Abstract
Purpose of Review
Cancer therapeutics have seen tremendous growth in the last decade and have been effective in the treatment of several cancer types. However, with advanced therapies like kinase inhibitors and immunotherapies, there have been unintended consequences of cardiotoxicities. While traditional chemotherapy and radiation-induced cardiotoxicity have been well studied, further research is needed to understand the adverse effects of newer regimens.
Recent Findings
Both immune-mediated and non-immune-medicated cytotoxicity have been noted with targeted therapies such as tyrosine kinase inhibitors and immune checkpoint inhibitors. In this manuscript, we describe the pericardial syndromes associated with cancer therapies and propose management strategies. Pericardial effusion and pericarditis are common presentations in cancer patients and often difficult to diagnose. Concomitant myocarditis may also present with pericardial toxicity, especially with immunotherapies. In addition to proper history and physical, additional testing such as cardiovascular imaging and tissue histology need to be obtained as appropriate. Holding the offending oncology drug, and institution of anti-inflammatory medications, and immunosuppressants such as steroids are indicated.
Summary
A high index of suspicion, use of standardized definitions, and comprehensive evaluation are needed for early identification, appropriate treatment, and better outcomes for patients with cancer treatment-associated pericardial disease. Further research is needed to understand the pathophysiology and to evaluate how the management of pericardial conditions in these patients differ from traditional management and also evaluate new therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a revolution in cancer therapeutics in the last decade with the advent of targeted and immune-based therapies. This has led to a significant improvement in the survival of cancer patients. These palpable benefits in outcomes of cancer patients have opened new and different spectrum of management issues, including varying side effects and long-term manifestations of both the traditional and new cancer interventions. Cardiovascular complications of cancers and cancer therapeutics are important components of modern oncologic care leading to a new field of collaborative medicine referred to as cardio-oncology [1]. Cardio-oncology encompasses various intersections between cardiovascular disease and cancer but has blossomed as a field due to myocardial, vascular, and metabolic sequelae of cancer therapies [2]. In this manuscript, we will focus on the pericardial complications of cancer and cancer treatments with an emphasis on the new cancer therapeutics.
Pericardial Disease in Cancer Patients
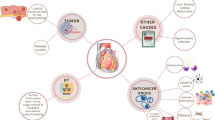
Pericardial disease including pericardial effusion can occur frequently in cancer patients (Fig. 1). Pericardial effusion is most often noted as an incidental finding. Cardiac tamponade may develop if the effusion grows rapidly, if there is prior fibrosis of the pericardium or if there is any bleeding into the pericardial sac, which may be fatal. Acute, recurrent, and chronic pericarditis may also develop. Acute pericarditis is defined as the first episode of pericardial inflammation with presence of at least 2 of 4 criteria. These include pericarditic chest pain; pericardial rub; widespread electrocardiographic changes, including ST elevation and/or PR depression; and new or worsening pericardial effusion. In addition, laboratory and imaging findings (e.g., elevated inflammatory markers and delayed hyperenhancement on magnetic resonance imaging) may support the diagnosis as recommended by the 2015 European Society of Cardiology Pericardial Disease Guidelines [3]. Incessant pericarditis is defined as pericarditis lasting more than 4–6 weeks and up to 3 months while chronic pericarditis is defined as any pericarditis lasting more than 3 months. Any relapse of symptoms after a quiet period of 4–6 weeks is referred to as recurrent pericarditis. Finally, constrictive pericarditis, where the pericardial compliance is reduced due to fibrosis and/or calcification may be the initial presentation or long-term complication. Effusive-constrictive pericarditis, where tense pericardial effusion occurs along with visceral pericardial constriction may also be seen [4]. Importantly, pericardial disease itself may be a sign of occult cancer. A Danish national database study reported that patients with pericarditis had a 50 % higher occurrence of subsequent cancer diagnosis. In this study, pericarditis portended an increased mortality after a cancer diagnosis [5].
Cancer itself can involve the heart and pericardium [6]. Primary malignancies involving the pericardium such as mesothelioma, fibrosarcoma, and angiosarcoma are rare while secondary or metastatic disease is much more common [7,8,9]. The pericardium may be involved by direct extension of the tumor or much more frequently by metastatic disease such as from the lung, breast, lymphoma, and melanoma [10]. The overall incidence of both malignant and benign (fibroma, lipoma) primary pericardial tumors is 0.02 % in the USA based on autopsy data. Reported prevalence is 0.017 % to 0.028 % and approximately 0.25 to 0.33 % of primary heart and pericardial tumors are malignant. Paraneoplastic involvement or general metabolic derangements such as transudative pericardial effusions are also seen. Furthermore, as life expectancy improves, primary pericardial conditions may manifest independent of cancer involvement which may complicate the diagnosis and management. In about two-thirds of patients with documented malignancy, pericardial effusion is caused by non-malignant causes like radiation, other therapies, or infections.
