Abstract
Purpose of Review
This review aims to provide an updated overview and a clinical perspective on novel transcatheter tricuspid valve interventions (TTVI), highlighting potential challenges and future directions.
Recent Findings
Severe tricuspid regurgitation (TR) is a predictor of mortality. However, a sizeable number of patients remain untreated until the end–stage when cardiac surgery presents a prohibitive risk. The emergent need in finding a treatment for patients with TR, deemed for surgery options, has encouraged the development of TTVI. These procedures mimic classical surgery techniques and are mainly divided in four categories: annuloplasty and coaptation devices, edge-to-edge techniques and transcatheter tricuspid valve replacement. Early studies showed promising results, but long-term follow-up data are not available.
Summary
For patients with severe TR and high surgical risk, several percutaneous options are available. However, these therapies are in a growing phase and bigger studies and long term follow-up are needed to prove their efficacy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past two decades, the interest in tricuspid valve treatment has increased [1, 2], nevertheless only 5% of the population with severe tricuspid regurgitation receives a surgical treatment [3]. Patients with untreated TR have a poor prognosis [4, 5], and most of them receive lifetime medical therapy until intractable right heart failure and end-organ dysfunction appears. Regurgitation remains the principal pathology of the tricuspid valve and it is more often secondary rather than caused by a primary valve lesion [6]. Annular dilatation and increased tricuspid leaflet tethering in relation to right ventricular pressure and/or volume overload cause secondary TR. Left-sided heart disease, atrial fibrillation, or pulmonary hypertension are frequently involved in the pathogenesis of tricuspid regurgitation [7••]. All this evidence changed the management of tricuspid regurgitation to a more aggressive surgical approach, and the most recent guidelines recommend surgical repair of concomitant replacement during left valve surgery also in patients with tricuspid annular dilatation or recent signs of right heart failure with non-severe TR [8].
Despite the improvement in operative techniques, the in-hospital mortality in patients with combined surgery or isolated tricuspid regurgitation who underwent surgical replacement (12.6% respectively 7.1%) or repair (10.8% respectively 8.1%) is still high [9]. Moreover previous tricuspid valve surgery recurrence of moderate or severe TR may be as high as 60% at 5 years [10], and reoperation is necessary in approximately 20% of patients within 10 years after tricuspid valve surgery [11]. While redo surgery is the treatment of choice for a degenerated bioprothesis or deterioration of a ring annuloplasty, it may be associated with a very high mortality rate, reaching 35% at 30 days [12], particularly in patients with comorbidities.
Patients with tricuspid regurgitation, and high risk for surgery, were until recently predestined to conservative treatment. The promising results in aortic and mitral valve percutaneous interventions in high-risk patients have encouraged the development of percutaneous tricuspid interventions.
Tricuspid Valve Anatomy
The TV is nearly vertical and oriented at approximately 45° to the sagittal plane, so that the margins of the valve are antero-superior, inferior and septal. Classically, three leaflets were described. However, the advancement in the field of percutaneous tricuspid procedures have generated renewed interest in the anatomy of the tricuspid valve complex. Several studies showed an important variability of the tricuspid leaflets number [13, 14]. Lama et al. showed that only 17% of patients presented three leaflets tricuspid valve, and most of them presented five leaflets [15]. This detail is of special interest during the edge-to-edge techniques. Moreover, the anterior leaflet is the largest, with a semi-circular shape and is almost always anchored to a single papillary muscle, which is attached to the free wall or the anterior wall of the right ventricle (RV). In functional tricuspid regurgitation, this zone is more prone to annular dilatation.
The tricuspid annulus (TA) is a dynamic structure, which changes its shape and size during the cardiac cycle (approximately 20% reduction in annular circumference with atrial systole) [16]. Changes, secondary to alterations in RV size and function, can determine enlargement of the antero-posterior diameter of the TA, losing the saddle shape and becoming more circular. Malcoaptation occurs primarily between the antero-posterior and postero-septal commissures, and this fact has therapeutic implications for TV repair, especially for leaflet-based approaches [7••].
