myocardium would suggest Takotsubo cardiomyopathy, while diffuse or focal abnormalities in a non-coronary artery distribution may indicate myocarditis. The findings of a pericardial effusion should lead to consideration of myopericarditis, while right ventricular dilation and hypokinesis might lead one to consider pulmonary embolism (Fig. 6). In some cases, transesophageal echocardiography can be complimentary to the transthoracic echocardiogram for the identification of sources of emboli (left atrial or ventricular thrombi, tumors, or valve abnormalities such as fibroelastoma and endocarditis) that may result in MINOCA from distal embolization of the microcirculation. It is important to note that while the echocardiogram is useful in providing information regarding the possible etiology for a patient’s myocardial injury, cardiac MRI (CMR) is often necessary to characterize the etiology and degree of myocardial damage.
A 56-year-old male presenting with chest tightness and shortness of breath, sinus tachycardia, and nonspecific ST-T on ECG, with elevated troponin. Coronary angiogram showed normal coronary arteries. Bedside transthoracic echocardiogram a in the short-axis view showed right ventricle dilation and D shape septum suggestive of right ventricle pressure overload and b in the apical 4-chamber view showed right ventricle dilation with right ventricle free wall hypokinesia. Chest CT confirmed this diagnosis of pulmonary emboli
Cardiac MRI
CMR is one of the most important diagnostic tools employed for assessing patients with MINOCA as it can establish a definitive diagnosis (myocardial necrosis, myocarditis, or Takotsubo cardiomyopathy) and influence clinical management. Previous studies in patients with suspected MINOCA have shown that CMR leads to a change in diagnosis in 54% of patients and a change in management in 41% of patients [22••]. Advantages of CMR are high spatial resolution and the lack of ionizing radiation, along with the ability to perform a comprehensive assessment of cardiovascular structure, function, and tissue characterization, when feasible CMR should be performed in all patients with suspected MINOCA and is best when performed early after presentation. It is important to note however that while CMR might support a diagnosis of MINOCA in cases where there are findings of late gadolinium enhancement, the absence of necrosis on CMR does not exclude a diagnosis of MINOCA. In one report [14] among 16 patients with MINOCA who were found to have plaque disruption on IVUS, only one (6%) patient was found to have evidence of late gadolinium enhancement. Other studies have also shown discrepancies in the proportion of patients with plaque rupture and findings on CMR [15]. It is unclear why this is noted, and may be related to the limitations in CMR in identifying very small areas of necrosis, or alternatively may be related to differences in timing of the CMR acquisition following the acute event [23].
In patients with MINOCA, CMR can detect small areas of sub-endocardial infarction. Myocardial infarctions may be caused by the rupture of unstable plaques with subsequent embolization of cholesterol material and superimposed thrombi to the periphery of the coronary artery. Detection of such plaque ruptures may be difficult by conventional invasive coronary angiography which makes CMR a very helpful additional tool in the workup of such patients (Figs. 7 and 8). CMR can also be used to quantify myocardial perfusion in patient with microvascular dysfunction.
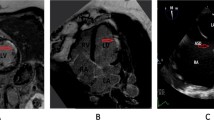
A 50-year-old female with a history of hypertension, hyperlipidemia, and diabetes presenting with chest pain, and nonspecific ST and T wave changes on ECG. Cardiac enzymes were elevated. Angiogram showed non-obstructive lesion. Cardiac MRI showed abnormal late gadolinium enhancement and edema of the lateral wall consistent with AMI. a T2-weighted imaging with myocardial edema of the mid-ventricular lateral wall consistent with acute myocardial injury (red arrowhead); and b, c post-contrast imaging demonstrates late gadolinium enhancement (green and blue arrows) indicative of a small-sized myocardial infarct involving predominantly the mid-ventricular lateral wall
A 42-year-old female with a history of hypertension presenting with severe chest pain. Electrocardiogram demonstrates T wave inversion in the precordial leads, and she has elevated cardiac enzymes. Coronary angiogram revealed normal coronary artery. Cardiac MRI showed abnormal rest perfusion defect and late gadolinium enhancement in the antero-apical region with sub-endocardial involvement consistent with myocardial infarction in the LAD territory. a Resting perfusion defect of antero-apical wall (red arrowhead); b, c post-contrast imaging demonstrates late gadolinium enhancement indicative of a small-sized myocardial infarction involving predominantly the antero-apical wall (green and blue arrowheads)
Finally, CMR will also help to identify other potential causes for the clinical presentation. CMR is the most comprehensive and accurate diagnostic tool for the noninvasive diagnosis of myocarditis, which is difficult to make on clinical grounds alone, and often mimics a MINOCA presentation. Myocardial inflammation leads to pathophysiological changes that can be detected using conventional CMR techniques. These include hyperemia and capillary leakage (early gadolinium enhancement), edema, and myocyte necrosis and fibrosis (late gadolinium enhancement) (Fig. 9). CMR is also ideally suited to detect the typical wall motion abnormalities and absence of late gadolinium enhancement associated with stress cardiomyopathy. In the acute phase of stress cardiomyopathy, the T2-weighted CMR shows a high signal intensity of myocardial edema. In stress cardiomyopathy, the area of edema usually corresponds to wall motion abnormalities different from those seen in acute coronary syndrome, where the distribution of the edema follows the epicardial coronary artery territory. In general, absence of macroscopic fibrosis as documented by the lack of a gadolinium enhancement is a classic hallmark for stress cardiomyopathy, helping to differentiate it from acute coronary syndrome.
