Abstract
Purpose of the Review
Proinflammatory cytokines are consistently elevated in congestive heart failure. In the current review, we provide an overview on the current understanding of how tumor necrosis factor-α (TNFα), a key proinflammatory cytokine, potentiates heart failure by overwhelming the anti-inflammatory responses disrupting the homeostasis.
Recent Findings
Studies have shown co-relationship between severity of heart failure and levels of the proinflammatory cytokine TNFα and one of its secondary mediators interleukin-6 (IL-6), suggesting their potential as biomarkers. Recent efforts have focused on understanding the mechanisms of how proinflammatory cytokines contribute towards cardiac dysfunction and failure. In addition, how unchecked proinflammatory cytokines and their cross-talk with sympathetic system overrides the anti-inflammatory response underlying failure.
Summary
The review offers insights on how TNFα and IL-6 contribute to cardiac dysfunction and failure. Furthermore, this provides a forum to begin the discussion on the cross-talk between sympathetic drive and proinflammatory cytokines and its determinant role in deleterious outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure is a complex progressive pathology, a phenotype reflective of an end organ damage as a consequence of insults/injuries, including hypertension, dyslipidemia, diabetes, ischemic heart disease, post-partum cardiomyopathy, and congenital disorders [1,2,3,4]. In response to the tissue injury from the diverse array of insults, the heart initiates tissue repair mechanisms by engaging the innate immune system. Consistent with the engagement of innate immunity, significant increase in the repertoire of proinflammatory cytokines is observed following cardiac insult [5, 6, 7••]. Tumor necrosis factor-α (TNFα), transforming growth factor β (TGFβ), and family of interleukins, including IL-1, -12, -8 and -18 are among the most common proinflammatory cytokines observed following cardiac stress [8, 9••]. The increase in the proinflammatory cytokine profile is a responsive tissue repair mechanism that is considered to provide beneficial effects by mediating cardiac remodeling. Accumulating evidence indicates that the acute proinflammatory stage is classically followed by the anti-inflammatory response to resolve the injury [10, 11]. However, given the diverse and unique nature of cardiac stress, the proinflammatory response is accentuated due to localized smooth muscle injury associated with increased extravasation of leukocytes that prolongs the proinflammatory cycle [7••, 12, 13]. The prolonged proinflammatory process if unchecked by the anti-inflammatory mechanisms transitions to chronic inflammation, aided in part by the constant cardiac stress mediated by co-morbid conditions like hypertension, diabetes, etc. [6, 14]. A key component in sustaining chronic inflammation is the infiltration of cardiac tissue by macrophages, which forms the nucleating site for generation of proinflammatory cytokines. Thus, there is excessive production and release of these cytokines into circulation, which in turn could have adverse effects on remote organs [15, 16••]. Consistent with their deleterious effects, multiple studies have suggested the use of TNFα and IL-6 as potential markers for heart failure [16••, 17,18,19]. Furthermore, high levels of proinflammatory cytokines like TNFα can impact cardiac function by mediating signals that underlie deleterious cardiac remodeling and negative inotropic effects [20,21,22]. In addition to the negative inotropic influence on the heart, there is also growing evidence of dynamic cross-regulation between sympathetic system and immune response [23]. Understanding the cross-talk and regulation becomes important given that cardiac function is tightly regulated by sympathetic signaling mechanisms [24]. In this context, we hope that our succinct review provides critical insights into immune responses focusing on integrating the cross-regulation between sympathetic systems and inflammation that mediate cardiac function/dysfunction and failure.
Innate Immune Response in Cardiac Remodeling and Heart Failure
The heart maintains cardiac tissue homeostasis like any other organ/tissue systems by engaging the innate and adaptive inflammatory responses. Traditionally, the innate immune response is associated with infections [25], and thus, difficult to envision its role as a part of initial inflammatory response [26]. However, it has come to be recognized that heart also encodes the classical germ-line encoded pattern recognition receptors that initiate repair upon detecting pathogen-associated molecular patterns (PAMPs or damage-associated molecular patterns (DAMPs) [6, 27, 28]. There is increasing appreciation that the pattern recognition receptors can detect molecules of self-origin like DAMPs providing the link between cardiac tissue injury/damage to the initial proinflammatory response [7••, 29]. In the context of cardiac injury, the DAMPs could include mitochondrial components, dsRNA, heart shock proteins released from damaged/necrotic cardiomyocytes, or degraded extracellular matrix components like Hyaluronan fragments [6, 30, 31]. These molecules engage the recognition pattern receptors to mediate a robust inflammatory response with infiltration of neutrophils and monocytes that initiate tissue repair. Given the robustness of the inflammatory response “sans,” the pathogenic infection in the cardiac tissue, it has been referred to as “sterile infection.” Thus, the innate inflammatory response is mediated by an ensemble of components involving macrophages, dendritic cells, and poly-morphonuclear granulocytes through cytokines, chemokines, and activation of complement system [32,33,34].