Mechanism of Tricuspid Regurgitation
The pathophysiology of secondary or functional TR can be divided into three phases. In the first phase, left-side heart disease, pulmonary hypertension and atrial fibrillation may determine impairment of RV, with progressive dilatation, which can lead to dilatation of the tricuspid annulus. The coaptation is not affected (“body-to-body”) and the TR is not significant. In the second phase, the progressive dilation of the RV and TA can result in a poor leaflet coaptation (“edge-to-edge”), leading to progressive, significant TR. Finally in the third phase, continuous distortion of RV geometry, especially on the anterior wall associated with tethering of the leaflets, will get worse with the degree of TR. The anterior and posterior leaflets, will lose contact (“non coaptation”), determining dilation of the TA along its antero-posterior plane. The septal leaflet, anchored to the fibrous skeleton, is only partially involved in the dilation of the TA [17].
Imaging Evaluation
Imaging plays a key role in both diagnostic and procedure guiding.
Echocardiography is the main tool for TR evaluation. It assesses the “Carpentier’s triade”, i.e. valve anatomy, lesions and dysfunction and the severity of the regurgitation using an integrative approach combining semi-quantitative and quantitative measurements. A new scale for severity, adding “massive and torrential” degrees, has recently been proposed but the additional prognostic value of these new grades remains to be proven [18•].
The evaluation of RV function using cardiac magnetic resonance (CMR) is very important in the decision making process. CMR imaging represents the gold standard for quantifying right ventricular volumes and function [19].
Computed tomographic (CT) imaging has become one of the most important imaging modalities during pre-procedural planning for TTVI, because it provides valuable anatomic information of the TV apparatus, which often is difficult to assess by echocardiography owing to its complex geometry and anterior position in the chest. The CT gives information regarding tricuspid apparatus morphology, landing zone geometry, annular dimension, presence of calcification, anatomic relationships with the surrounding structures, evaluation of the risk of right ventricular outflow tract obstruction, and it can also predict the best fluoroscopic projection [20].
Right heart catheterisation should be performed when needed to evaluate the pulmonary vascular pressures/resistances.
Finally, imaging during TTVI remains challenging and multimodality imaging should be encouraged.
Target Patients for Percutaneous Interventions
Tricuspid regurgitation is a silent disease and the symptoms appear in later stage, which leads to a delayed referral. Usually, those patients present advanced age, previous cardiac surgery and often they have right ventricular dysfunction. Left untreated, those patients with severe tricuspid regurgitation, even isolated, have a very poor prognosis [4]. On the basis of all this evidence, there is an emergent need to find an adequate treatment and a proper moment to treat patients with tricuspid regurgitation, deemed for surgery options. Those treatments should take into consideration not only the tricuspid valve, but also the entire tricuspid apparatus.
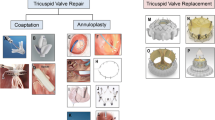
More than 18 devices have been developed or are under development for the pathologic tricuspid apparatus treatment (Fig. 1).
Transcatheter tricuspid devices. Tricuspid annuloplasty devices: “suture” based: Tricinch, Trialign, MIA. “rings” based: Cardioband, TRAIPTA, Millipede, Da Vingi. Coaptation devices: Forma. Edge-to edge devices: Mitraclip, Pascal, Pasta. Replacement devices: Orthotopic valves: Sapien*, Melody, NaviGate, Trisol, Lux-valve. Heterotopic valves: TRICvalve, Sapiens**, Tricento. Permissions for Figure 1 are from the following sources: For the TRICValve: (From: Lauten A, et al. Circ Cardiovasc Interv 2018 2018;11:e006061, with permission from Wolters Kluwer Health, Inc.) [21]. For the NaviGate valve: (From: Navia JL, et al. JACC: Basic to Translational Science 2018;3:67-79, with permission from Elsevier) [22]. For the TRAIPTA device: (From: Rogers T, et al. Transatrial JACC Cardiovascular Interventions 2015;8:483-491, with permission from Elseiver) [23]. For the Pascal device: (From: Fam NP, et al. JACC-Cardiovasc Inte. 2018;11:407-408 with permission from Elseiver) [24]. For the Triciento: (Reprinted from EuroIntervention: Toggweiler S, et al. Eurointervention 2018, in press, with permission from Europa Digital & Publishing) [25]. For the other images: (From: Asmarats L, et al. JACC 2018;71:2935-2956, with permission from Elseiver) [7••]
They are mainly divided in four categories: TV annuloplasty devices, coaptation devices, edge-to-edge techniques and transcatheter tricuspid valve replacement (orthotopic and heterotopic-caval valve implantation). Principal characteristics and early results are presented in Table 1.