Cancer treatment modalities themselves may affect the heart and pericardium (Fig. 1). The effects of traditional cancer therapies such as radiation and anthracyclines on the heart have been well studied and well described [11, 12]. Hodgkin’s disease patients who have received radiation therapy can present decades later with constrictive pericarditis, valvular heart disease, or premature coronary artery disease [13]. While strategies to minimize radiation-induced cardiotoxicity (such as reducing the dose and area of radiation) have helped, newer targeted therapies, cancer immunotherapies, and combination therapies have led to more heterogeneous cardiac and pericardial complications. In this manuscript, we will concentrate on the presentation, diagnosis, management, and future direction of the newer cancer treatment modalities-associated pericardial disease.
Cancer Treatment-Associated Pericardial Disease
The epidemiology of cancer treatment-associated pericardial disease is limited. Only about 0.2 % of all cardiovascular admissions are due to acute pericarditis and it is estimated that many cases may be subclinical, missed diagnostically, or underreported [14]. This is probably also true of pericardial disease in cancer patients, which may be underreported due to lack of awareness and structured testing before, during, and after treatment.
Radiation Therapy
Radiation therapy is a common cause of pericardial disease with cancer therapies. The mantle radiation that used to be the norm for Hodgkin’s disease may have extensive involvement of the heart, pericardium, and mediastinal structures [15]. Among solid tumors, radiation is most commonly used for breast and lung cancer. Radiation may cause large effusions or hemorrhagic effusions or even tamponade. Though early pericarditis that occurs soon after radiation may be amenable to anti-inflammatory therapy, most of radiation-associated complications are delayed and occur after one to two decades [16]. Effusive-constrictive pericarditis or classic constriction may be seen in about 4–20 % of patients and the incidence seem to be dose-dependent. Absolute cumulative incidence is 2–5 % [3]. These patients can present with fibrotic constrictive pericarditis encasing the heart along with restrictive cardiomyopathy, conduction disease, valvular heart disease, and accelerated coronary artery disease, which increases morbidity and contributes to increased mortality [17, 18]. Survival is poor, even with pericardiectomy, leaving few options to effectively manage these patients [19,20,21].
Radiation dose-reduction strategies, shielding, and more narrow fields of radiation have helped reduce the incidence of complications from 20 to 2.5 %, but the number of patients presenting few decades after Hodgkin’s disease has increased, though this is expected to decrease over time given safer radiation treatment strategies [22].
Traditional Chemotherapy
Traditional chemotherapy medications have been associated with pericardial diseases but much of this appears in case reports or cases series and has not been systematically studied. Isolated pericarditis or pericardial effusions have been reported with high dose cytarabine [23,24,25,26,27,28]. Pericardial and endomyocardial fibrosis has been described with busulfan [29]. Myo-pericarditis has been reported with cyclophosphamide which has also be associated with pericardial effusion and pericarditis [30,31,32,33,34]. Although used to manage malignant pericardial effusion, bleomycin has been associated with life-threatening pericarditis [35, 36]. Acute pericarditis and pericardial effusion are also seen with anthracycline-based chemotherapies such as daunorubicin or doxorubicin [37, 38].
Targeted Therapies
Targeted therapy has also revolutionized cancer therapy. Specifically, the observation that kinase mutations can lead to cancer has led to the development of kinase inhibitors for the treatment of cancer. Interestingly, kinase inhibitors can lead to heterogenous cardiovascular disease including pericardial disease [39, 40]. Pericardial disease has been best described with dasatinib, a small molecule inhibitor with activity against multiple kinases, including which is aberrantly activated in certain leukemias, such as chronic myelogenous leukemia (CML). Dasatinib has been also associated with both pleural and pericardial effusions and cardiac tamponade has been reported [41, 42]. Interestingly, other kinases in this class such as imatinib can also cause serosal inflammation although minimal cardiac effects have been reported with imatinib [43,44,45].
Cancer Immunotherapy
Immune system modulation has been used for decades to treat various conditions including cancer. Nevertheless, in the last 5 years, several new treatments have led to a revolution in cancer therapy. Depending on the form of immunotherapy, pericardial diseases can be observed.
- a.
IL-2 Therapy
Cytokines are small glycoproteins which bind to surface receptors and influence the development, and function of immune cells. IL-2 is a T-lymphocyte growth factor and in the 1970s was developed as a drug to treat certain cancers including renal cell carcinoma and melanoma. IL-2 has a short half-life in minutes due to rapid metabolism and elimination by the kidney; for this reason, it was previously used in high doses. A number of vascular issues including capillary leak syndrome (perhaps due to lymphoid infiltration into tissues) were observed after IL-2 treatment [46, 47]. Myocarditis and pericarditis are observed in 2.5–5 % of patients and can present as asymptomatic temporary left ventricular dysfunction.
- b.