A key determinant in the robust innate inflammatory response is the pattern recognition receptors that are activated by DAMPs or PAMPs, which are primarily represented by Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) [35,36,37]. In addition to these classical pattern recognition receptors, there are also intercellular receptors like retinoic acid-inducible gene-1-like receptors (RLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) [34, 38, 39]. Together, this repertoire of pattern recognition receptors senses the DAMPs and/or PAMPs to mediate the initial repair response to resolve the damage. Although the roles of pattern recognition receptors are well described in immune cells, less is known about their roles in cardiac systems, especially in cardiomyocytes. In this context, it is important to note intense studies are in progress defining the roles of pattern recognition receptors in human hearts and in conditions of failure. It is known that human hearts express the ensemble of pattern recognition receptors, including TLRs, CLRs, and NLRs [38, 40, 41]. Thus, expression of these set of receptors in human heart may provide a quick and robust inflammatory repair response to cardiac stressors, including injury to cardiac tissue or infection [34, 37, 42].
The primary purpose of this response is to resolve the source underlying disruption of homeostasis with aim to restore cardiac function. However, given that co-morbidities like hypertension, dyslipidemia, and diabetes are sustained cardiac stressors, in turn, ensues in prolonged inflammatory response leading to persistent low-grade inflammation. This low-grade inflammation that occurs in the absence of any identifiable infection is termed “parainflammation” [43]. Occurrence of this low-grade inflammation suggests that the heart is not able to restore homeostasis by resolving the initial inflammatory response [44]. Consistent with this idea, human heart failure has long been known to be associated with elevated levels proinflammatory cytokines (TNFα and IL-6) [19, 45] and reduced level of circulating regulatory T cells (Tregs, known to suppress immune response) [46,47,48]. In addition, Tregs from heart failure patients have innately reduced functional capability to suppress cytokines production [46, 47]. Accordingly, rodent studies have shown that transfer of Tregs from healthy to hypertension-induced cardiac hypertrophic rats results in reverse remodeling and amelioration in cardiac function [48]. This supports the idea that resolution of the inflammatory response may not occur efficiently paving the way for a low-grade inflammation that could transition into a sustained low-grade pathological proinflammatory response.
An integral part of the inflammatory response with progression of heart failure is the simultaneous observation of graded increase in the proinflammatory cytokine profile. However, due to the complexity of the immune response, a key question that remains to be addressed is whether proinflammatory cytokines are the “cause” or “effect” of HF. Thus, despite evidence that inflammatory cytokines are associated with heart failure, anti-cytokine therapies have not achieved significant success [49, 50], in part, due to incomplete understanding of the beneficial vs. deleterious role these play in cardiac remodeling [51]. It is known that proinflammatory cytokines bind to their cognate receptors to mediate their effects through a key transcription factor—nuclear factor kappa B (NF-κB) [52, 53]. NF-κB is engaged by both the innate immune response as well as by the proinflammatory cytokines [6, 54]. Increasing evidence shows that NF-κB can be activated by canonical and non-canonical pathways reviewed in-depth in Bartekova et al. [9••]. Canonical activation of NF-κB involves the activation of IkB kinase that predominantly mediates p65/p50 dimer to be translocated to the nucleus to initiate transcription [9••, 55, 56]. Non-canonical activation involves IKKα kinase complex predominantly mediating p52/RelB complex translocation to the nucleus [57, 58]. Despite these complexities in activation, NF-κB is well-known to mediate both beneficial and deleterious effects reflecting the beneficial vs. deleterious role of inflammation in cardiac remodeling and failure.