A few of them were previously successfully used in percutaneous mitral valve interventions [26, 27], and they were transferred to tricuspid valve. Nevertheless, the majority of these devices are in the initial phase.
Tricuspid Valve Annuloplasty Devices
The TriCinch System Device
The 4Tech TriCinch™ Coil System (4Tech Cardio Ltd, Galway, Ireland) is a novel percutaneous device for severe functional TR designed to reduce tricuspid annular dimensions. The device consists of an anchoring system (placed in the TA at the level of antero-posterior commissure), a nitinol self-expandable stent (27, 32, 37 or 43 mm) and a Dacron band connecting both. In the PREVENT trial, the procedural success was 75%. At the 6-month follow-up, improvements in quality of life were reported, and 75% of patients showed functional class I or II [28].
The second generation of the TriCinch device seems to solve the early detachment problems (the anchoring system is placed in the pericardial space in order to ensure a better stability). During the device implantation, the expansion and pericardial space visualization is obtained using a controlled pneumopericardium with CO2 inflation [29].
Clinical trial “Evaluation of the Percutaneous 4Tech TriCinch Coil Tricuspid Valve Repair System” (NCT03294200) will include 90 patients with significant functional TR and high risk for surgery, and the main objective is to prove safety and performance of the Tricinch Coil System device.
Trialign Device
The Trialign system (Mitralign Inc., Tewksbury, Massachusetts) attempts to replicate the results of the current modified Kay (conversion of an incompetent TV into a competent bicuspid valve) by placing percutaneous pledgets at the level of the TA in the posteroanterior and posteroseptal positions [30]. In the SCOUT I trial, only one pair of pledgets were implanted for each patient. Later, in patients with very large annulus, multiple pledgets were implanted (“side by side” or “in series”).
The US early feasibility study SCOUT I [26] showed 93% acute procedural success and 80% technical success at 30-day follow-up. The SCOUT II CE mark study (NCT03225612) is enrolling 60 patients in different centres of Europe and the U.S, and preliminary results are expected at the beginning of 2019.
MIA (Micro Interventional Devices, Inc) Device
MIATM is a transcatheter tricuspid annuloplasty device designed to replicate tricuspid repair remotely. It is composed of a thermoplastic elastomer (MyoLast) and low mass polymeric, compliant, self-tensioning anchors (PoliCor) allowing the annular reduction. The catheter-based system provides a customizable number of implants deployed to the target annulus allowing further catheterization or surgery if it is needed. The device is surgically implanted through a 16 F steerable delivery system. The STTAR (Study of Transcatheter Tricuspid Annular Repair) will enroll 40 patients to assess the safety and efficacy of this technique [7••].
Cardioband Device
The Cardioband system (Edwards Lifesciences) was initially designed for functional mitral regurgitation treatment, and the results were promising [31]. Its usefulness has also been proven in functional TR, becoming the only transcatheter device for tricuspid regurgitation with CE Mark approval. The results of the TRI-REPAIR (NCT02981953) study were recently presented, showing echocardiographic and clinical parameter improvements at 6 months follow-up [32].
The device is designed as a percutaneous annuloplasty band implanted in a clockwise way from the antero-septal commissure to the first part of the septal annulus after the coronary sinus.