A 70-year-old female with a history of hypertension presenting with chest pain. Her electrocardiogram showed nonspecific ST and T wave abnormalities and cardiac enzymes were elevated. Coronary angiogram revealed normal coronary artery. Cardiac MRI showed abnormal mid-myocardium late gadolinium enhancement with myocardial edema at the same territory consistent with acute myocarditis. a Edema-sensitive image (T2-weighted imaging) with increased signal intensity in the mid-myocardium (red arrow) indicates myocardial edema consistent with acute ischemic injury, and b, c post-contrast imaging demonstrates mid-myocardial late gadolinium enhancement with sparing of the sub-endocardium indicating a non-coronary etiology of the myocardial scar (green and blue arrows)
Coronary Computed Tomography Angiography
At the current time, a working diagnosis of MINOCA is traditionally made using conventional invasive coronary angiography and coronary computed tomography angiography (CCTA) should not be used interchangeably with coronary angiography when diagnosing MINOCA in a patient with AMI and non-obstructive CAD. With that in mind, as technologies evolve, it is possible that CCTA will play a greater role in diagnosing MINOCA and said etiologies. CCTA has a suite of strengths including assessment of plaque burden, identification of plaque characteristics, and perfusion data. In one study [24] of 50 patients with CMR-confirmed MINOCA, CCTA identified 101 plaques across all coronary segments, which was notably more than the 41 identified with invasive coronary angiography. Additionally, CCTA has been shown to be a better tool than coronary angiography alone in understanding the composition of non-obstructive coronary artery plaques [25]. CCTA can identify high-risk plaques as defined by large plaque burden, spotty calcium, positive remodeling, low Hounsfield plaque, and napkin ring sign (Fig. 10) [26]. However, at the current time due to limitations in resolution, CCTA cannot reliably identify plaque disruption, and invasive intracoronary imaging remains the gold standard. When exploring possible etiologies for MINOCA, cardiac CT (with or without coronary angiography) may be useful in identifying LV or LA thrombi or tumors (which may be potential sources of emboli). Additionally, cardiac CT may be useful in assessing for the presence of myocarditis although CMR remains the gold standard for evaluating this condition [27].
A 56-year-old female presenting with chest pain. Her electrocardiogram showed nonspecific ST and T wave abnormalities and cardiac enzymes were elevated. Coronary angiography was normal. CCTA showed non-obstructive calcified and high-risk non-calcified plaque of the left anterior descending artery that was not well seen in coronary angiography. a Non-obstructive calcified plaque noted in the mid left anterior descending artery (red arrow). b Non-calcified high-risk plaque at proximal left anterior descending artery (green arrow)
Conclusion
MINOCA is an important clinical entity that has gained recent recognition over the past decade. There are myriad conditions that can lead to MINOCA. For this reason, in patients with a working diagnosis of MINOCA, it is critical that additional testing be performed to exclude other underlying causes of myocardial injury. In cases where MINOCA remains the most likely diagnosis, novel modalities of cardiovascular imaging may assist to identify the etiologic cause. A systematic approach to the diagnostic evaluation will help to uncover the underlying diagnosis, guide therapy, and provide appropriate feedback to the patient and their families.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–426.
Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circulation Cardiovascular quality and outcomes. 2017;10:e003443.
Safdar B, Spatz E, Dreyer R, Beltrame J, Lichtman J, Reynolds H, et al. Presentation, clinical profile and prognosis of young patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) - results from the variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study. J Am Heart Assoc. 2018;7(13):e009174. https://doi.org/10.1161/JAHA.118.009174.
Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–70.