Interferon Alpha
Interferon alpha was shown to have cytogenic activity and has been previously used for the treatment of melanoma and chronic myelogenous leukemia. However, pericarditis and pericardial effusion can occur [48,49,50]. With the advent of targeted and immune-based therapies, interferon is rarely used for cancer treatment in 2019.
- c.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICI) are antibodies against “brakes” or checkpoints in immune cells—specifically T-lymphocytes. ICI include monoclonal antibodies against CTLA-4 (e.g., ipilimumab), PD-1 (nivolumab, pembrolizumab, cemiplimab), and PD-L1 (atezolizumab, durvalumab, avelumab). By activating the immune system, ICI have revolutionized treatment for several cancer types [51]. In 2018, the Nobel Prize in medicine was awarded to Drs. James P. Allison and Tasuku Honjo for discovering these receptors and their potent role in cancer regulation. Initially approved in metastatic melanoma, renal cell cancer, and non-small cell lung cancer, they are being used for many cancer types. In 2017, there were over 3000 active clinical trials with more than 600,000 patients involving ICI [52]. Increasingly, ICI are being used in combination for more effective therapies. In addition, for many cancer types, ICI are being combined with existing traditional or targeted therapies. Occasionally, ICI can cause immune-related toxicities, which include the cardiovascular systems. In 2016, Johnson and colleagues reported two cases of fulminant myocarditis following treatment with ipilimumab and nivolumab [53]. Since then, ICI have been associated with a number of other cases of myocarditis, but also pericardial disease, including pericarditis, vasculitis, or arrhythmia [54•]. Pharmacovigilance databases suggest that close to 1 % of patients with combination ICI treatment can have cardiovascular toxicities [55, 56]. ICI-associated cardiovascular toxicities, especially myocarditis, can portend a poor prognosis with 50 % mortality [55].
Pericardial conditions were not recognized in the early clinical trials with ICI but are increasingly reported and include pericarditis, pericardial effusion, and tamponade, and can be associated with myocarditis [57,58,59,60]. Based on the large World Health Organization (WHO) pharmacovigilance database (Vigibase), ICI-associated pericardial disease can present after a median time of 30 days (IQR 8.5–90), often 1 or 2 ICI administrations following initiation of therapy [54, 61••]. The fatality rate of ICI-associated pericarditis was 21.1 %. More pericarditis was reported in men; however, this may be due to more men receiving ICI in both clinical trial and real-world population settings. Further characterization of ICI-associated pericardial diseases is needed [54•]. Interestingly, ICI, when used in lung cancer, appears to carry a higher risk of pericarditis [54•].
Pathophysiology of Pericardial Involvement
Cancer therapies can cause pericardial disease by a number of different mechanisms. Pericardial reaction to injury leads to fibrinous exudate, fluid, or cells and varies by the type of injury [62]. Over time, fibrosis may occur though the extent of which may depend on the etiology and lead to constriction. Radiation exposure leads to T cell-mediated injury [15]. With acute pericarditis, neutrophilic or macrophage infiltration may be observed. In constrictive pericarditis, exudates, fibroblast proliferation, and neovascularization have been observed [63]. In patients with pericarditis due to ICI, fibrinous exudates with mostly lymphocytes, plasma cell, and macrophage infiltration have been observed. Recently, Altan et al. reported 3 patients with ICI-associated pericarditis with histopathological evidence of lymphocytic and macrophage infiltration. Further characterization of immune infiltrates revealed increased CD68 expression in ICI-pericarditis consistent with macrophages. In addition, CD 68+ cells had increased PDL1 expression [64]. These observations are consistent with histological examination of ICI-associated myocarditis [53, 65]. Further molecular characterization of ICI-associated pericarditis is warranted.
Special Conditions
Worsening of Pre-existing Pericardial Disease
While cancer therapies may cause de novo pericardial diseases, they may also worsen pre-existing conditions. For example, radiation or medications may cause recurrences of pericarditis or worsen pre-existing pericardial effusion. It is important to obtain proper baseline cardiac status before initiation of treatment to assess if any change is attributable to the cancer therapy or due to other comorbidities.
Combination Therapies
Multi-modal treatment is common in cancer therapeutics and the different combinations of medications, radiation, and chemotherapy may have additive or synergistic deleterious effect on the pericardium or heart. Further combination ICI therapies are being used more and more often. In addition to cancer therapeutics, immune reconstitution and opportunistic infections may lead to worsening or new problems.
Clinical Presentation and Diagnosis of Pericardial Disease Associated with Cancer Treatment
History
A careful cardiac history of baseline cardiac and other pre-existing conditions must be obtained in every patient undergoing cancer therapy. This is an important consideration for some medications given cyclically such as ICI therapy, since the condition may not present until after several doses have been administered.
Physical Exam
Patients should undergo a thorough physical examination and be evaluated for vital signs, pericardial rub, or signs of tamponade or constriction including volume status, jugular venous distension, Kussmaul’s sign, pulsus paradoxus, pericardial rub, pericardial knock, and pedal edema.