TRAIPTA Device
The action mechanism of the TRAIPTA device is based on an extracardiac tricuspid annuloplasty, and it is positioned in the pericardial space and delivered by puncture through the right atrial appendage (transatrial intrapericardial tricuspid annuloplasty [23]. Pre-clinical experience in animals showed the safety of the implant with significant annular area reduction [23]. Actually, patients with previous surgery are excluded owing to the fact that this approach requires a free pericardial space.
The Millipede System
The Millipede system (Millipede, LLC, Ann Arbor, Michigan) is a repositionable and retrievable complete ring, which can be implanted surgically or transcatheter on the atrial side of the native tricuspid annulus, in order to restore its shape and diameter. Designed initially for the mitral valve, it was used successfully in two cases for tricuspid regurgitation with significant reduction of annulus dimension (36%) and tricuspid regurgitation grade [33].
DaVingiTM TR System
The DaVingi TR System is a novel annuloplasty approach using a tissue-healing process to achieve a strong neoannulus, allowing an aggressive annular reduction. It is a percutaneous device that enables a complete, direct annuloplasty with a single–shot delivery of a ring implant. Predictable annular physiologic constriction using an adjustment tool take place in a second stage (90 days) after a period of tissue healing. To date, four cases were performed in a first-in-human study [34].
Coaptation Devices
FORMA Device
The FORMA Repair System (Edwards Lifescience, Irvine, USA) is a valve spacer, which is positioned into the regurgitant orifice in order to create a platform for native leaflet coaptation. The device is delivered through axillary venous access and is then distally anchored to the RV apex. The SPACER-trial included 78 patients, and the results of 1-year follow-up are available in 18 patients. Reduction of tricuspid regurgitation and improvement in NYHA, 6MWT was observed [35].
MitraClip Device
More than 650 procedures have been performed worldwide, and the multicentre TriValve registry showed that the Mitraclip is so far the most common technique applied for interventional TR treatment [36••]. Preliminary evidence suggests it is a safe, feasible procedure. In a recent study, the procedural rate success was of 81%. Small TR coaptation gap size and central/antero-septal TR jet locations were identified as independent predictors of procedural success and coaptation gap >10 mm, ERO >0.6 cm2 tenting area >2.1 cm2 and TV vena contracta >11 mm as predictors of unfavourable TR repair [37]. In patients with severe mitral and tricuspid regurgitation, both mitral and tricuspid Mitraclip seems to improve functional status and biventricular hemodynamics early after the intervention and in mid-term follow-up [27].
The TRILUMINATE CE Mark trial is still enrolling patients from over 25 centres in Europe, Canada and the U.S., and preliminary results will be available at the end of 2018.
PASCAL Device
The Edwards Pascal transcatheter mitral valve repair system (Edwards Lifesciences) integrates technical aspects from the Forma and the MitraClip devices by combining a 10-mm central spacer and two paddles (25 mm width) and clasps (10 mm length) that attach the device to the valve leaflets, thus overcoming possible limitations of the former devices separately. In patients with severe mitral regurgitation, the device showed to be a feasible option in preliminary efficacy data [38]. The first successful case treating severe TR with PASCAL devices was recently reported [24].
PASTA Device
PASTA device reduces the tricuspid valve orifice by opposing septal and lateral targets on the tricuspid annulus using percutaneously delivered pledgeted sutures. The specific annular targets for PASTA are the mid-anterior leaflet and the posterior-septal leaflet commissure, avoiding the AV node and bundle of His. The result is a double-orifice tricuspid valve. Preliminary studies in animals showed reduction in annular area and tricuspid regurgitation [39].
Transcatheter Tricuspid Valve Replacement (Orthotopic Concept)
Melody and Sapiens Tricuspid Valve for Valve-in-valve and Valve-in-ring
Two different valves have been successfully implanted during tricuspid valve-in-valve or valve-in-ring procedures: the SAPIEN transcatheter aortic valve and the Melody valve (Medtronic, Minneapolis, Minnesota, United States). The biggest registry of transcatheter tricuspid valve replacement included 306 patients (284 patients with valve-in-valve and 22 patients with valve in ring) [40]. After procedure 83% of the patients presented no trivial tricuspid regurgitation. During follow-up (3-years), 31 patients (10%) underwent reintervention on the TV, and survival rate was 83%.