Barr PR, Harrison W, Smyth D, Flynn C, Lee M, Kerr AJ. Myocardial infarction without obstructive coronary artery disease is not a benign condition (ANZACS-QI 10). Heart, lung & circulation. 2018;27:165–74.
•• Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–908 This paper suggests that the optimal assessment for patients with a MINOCA diagnosis should be aimed at determining the specific cause for each patient, after excluding other causes for elevation of troponin, so that targeted therapies can be used.
Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart JEur Heart J. 2017;38:143–53.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. The executive group on behalf of the joint ESC/ACC/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20):e618–51. https://doi.org/10.1161/CIR.0000000000000617.
Paolisso P, Bergamaschi L, Saturi G, D'Angelo EC, Magnani I, Toniolo S, et al. Secondary prevention medical therapy and outcomes in patients with myocardial infarction with non-obstructive coronary artery disease. Front Pharmacol. 2019;10:1606.
Choo EH, Chang K, Lee KY, Lee D, Kim JG, Ahn Y, et al. Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J Am Heart Assoc. 2019;8:e011990.
Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–9.
Ouldzein H, Elbaz M, Roncalli J, Cagnac R, Carrie D, Puel J, et al. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris). 2012;61:20–6.
Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25.
Opolski MP, Spiewak M, Marczak M, Debski A, Knaapen P, Schumacher SP, et al. Mechanisms of myocardial infarction in patients with nonobstructive coronary artery disease: results from the optical coherence tomography study. JACC Cardiovasc Imaging. 2019 Nov;12(11 Pt 1):2210–21. https://doi.org/10.1016/j.jcmg.2018.08.022.
Jia H, Kubo T, Akasaka T, Yu B. Optical coherence tomography guidance in management of acute coronary syndrome caused by plaque erosion. Circ J. 2018;82:302–8.
Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073–9.
Iqbal SN, Feit F, Mancini GB, Wood D, Patel R, Pena-Sing I, et al. Characteristics of plaque disruption by intravascular ultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am Heart J. 2014;167:715–22.
Jang JH, Kim DH, Yang DH, Woo SI, Kwan J, Park KS, et al. Spontaneous coronary artery dissection by intravascular ultrasound in a patient with myocardial infarction. Korean J Internal Med. 2014;29:106–10.
Buxton A, Goldberg S, Hirshfeld JW, Wilson J, Mann T, Williams DO, et al. Refractory ergonovine-induced coronary vasospasm: importance of intracoronary nitroglycerin. Am J Cardiol. 1980;46:329–34.
Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, et al. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart JEur Heart J. 2018;39(2):91–8.
•• Dastidar AG, Baritussio A, De Garate E, Drobni Z, Biglino G, Singhal P, et al. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging. 2019;12(10):1973–82 This study highlights the potential benefit of using additional tests to further evaluate patients with MINOCA for better cause-based, tailored treatment. CMR is increasingly available and can confirm MI to categorize patients accurately according to MINOCA’s contemporary definition.
Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–53.
Aldrovandi A, Cademartiri F, Arduini D, Lina D, Ugo F, Maffei E, et al. Computed tomography coronary angiography in patients with acute myocardial infarction without significant coronary stenosis. Circulation. 2012;126:3000–7.
Evangelos K, OikonomouHenry W. West,Charalambos Antoniades Cardiac computed tomography assessment of coronary inflammation and other plaque features. Arterioscler Thromb Vasc Biol. 2019;39(11):2207–19.
Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2:153–60.
Ko SM, Hwang SH, Lee HJ. Role of cardiac computed tomography in the diagnosis of left ventricular myocardial diseases. J Cardiovasc Imaging. 2019 Apr;27(2):73–92.
Acknowledgments
We would like to thank Dr. Emmanouil Brilakis for providing the images for Fig. 3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any financial or other relations that could lead to a conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Management of Acute Coronary Syndromes
Electronic supplementary material
Video 1
Cine-angiogram of a 54-year-old patient with known history of non-obstructive CAD, presenting with chest pain and elevated cardiac biomarkers. Angiogram of the RCA demonstrates distal occlusion of the second right posterior-lateral artery, a finding that was not noted on initial review of the angiogram. (AVI 1034 kb)
Rights and permissions
About this article
Cite this article
Talebi, S., Moreno, P., Dominguez, A.C. et al. The Imaging Toolbox to Assess Patients with Suspected Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease (MINOCA). Curr Cardiol Rep 22, 134 (2020). https://doi.org/10.1007/s11886-020-01379-x
Published:
DOI: https://doi.org/10.1007/s11886-020-01379-x