Laboratory
Inflammatory biomarkers such as sedimentation rate and C-reactive protein have been studied in the diagnosis and management of pericarditis. Though nonspecific, elevated inflammatory markers are supportive of the diagnosis of pericarditis and may help manage the condition. Serial monitoring of inflammatory markers gives important information about when to taper or discontinue anti-inflammatory therapy.
Elevated troponin may be seen in patients who have myo-pericarditis (predominant pericardial involvement) or peri-myocarditis (predominant myocardial involvement) in addition to patients with acute coronary syndromes. Patients with elevated troponin have shown to have poor prognosis [56].
Electrocardiogram (EKG)
An electrocardiogram is a first-line investigation in the diagnosis of suspected pericarditis. EKG changes such as diffuse ST elevation, PR depressions may be evolutionary and may be missed.
Imaging
The American Society of Echocardiography and the European Association of Cardiovascular Imaging expert consensus document on the multimodality imaging of patients during and after cancer therapy, highlights the importance of multimodality imaging of patients undergoing cancer therapy. Multimodality imaging will help clarify patients who have constrictive pericarditis or restrictive cardiomyopathy or a combination of both such as in radiation-induced constriction [66].
Echocardiogram
The American Society of Echocardiography expert consensus statement recommends echocardiography as the first imaging study to evaluate pericardial conditions [67]. The presence and quantity of pericardial effusion, thickness of the pericardium, diastolic collapse of right-sided chambers in tamponade and signs of constriction including diastolic septal bounce, respiratory variation across mitral and tricuspid valves, respirophasic shift, and tethering may be identified, which will help in diagnosing and managing these patients. An echocardiogram is non-invasive with minimal risks and can be repeated to follow the course of pericardial disease.
CT Scan
Most often, patients are incidentally diagnosed with a pericardial condition after a CT scan is obtained as part of evaluation or treatment for their malignancy. A CT scan may help evaluate pericardial effusion, pericardial calcification, and thickening.
MRI
Cardiac MRI with contrast is more helpful and is indicated in specific situations. Pericardial edema may be identified by T2W STIR (Short T1 Inversion Recovery) imaging and late gadolinium enhancement of the pericardium indicating active inflammation (Fig. 2). MRI is also useful to identify constrictive physiology, including tethering, conical or tubular ventricular deformity, abnormal septal motion, and respirophasic septal shift.
Histopathology
Patients who undergo pericardiocentesis or pericardial window should have fluid/tissue tested for chemistry and histology. In the event of patient death, an autopsy could be considered for further evaluation to confirm the diagnosis and etiology.
General Management of Pericardial Conditions
Diagnosis and management of pericardial diseases due to cancer therapy are challenging for a number of reasons. First, the presentation may be atypical; signs and symptoms may be attributed to other etiologies. As a result, prompt diagnosis may not occur, and so an increased awareness of this condition is critical. Second, identifying the etiology of pericardial conditions and determining if the pathology is due to cancer therapy, cancer itself or due to other reasons can be difficult. A careful examination of the patient’s baseline cardiac testing, as well as combination of an EKG, cardiac biomarkers, cardiac imaging, and a proper history and physical exam is necessary to delineate the chronology of events that will help better classify the nature of the pericardial disease. Finally, although there has been an interest in newer therapies for pericarditis, there is a paucity of data as how to treat the patients with pericardial diseases.
Dose-reduction strategies are useful in preventing complications for traditional chemotherapy and radiation therapy [17]. In some cases, cancer therapy may be held. The decision to stop the offending drug depends on the overall cancer prognosis and cancer response to the individual therapy. If ICI-associated myocarditis is suspected, complete drug discontinuation may be needed due to potentially fulminant course.
Pericardial Effusion
Mild and moderate pericardial effusion may be conservatively monitored with regular echocardiograms. In cases where there is a need to determine etiology (e.g., malignant effusion or treatment related effusion), then a diagnostic pericardiocentesis is recommended. Depending on the size of effusion, this can be done using echo guidance at the bedside, using fluoroscopy guidance in the cath lab or with CT guidance in interventional radiology. Knowing the techniques and expertise available at your institution is paramount. Large pericardial effusions typically benefit from therapeutic pericardiocentesis or pericardial window placement. Malignant effusions may be treated with intrapericardial instillation of agents such as bleomycin [68,69,70]. Recurrent effusions are managed by pericardial window placement. Pericardial fluid evaluation and pericardial window placements allow tissue diagnosis and rule out any bleeding into the pericardial sac. Pericardial effusion related to pericarditis is treated by treating the pericardial inflammation as described below.
Cardiac Tamponade
Cardiac tamponade is a medical emergency and is suspected clinically. Diagnosis is usually confirmed by physical examination and focused echocardiogram. Immediate intervention is needed to prevent hemodynamic collapse and possible death.