NaviGate, Trisol, LUX-valve in Tricuspid Native Valve
NaviGate Valve
The NaviGate bioprosthesis is a novel self-expanding valved stent designed to treat functional tricuspid regurgitation. Actually, 27 patients received the NaviGate valve with excellent results and a low rate of PVL. Although the preliminary results are promising, there are some pitfalls that still have to be addressed, such as device anchoring and sealing (large asymmetric annulus, minimal calcium), delivery system size (42 OD) and leaflet durability (risk of thrombosis) [22, 41].
TRISOL Valve
TRISOL valve is a percutaneous valve design for tricuspid regurgitation taking into consideration the right ventricle afterload after the valve implantation.
The valve is one piece of pericardium divided into two leaflets, and its opening starts at the periphery. During the systole, the pericardium (one big leaflet) acquired the dome shape, which increases the closing right ventricle volume with pressure relief and function preservation. Further investigations are necessary before first-in-human studies [42].
LUX-valve
The LUX-valve (Jenscare Biotechnology, Ningbo, China) is a self-expanding bovine pericardial tissue valve mounted on a nitinol stent frame with transatrial access.
The device has a self-adaptive skirt to minimize paravalvular leak and a special anchoring mechanism for secure anchoring within the right ventricle. To date, only experimental data are available [7••].
Caval Valve Implantation (Heterotopic Concept)
In patients with limited tricuspid therapeutic options, an alternative approach to percutaneous treatment of TV is to implant transcatheter prosthesis in IVC (single valve approach) or in combination with a superior vena cava (SVC) valve (dual valve) to prevent caval backflow of TR and mitigate systemic venous congestion. Actually, more than 40 patients benefited from Caval Valve Implantation (CAVI) prosthesis using SAPIENS valve or TRICVALVE, and the majority were implanted in compassionate use cases [21]. Published data [21], in a first-in-human study, including 25 patients treated with Sapiens or TricValve, showed improvement in NYHA class and hemodynamic parameters. However, 1-year mortality was high (63%).
The safety and efficacy of SAPIEN valve implantation at the inferior vena cava is currently being studied in the TRICAVAL (NCT02387697) and HOVER (NCT02339974) trials.
TRICENTO Device
It is composed of a bicavally anchored covered stent with a lateral bicuspid valve element (to the right atrium) made of thin porcine pericardium leaflets requiring only a low closing pressure. The device aims to abolish the systolic backflow in both the inferior and superior caval veins. The FIM was recently published showing reduction of caval vein regurgitant volume with stable position in the follow-up [25].
Clinical Perspectives on Transcatheter Tricuspid Interventions
There is no doubt of the emergent necessity to find treatment options for severe tricuspid regurgitation. In the past decade, the number of devices destined to treat severe TR multiplied. The tricuspid valve is no longer the “forgotten valve”, and we are witnesses of a real device parade (Fig. 1).
Some of the devices were successfully used in mitral or aortic valve percutaneous treatment (Mitraclip, Cardioband, Mitralign, Pascal, Millipede devices, Sapiens and Melody valve).
Moreover, a big proportion of devices are still in early development stages and long-term follow-up data is not available. The only device with CE Mark for treating functional tricuspid regurgitation is Cardioband. Others as Tricinch, Trialign, Mitraclip or Forma are still enrolling patients in CE mark Trials.
Percutaneous annuloplasty devices reproduce well-established surgical techniques, and they are divided into suture-based and rings. The acute results are encouraging, showing reduction in annulus dimension and quality of life and symptoms improvement (Table 1).
For those cases with massive TR and a big gap between the leaflets, the Forma device also showed improvement in quality of life parameters and symptomatology.
The edge-to-edge technique, particularly Mitraclip, has become the first-choice approach for high-risk patients with functional TR. Two other devices (the Pascal and the Pasta device) are still in the early stage of development.
Moreover, five valves were designed for percutaneous tricuspid replacement (two of them are also available for valve-in-valve and valve-in-ring procedures). Special precautions are taken into consideration during valve implantation, and different strategies were proposed to avoid AV node conduction system damage. Despite the complex anatomy of the tricuspid annulus, only a small percentage of cases presented with residual TR after valve implantation (Table 1).
Three heterotopic implanted valve designs were designed to relieve the symptoms and reduce the back-flow in the caval veins. However, this therapy was used in pluripathologic patients, the majority in compassionate use cases, and the mortally rate was more than 50% at 1-year follow-up due to patient pre-existing conditions.
All these therapies showed promising results in terms of acute procedural success, but long-term follow-up data (durability, outcomes, etc.) are not available yet. Moreover, technical details, such as sheath size, possible anatomical complications, pre-existing leads in RV and antithrombotic therapy, should be taken into consideration before TTVI.
Finally there is a paradox between the prevalence of tricuspid regurgitation and the number of performed surgical and percutaneous procedures. Only a few patients with severe tricuspid regurgitations, high risk and deemed for surgery are suitable for FIH or early feasibility studies. The majority of the enrolling studies are excluding real symptomatic patients with pulmonary hypertension, severe right ventricle dysfunction or severe left ventricle dysfunction and patients with pacemaker leads (Table 2).
Conclusions
The tricuspid valve is no longer “the forgotten valve”. For patients with severe tricuspid regurgitation and high surgical risk, several percutaneous options are available. However, all these therapies are in a growing phase and not all possible candidates are suitable for these new techniques.
Abbreviations
- 6MWT:
-
6 minute walk test
- CMR:
-
Cardiac magnetic resonance
- CT:
-
Computer tomography
- FIH:
-
First in human
- IVS:
-
Inferior vena cava
- HOVER:
-
Heterotopic Implantation of the Edwards-Sapien Transcatheter Aortic Valve in the Inferior VEna Cava for the Treatment of Severe Tricuspid Regurgitation
- NYHA:
-
New York Heart Association
- PREVENT:
-
Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System™
- RV:
-
Right ventricle
- SCOUT:
-
Early Feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System (PTVAS) Also Known as TriAlign™
- SPACER:
-
Repair of Tricuspid Valve Regurgitation Using the Edwards TricuSPid TrAnsCatheter REpaiR System (SPACER)
- STTAR:
-
Study of Transcatheter Tricuspid Annular Repair
- TA:
-
Tricuspid annulus
- TR:
-
Tricuspid regurgitation
- TRILUMINATE:
-
Evaluation of Treatment With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater Tricuspid Regurgitation
- TRICAVAL:
-
Treatment of Severe Secondary TRIcuspid Regurgitation in Patients With Advance Heart Failure With CAval Vein Implantation of the Edwards Sapien XT VALve
- TRI-REPAIR:
-
TrIcuspid Regurgitation RePAIr With CaRdioband Transcatheter System
- TV:
-
Tricuspid valve
- TTVI:
-
Transcatheter tricuspid valve interventions
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–9.
Zack CJ, Fender EA, Chandrashekar P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. 2017;70:2953–60.
Stuge O, Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg. 2006;132:1258–61.
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–9.
Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–94.
Agarwal S, Tuzcu EM, Rodriguez ER, Tan Carmela D, Rodriguez LL, Kapadia Samir R. Interventional cardiology perspective of functional tricuspid regurgitation. Circ Cardiovasc Interv. 2009;2:565–73.
•• Asmarats L, Puri R, Latib A, Navia JL, Rodés-Cabau J. Transcatheter tricuspid valve interventions: landscape, challenges, and future directions. J Am Coll Cardiol. 2018;71:2935–56 This study provides a complete review of tricuspid valve evaluation and therapies.
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91.