Acute Pericarditis
Acute pericarditis is treated with anti-inflammatory medications such as high-dose aspirin or non-steroidal anti-inflammatory drugs (NSAID) with exercise restriction. Colchicine may be added to prevent recurrences and is considered first-line therapy in acute or recurrent pericarditis [3]. Steroids are usually avoided or used as a second-line at low to moderate doses for cases not improving with NSAIDs and colchicine but may lead to steroid dependence. However, in patients with ICI-related pericarditis, in addition to stopping the drugs, high-dose steroids may be needed especially if concomitant myocarditis and other ICI-associated toxicities are suspected. In patients with cancer, some of these therapies may have to be altered. For example, aspirin may increase the risk of bleeding in cancer patients with thrombocytopenia.
Recurrent Pericarditis
Chronic or recurrent pericarditis is treated with a combination of anti-inflammatory medications’ aspirin or NSAIDs with colchicine and exercise restriction as a first-line. Resistant cases are treated with low-to-moderate-dose steroids. In idiopathic recurrent pericarditis, high-dose steroids have been shown to increase side effects and chance of recurrences; however, the pathophysiology of cancer treatment-related pericarditis may be different as discussed above in cancer immunotherapy and may need treatment with high doses [71]. Steroid-dependent or difficult to treat patients may need treatment with immunosuppressive drugs such as azathioprine or interleukin-1 blocker such as anakinra [72]. While these drugs have not been specifically studied in the treatment of cancer treatment-related pericarditis, they are promising supportive medications.
Constrictive Pericarditis
Constrictive pericarditis is most commonly seen in patients who received radiation therapy. Transient constriction or rarely chronic constriction may develop in patients treated with other modalities. Initial treatment options include management of heart failure symptoms with diuretics, anti-inflammatory therapy in the presence of pericardial inflammation or elevated inflammatory markers, and finally pericardiectomy. The prognosis of pericardiectomy itself varies based on the etiology with radiation-induced constriction having the worst prognosis [20, 73, 74]. These patients have coexisting valvular pathology, cardiomyopathy, and accelerated coronary disease leading to worse outcomes.
Management of Cardiac Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors
There are no concrete guidelines recommended for management of cardiac immune adverse events. American Society of Clinical Oncology clinical practice guideline offers guidance on management of immune-related adverse events, though there is paucity of evidence-based therapy. Much of the recommendations extrapolate to similar non-cancer-related diseases and following ACC/AHA guidelines under the guidance of a cardiologist [75]. Though cardiac immune-related adverse events are rare, they may be fatal. Therefore, it is recommended that diagnosis be made promptly and definitively, and immunotherapy be held. High-dose steroids are recommended for myocarditis; there are case reports of patients with pericarditis treated with high-dose steroids. Anti-inflammatory medications including aspirin or non-steroidal and colchicine should be considered in patients with ICI-associated pericarditis [61••]. Pericardiocentesis and pericardial windows may be needed if patient presents with tamponade.
Conclusions and Future Direction
The recent advances in cancer therapeutics have resulted in advances for cancer care and improved survival of oncology patients. Newer regimens, including targeted therapies and immunotherapies such as immune checkpoint inhibitors, have dramatically improved the life expectancy for certain cancers. Multiple studies are underway for combination treatments for even better treatment efficacy, leading to an even greater number of patients being treated. Unintended consequences of cardiotoxicity have resulted in the new field of cardio-oncology. Immune checkpoint inhibitors are especially intriguing because of adverse immune reactions including pericardial effusions or pericarditis. A high degree of clinical suspicion is needed to treat patients. Because of the novelty of these drug effects, research is needed to better understand the underlying pathophysiology and to standardize cardiac evaluation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457–67. https://doi.org/10.1056/NEJMra1100265.
Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol. 2015;66(10):1160–78. https://doi.org/10.1016/j.jacc.2015.07.025.
Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–64. https://doi.org/10.1093/eurheartj/ehv318.
Sagrista-Sauleda J, Angel J, Sanchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. N Engl J Med. 2004;350(5):469–75. https://doi.org/10.1056/NEJMoa035630.
Søgaard KK, Farkas DK, Ehrenstein V, Bhaskaran K, Bøtker HE, Sørensen HT. Pericarditis as a marker of occult cancer and a prognostic factor for cancer mortality. Circulation. 2017;136(11):996–1006. https://doi.org/10.1161/circulationaha.116.024041.
Maleszewski JJ, Anavekar NS, Moynihan TJ, Klarich KW. Pathology, imaging, and treatment of cardiac tumours. Nat Rev Cardiol. 2017;14(9):536–49. https://doi.org/10.1038/nrcardio.2017.47.
Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol. 2002;84(1):69–75.