Alqahtani F, Berzingi CO, Aljohani S, Hijazi M, Al-Hallak A, Alkhouli M. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc. 2017;6:e007597.
Kim JB, Jung S-H, Choo SJ, Chung CH, Lee JW. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg. 2013;146:278–84.
Guenther T, Noebauer C, Mazzitelli D, Busch R, Tassani-Prell P, Lange R. Tricuspid valve surgery: a thirty-year assessment of early and late outcome. Eur J Cardiothorac Surg. 2008;34:402–9.
Bernal JM, Morales D, Revuelta C, Llorca J, Gutiérrez-Morlote J, Revuelta JM. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg. 2005;130:498–503.
Kawada N, Naganuma H, Muramatsu K, Ishibashi-Ueda H, Bando K, Hashimoto K. Redefinition of tricuspid valve structures for successful ring annuloplasty. J Thorac Cardiovasc Surg. 2018;155:1511–1519.e1.
Athavale S, Deopujari R, Sinha U, Lalwani R, Kotgirwar S. Is tricuspid valve really tricuspid? Anat Cell Biol. 2017;50:1–6.
Lama P, Tamang BK, Kulkarni J. Morphometry and aberrant morphology of the adult human tricuspid valve leaflets. Anat Sci Int. 2016;91:143–50.
Huttin O, Voilliot D, Mandry D, Venner C, Juillière Y, Selton-Suty C. All you need to know about the tricuspid valve: Tricuspid valve imaging and tricuspid regurgitation analysis. Arch Cardiovasc Dis. 2016;109:67–80.
Dreyfus GD, Martin RP, Chan KMJ, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015;65:2331–6.
• Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18:1342–3 This study provides a new grading scale of tricuspid regurgitation.
Saremi F, Hassani C, Millan-Nunez V, Sánchez-Quintana D. Imaging evaluation of tricuspid valve: analysis of morphology and function with CT and MRI. Am J Roentgenol. 2015;204:W531–42.
Pighi M, Thériault-Lauzier P, Alosaimi H, et al. Fluoroscopic anatomy of right-sided heart structures for transcatheter interventions. J Am Coll Cardiol Intv. 2018;11:1614–25.
Lauten A, Figulla HR, Unbehaun A, Fam N, Schofer J, Doenst T, et al. Interventional treatment of severe tricuspid regurgitation: early clinical experience in a multicenter, observational, first-in-man study. Circ Cardiovasc Interv. 2018;11:e006061.
Navia JL, Kapadia S, Elgharably H, et al. Transcatheter tricuspid valve implantation of NaviGate bioprosthesis in a preclinical model. JACC-Basic Transl Sci. 2018;3:67–79.
Rogers T, Ratnayaka K, Sonmez M, et al. Transatrial intrapericardial tricuspid annuloplasty. J Am Coll Cardiol Intv. 2015;8:483–91.
Fam NP, Ho EC, Zahrani M, Samargandy S, Connelly KA. Transcatheter tricuspid valve repair with the PASCAL system. J Am Coll Cardiol Intv. 2018;11:407–8.
Toggweiler SBB, Brinkert M, Buhmann R, Bossard M, Kobza R, Cuculi F. First-in-man implantation of the Tricento® transcatheter heart valve for the treatment of severe tricuspid regurgitation. Eurointervention. 2018, in press.
Hahn RT, Meduri CU, Davidson CJ, et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty. SCOUT Trial 30-Day Results. J Am Coll Cardiol. 2017;69:1795–806.
Besler C, Blazek S, Rommel K-P, et al. Combined mitral and tricuspid versus isolated mitral valve transcatheter edge-to-edge repair in patients with symptomatic valve regurgitation at high surgical risk. J Am Coll Cardiol Intv. 2018;11:1142–51.
Denti P. 4Tech—Clinical outcomes and current challenges. Presented at: PCR London Valves; September 26, 2017; London, UK.
Greenbaum AB, Rogers T, Paone G, et al. Intentional right atrial exit and carbon dioxide insufflation to facilitate subxiphoid needle entry into the empty pericardial space: first human experience. JACC Clin Electrophysiol. 2015;1:434–41.
Besler C, Meduri CU, Lurz P. Transcatheter treatment of functional tricuspid regurgitation using the trialign device. Interv Cardiol. 2018;13:8–13.
Latib A. The Edwards Cardioband system - reducing valve regurgitation through annular reduction: Edward´s solution for patients suffering from mitral regurgitation with anular dilatation. Presented at: EuroPCR 2018; May 22-25, 2018; Paris [Accessed 21 August 2018] Available at: https://wwwpcronline.com/Cases-resources-images/Resources/Course-videos-slides/2018/The-Edwards-Cardioband-system-reducing-valve-regurgitation-through-annular-reduction.
Bardeleben RSV. The Edwards Cardioband system - reducing valve regurgitation through annular reduction: how cardioband tricuspid system can benefit my patients. Presented at: EuroPCR 2018; May 22-25, 2018; Paris. [Accessed 21 August 2018]. Available at: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2018/The-Edwards-Cardioband-system-reducing-valve-regurgitation-through-annular-reduction.
Rogers JH, Boyd WD, Smith TWR, Ebner AA, Grube E, Bolling SF. Transcatheter annuloplasty for mitral regurgitation with an adjustable semi-rigid complete ring: initial experience with the millipede IRIS device. Struct Heart. 2018;2:43–50.
Kreidel F. Device parade:tricuspid repair and replacement. Cardiac implant complete ring. Presented at: CSI 2018; Frankurt 27-30 June, 2018; Frankfurt. [Accessed 21 August 2018].
Perlman G, Praz F, Puri R, et al. Transcatheter tricuspid valve repair with a new transcatheter coaptation system for the treatment of severe tricuspid regurgitation. 1-year clinical and echocardiographic results. J Am Coll Cardiol Intv. 2017;10:1994–2003.
•• Taramasso M, Hahn RT, Alessandrini H, et al. The international multicenter TriValve registry: which patients are undergoing transcatheter tricuspid repair? J Am Coll Cardiol Intv. 2017;10:1982–90 This study provides the results of multicenter registry of tricuspid valve interventions.
Besler C, Orban M, Rommel K-P, et al. Predictors of procedural and clinical outcomes in patients with symptomatic tricuspid regurgitation undergoing transcatheter edge-to-edge repair. J Am Coll Cardiol Intv. 2018;11:1119.
Praz F, Spargias K, et al. Compassionate use of the Pascal transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet. 2017;390:773–80.
Khan JM, Rogers T, Schenke WH, et al. Transcatheter pledget-assisted suture tricuspid annuloplasty (PASTA) to create a double-orifice valve. Catheter Cardiovasc Interv. 2018:1–10.
Cabalka A. Device Parade:Tricuspid repair and replacement. Tricuspid valve in valve. Presented at: CSI 2018; Frankurt 27-30 June, 2018; Frankfurt.
Kodali S. Device Parade:tricuspid repair and replacement. Tricuspide valve in native. Presented at: CSI 2018; Frankurt 27-30 June, 2018; Frankfurt.
Weisz G. Device Parade:Tricuspid repair and replacement. Percutaneous tricuspid valve replacement with RV consideration. Presented at: CSI 2018; Frankurt 27-30 June, 2018; Frankfurt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Livia Gheorghe, Benno J.W.M. Rensing, Frank D. Eefting, Martijn C. Post and Bushra Rana declare that they have no conflict of interest.
Jan A.S. Van der Heyden and Martin J. Swaans are both faculty members of the Abbott’s Crossroads training facility.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Structural Heart Disease
Rights and permissions
About this article
Cite this article
Gheorghe, L., Rensing, B.J.W.M., Van der Heyden, J.A.S. et al. Transcatheter Tricuspid Valve Interventions: An Emerging Field. Curr Cardiol Rep 21, 37 (2019). https://doi.org/10.1007/s11886-019-1119-7
Published:
DOI: https://doi.org/10.1007/s11886-019-1119-7