Burazor I, Aviel-Ronen S, Imazio M, Markel G, Grossman Y, Yosepovich A, et al. Primary malignancies of the heart and pericardium. Clin Cardiol. 2014. https://doi.org/10.1002/clc.22295.
Restrepo CS, Vargas D, Ocazionez D, Martinez-Jimenez S, Betancourt Cuellar SL, Gutierrez FR. Primary pericardial tumors. Radiographics. 2013;33(6):1613–30. https://doi.org/10.1148/rg.336135512.
Klatt EC, Heitz DR. Cardiac metastases. Cancer. 1990;65(6):1456–9.
Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35(10):612–23. https://doi.org/10.1093/eurheartj/eht114.
Raber I, Asnani A. Cardioprotection in cancer therapy: novel insights with anthracyclines. Cardiovasc Res. 2019;115(5):915–21. https://doi.org/10.1093/cvr/cvz023.
Galper SL, Yu JB, Mauch PM, Strasser JF, Silver B, Lacasce A, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412–8. https://doi.org/10.1182/blood-2010-06-291328.
Kyto V, Sipila J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130(18):1601–6. https://doi.org/10.1161/circulationaha.114.010376.
Spetz J, Moslehi J, Sarosiek K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr Treat Options Cardiovasc Med. 2018;20(4):31. https://doi.org/10.1007/s11936-018-0627-x.
Lee MS, Finch W, Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112(10):1688–96. https://doi.org/10.1016/j.amjcard.2013.07.031;10.1016/j.amjcard.2013.07.031.
Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-associated cardiac disease: A Practical Approach to Diagnosis and Management. JACC Cardiovascular imaging. 2018;11(8):1132–49. https://doi.org/10.1016/j.jcmg.2018.04.028.
Szpakowski N, Desai MY. Radiation-associated pericardial disease. Curr Cardiol Rep. 2019;21(9):97. https://doi.org/10.1007/s11886-019-1192-y.
Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43(8):1445–52. https://doi.org/10.1016/j.jacc.2003.11.048.
Murashita T, Schaff HV, Daly RC, Oh JK, Dearani JA, Stulak JM, et al. Experience with pericardiectomy for constrictive pericarditis over eight decades. Ann Thorac Surg. 2017;104(3):742–50. https://doi.org/10.1016/j.athoracsur.2017.05.063.
Barbetakis N, Xenikakis T, Paliouras D, Asteriou C, Samanidis G, Kleontas A, et al. Pericardiectomy for radiation-induced constrictive pericarditis. Hell J Cardiol. 2010;51(3):214–8.
Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–40. https://doi.org/10.1093/ehjci/jet123.
Hermans C, Straetmans N, Michaux JL, Ferrant A. Pericarditis induced by high-dose cytosine arabinoside chemotherapy. Ann Hematol. 1997;75(1–2):55–7.
Vaickus L, Letendre L. Pericarditis induced by high-dose cytarabine therapy. Arch Intern Med. 1984;144(9):1868–9.
Gahler A, Hitz F, Hess U, Cerny T. Acute pericarditis and pleural effusion complicating cytarabine chemotherapy. Onkologie. 2003;26(4):348–50. https://doi.org/10.1159/000072094.
Reykdal S, Sham R, Kouides P. Cytarabine-induced pericarditis: a case report and review of the literature of the cardio-pulmonary complications of cytarabine therapy. Leuk Res. 1995;19(2):141–4.
Yamada T, Tsurumi H, Hara T, Sawada M, Oyama M, Moriwaki H. Cytarabine-induced pericarditis. Rinsho Ketsueki. 1998;39(11):1115–20.
Yang X, Liu W, Lyons A, Song Z, Zhai S, Hu K. Pericarditis associated with cytarabine therapy for acute myelocytic leukemia: a case report. Eur J Clin Pharmacol. 2018;74(2):181–2. https://doi.org/10.1007/s00228-017-2355-7.
Terpstra W, de Maat CE. Pericardial fibrosis following busulfan treatment. Neth J Med. 1989;35(5–6):249–52.
Bock J, Doenitz A, Andreesen R, Reichle A, Hennemann B. Pericarditis after high-dose chemotherapy: more frequent than expected? Onkologie. 2006;29(7):321–4. https://doi.org/10.1159/000093528.
Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141(6):758–63.
Wadia S. Acute cyclophosphamide hemorrhagic myopericarditis: dilemma case report, literature review and proposed diagnostic criteria. J Clin Diagn Res. 2015;9(11):OE01–OE3. https://doi.org/10.7860/JCDR/2015/15054.6758.
Yamamoto R, Kanda Y, Matsuyama T, Oshima K, Nannya Y, Suguro M, et al. Myopericarditis caused by cyclophosphamide used to mobilize peripheral blood stem cells in a myeloma patient with renal failure. Bone Marrow Transplant. 2000;26(6):685–8. https://doi.org/10.1038/sj.bmt.1702592.
Appelbaum F, Strauchen JA, Graw RG Jr, Savage DD, Kent KM, Ferrans VJ, et al. Acute lethal carditis caused by high-dose combination chemotherapy: a unique clinical and pathological entity. Lancet. 1976;1(7950):58–62. https://doi.org/10.1016/s0140-6736(76)90151-3.
Durkin WJ, Pugh RP, Solomon J, Rosen P, Pajak TF, Bateman JR. Treatment of advanced lymphomas with bleomycin (NSC-125066). Oncology. 1976;33(3):140–5. https://doi.org/10.1159/000225128.
Ben Yosef R, Gez E, Catane R. Acute pericarditis following bleomycin: a case report and literature analysis. J Chemother. 1990;2(1):70–1.
Tohda S, Kobayashi H, Suzuki T, Koyama T, Kamiyama T, Nakamura Y, et al. Acute pericarditis caused by daunorubicin in acute myelocytic leukemia. Rinsho Ketsueki. 1988;29(6):874–8.
Dazzi H, Kaufmann K, Follath F. Anthracycline-induced acute cardiotoxicity in adults treated for leukaemia: analysis of the clinico-pathological aspects of documented acute anthracycline-induced cardiotoxicity in patients treated for acute leukaemia at the University Hospital of Zurich, Switzerland, between 1990 and 1996. Ann Oncol. 2001;12(7):963–6. https://doi.org/10.1023/a:1011196910325.
Bellinger AM, Arteaga CL, Force T, Humphreys BD, Demetri GD, Druker BJ, et al. Cardio-oncology: how new targeted cancer therapies and precision medicine can inform cardiovascular discovery. Circulation. 2015;132(23):2248–58. https://doi.org/10.1161/CIRCULATIONAHA.115.010484.
Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33(35):4210–8. https://doi.org/10.1200/JCO.2015.62.4718.
Breccia M, Alimena G. Pleural/pericardic effusions during dasatinib treatment: incidence, management and risk factors associated to their development. Expert Opin Drug Saf. 2010;9(5):713–21. https://doi.org/10.1517/14740331003742935.
Wattal S, Rao MS, Chandra GN, Razak UK, Shetty KR. Dasatinib induced cardiac tamponade-a rare association. J Clin Diagn Res. 2017;11(2):Fd03–fd4. https://doi.org/10.7860/jcdr/2017/24633.9418.
Breccia M, D'Elia GM, D'Andrea M, Latagliata R, Alimena G. Pleural-pericardic effusion as uncommon complication in CML patients treated with Imatinib. Eur J Haematol. 2005;74(1):89–90. https://doi.org/10.1111/j.1600-0609.2004.00347.x.
Barton JC, Jones SC, Lamberth WC, Reymann MT, Scott VC. Cardiac tamponade associated with imatinib mesylate therapy of chronic myelogenous leukemia. Am J Hematol. 2002;71(2):139–40. https://doi.org/10.1002/ajh.10186.
Miura S, Murase K, Sakurada A, Takada K, Iyama S, Sato T, et al. A case of chronic myelogenous leukemia that developed fibrous pericarditis owing to nilotinib use. Gan To Kagaku Ryoho. 2017;44(6):529–31.
Jeong GH, Lee KH, Lee IR, Oh JH, Kim DW, Shin JW, et al. Incidence of capillary leak syndrome as an adverse effect of drugs in cancer patients: a systematic review and meta-analysis. J Clin Med. 2019;8(2). https://doi.org/10.3390/jcm8020143.
Zhang J, Yu ZX, Hilbert SL, Yamaguchi M, Chadwick DP, Herman EH, et al. Cardiotoxicity of human recombinant interleukin-2 in rats. A morphological study. Circulation. 1993;87(4):1340–53. https://doi.org/10.1161/01.cir.87.4.1340.
Atkins MB, Gollob JA, Sosman JA, McDermott DF, Tutin L, Sorokin P, et al. A phase II pilot trial of concurrent biochemotherapy with cisplatin, vinblastine, temozolomide, interleukin 2, and IFN-alpha 2B in patients with metastatic melanoma. Clin Cancer Res. 2002;8(10):3075–81.
Fava S, Luoni M, Stioui S. Pericarditis during interferon-alpha therapy in chronic myelogenous leukemia. Haematologica. 1996;81(5):484.
Benjamini O, Kimhi O, Lishner M. Severe pleuropericarditis and cardiomyopathy induced by high dose interferon alpha-2b. Isr Med Assoc J. 2007;9(6):486–7.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5. https://doi.org/10.1126/science.aar4060.
Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29(1):84–91. https://doi.org/10.1093/annonc/mdx755.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55. https://doi.org/10.1056/NEJMoa1609214.
• Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89. https://doi.org/10.1016/S1470-2045(18)30608-9 This observational study gives an overview of cardiovascular adverse events reported with immune checkpoint inhibitors.
Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. https://doi.org/10.1016/S0140-6736(18)30533-6.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64. https://doi.org/10.1016/j.jacc.2018.02.037.
De Almeida DVP, Gomes JR, Haddad FJ, Buzaid AC. Immune-mediated pericarditis with pericardial tamponade during nivolumab therapy. J Immunother. 2018. https://doi.org/10.1097/CJI.0000000000000217.
Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015;2015:794842. https://doi.org/10.1155/2015/794842.
Nesfeder J, Elsensohn AN, Thind M, Lennon J, Domsky S. Pericardial effusion with tamponade physiology induced by nivolumab. Int J Cardiol. 2016;222:613–4. https://doi.org/10.1016/j.ijcard.2016.08.023.
Kushnir I, Wolf I. Nivolumab-induced pericardial tamponade: a case report and discussion. Cardiology. 2017;136(1):49–51. https://doi.org/10.1159/000447053.
•• Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019. https://doi.org/10.1093/cvr/cvz026 This review gives an overview, discusses the pathophysiology, and significance of cardiovascular adverse effects of immune checkpoint inhibitors.
Roberts WC. Pericardial heart disease: its morphologic features and its causes. Proc (Bayl Univ Med Cent). 2005;18(1):38–55. https://doi.org/10.1080/08998280.2005.11928030.
Zurick AO, Bolen MA, Kwon DH, Tan CD, Popovic ZB, Rajeswaran J, et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. JACC Cardiovascular imaging. 2011;4(11):1180–91. https://doi.org/10.1016/j.jcmg.2011.08.011.
Altan M, Toki MI, Gettinger SN, Carvajal-Hausdorf DE, Zugazagoitia J, Sinard JH, et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14(6):1102–8. https://doi.org/10.1016/j.jtho.2019.02.026.
Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5(1):91. https://doi.org/10.1186/s40425-017-0296-4.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. https://doi.org/10.1016/j.echo.2014.07.012.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26(9):965–1012 e15. https://doi.org/10.1016/j.echo.2013.06.023.
Numico G, Cristofano A, Occelli M, Sicuro M, Mozzicafreddo A, Fea E, et al. Prolonged drainage and intrapericardial bleomycin administration for cardiac tamponade secondary to cancer-related pericardial effusion. Medicine (Baltimore). 2016;95(15):e3273. https://doi.org/10.1097/md.0000000000003273.
Kunitoh H, Tamura T, Shibata T, Imai M, Nishiwaki Y, Nishio M, et al. A randomised trial of intrapericardial bleomycin for malignant pericardial effusion with lung cancer (JCOG9811). Br J Cancer. 2009;100(3):464–9. https://doi.org/10.1038/sj.bjc.6604866.
Maruyama R, Yokoyama H, Seto T, Nagashima S, Kashiwabara K, Araki J, et al. Catheter drainage followed by the instillation of bleomycin to manage malignant pericardial effusion in non-small cell lung cancer: a multi-institutional phase II trial. J Thorac Oncol. 2007;2(1):65–8. https://doi.org/10.1097/JTO.0b013e31802c8260.
Shaheen S, Mirshahidi H, Nagaraj G, Hsueh CT. Conservative management of nivolumab-induced pericardial effusion: a case report and review of literature. Exp Hematol Oncol. 2018;7:11. https://doi.org/10.1186/s40164-018-0104-y.
Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316(18):1906–12. https://doi.org/10.1001/jama.2016.15826.
Gillaspie EA, Stulak JM, Daly RC, Greason KL, Joyce LD, Oh J, et al. A 20-year experience with isolated pericardiectomy: analysis of indications and outcomes. J Thorac Cardiovasc Surg. 2016;152(2):448–58. https://doi.org/10.1016/j.jtcvs.2016.03.098.
Bashi VV, John S, Ravikumar E, Jairaj PS, Shyamsunder K, Krishnaswami S. Early and late results of pericardiectomy in 118 cases of constrictive pericarditis. Thorax. 1988;43(8):637–41.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68. https://doi.org/10.1200/JCO.2017.77.6385.
Funding
Dr. Moslehi is supported by NIH grants (R56 HL141166 and R01 HL141166).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Chandra K. Ala declares no conflict of interest.
Allan L. Klein reports he is on the steering committee for Sobi and Pfizer, and he is also supported by a research grant from Kiniksa.
Javid J. Moslehi reports he has been a consultant for Pfzier, Novartis, Bristol-Myers Squibb, Audentes, AstraZeneca, and Nektar.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pericardial Disease
Rights and permissions
About this article
Cite this article
Ala, C.K., Klein, A.L. & Moslehi, J.J. Cancer Treatment-Associated Pericardial Disease: Epidemiology, Clinical Presentation, Diagnosis, and Management. Curr Cardiol Rep 21, 156 (2019). https://doi.org/10.1007/s11886-019-1225-6
Published:
DOI: https://doi.org/10.1007/s11886-019-1225-6