Given that human heart failure is associated with an elevated proinflammatory cytokine milieu, a key question studies have been focused are on determining whether biological effects of proinflammatory cytokines are adequate to provoke a phenotype of heart failure in experimental animals and in humans [7••]. However, increasing evidence supports the prevailing idea that heart failure progression occurs as a result of deleterious signaling exerted by proinflammatory cytokines secreted by the hearts in addition to the secondary effects accruing from circulating cytokines [59]. Thus, chronic sustained inflammation contributes to worsening of heart failure phenotype reminiscent of the sustained neurohormonal activation in heart failure. In this context, pathological effects of chronic proinflammatory cytokines have been extensively reviewed [23, 51], including their role in cardiomyocyte function [23] and ventricular remodeling [7••, 60]. Studies have shown that proinflammatory cytokines directly modulate cardiac contractile output, wherein this modulation can be mediated by immediate and delayed effects. The early immediate outcomes encompass effects on EC coupling [19, 28,29,30,31,32,33,34,35,36,37,38,39,40], NOS leading to nitric oxide generation [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78], sphingomyelinase signaling [67, 70, 72, 77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92], and phospholipase A2 (PLA2) and arachidonic acid (AA) activation [81, 93,94,95,96,97,98,99]. In contrast, the delayed effects also play a role in modulating contractile function by altering βAR signaling, wherein loss of βAR responsiveness to βAR agonists like isoproterenol underlies the reduction in contractile responses [22, 67, 100, 101].
Tumor Necrosis Factor-α (TNFα)
TNFα belongs to a large family of proinflammatory cytokine TNF ligands (Table 1) that play a key role in immune responses and are altered primarily in autoimmune diseases [102]. Although analysis has shown that some of the TNF family of ligands are elevated in heart failure [103, 104] (Table 1), less is understood about their role in heart failure. Among these TNF superfamily of ligands (TNFSF), one of the key members that has been extensively studied, including its use for therapeutic strategies and as a potential biomarker associated with heart failure is TNFα [19, 45, 49, 50]. One of the key mediators of TNFα signaling in the cells is through NF-κB, which again is reflective of the dual role TNFα plays in cardiac physiology and pathology [105]. The downstream cellular effects of TNFα are mediated by two cognate receptors, TNF receptor 1 or 2 (TNFR1 or 2) [106]. Classically, it is considered that TNFα activation of TNFR1 is deleterious, while TNFR2 is beneficial and the relative ratio of their expression in a given tissue system would potentially drive the phenotypes [106,107,108]. In addition to the divergent roles the receptors play in TNFα signaling, it is known that these receptors can be shed from various cells to generate soluble TNFR1 or 2 (sTNFRs). Circulating TNFα may bind to these receptors depleting the available pool of TNFα to bind and activate membrane TNFRs on cells [85, 109]. However, it is important to note that the role of these sTNFRs is not yet well understood in the overall cardiac pathophysiology [51]. Furthermore, TNFα as an inflammatory ligand has two forms: (a) the membrane bound form and (b) the secreted form [110, 111], adding another layer of complexity in the overall development of pathophysiology. Despite the complexities of TNFα signaling, multiple studies have shown that cardiomyocyte-specific expression of TNFα results in depressed cardiac function that is gene dosage dependent [112, 113]. Consistently, studies have shown that TNFα per se mediates negative inotropic effects in vitro and in vivo [114,115,116] and is discussed later in the review. Such findings indicate that the proinflammatory cytokine TNFα could cross-talk with the beta-adrenergic receptor (βAR) system, and thereby, underlie the negative inotropic phenotype observed in heart failure.
Cross-Talk Between Inflammatory Cytokines and Sympathetic System
Sympathetic overdrive and elevated levels of proinflammatory cytokines underlie the progression of heart failure and a key concern has been whether these parallel pathways cross-talk and contribute to the pathology. In this regard, many studies have consistently shown that an increase in proinflammatory cytokine TNFα leads to negative inotropy both in “in vitro” and “in vivo” settings [115, 116]. Given that βARs are key regulators of cardiac inotropic responses, these observations show that proinflammatory cytokines like TNFα could directly alter βAR function to induce negative inotropy. Similarly, chronic activation of βARs by sympathetic system results in induction of proinflammatory response characterized by increased expression of TNFα, IL-1β, and IL-6 in the hearts [117,118,119,120]. Consistently, antagonizing βARs using the β-blockers markedly reduces myocardial TNFα and IL-1β expression in the hearts [121] indicating a “quid-pro-quo” relationship between the sympathetic βAR activation and induction of inflammatory responses. As discussed in the earlier part of the review, upregulation of TNFα, IL-1β, and IL-6 in the myocardium occurs through the NF-κB dependent mechanisms [122, 123]. In addition, studies have shown an increase in the proinflammatory cytokine profile following sympathetic β-agonist is markedly blunted by epidermal growth factor receptor (EGFR) blocker gefitinib [124] indicating another layer of complexity in the dynamic cross-talk between inflammatory cytokines and sympathetic systems. Thus, accumulating evidence from multiple studies portrays the existence of a dynamic cross-talk between sympathetic activation and proinflammatory responses that determines the ultimate cardiac phenotype.
Proinflammatory milieu (TNFα) modulates cardiac sympathetic response
TNFα is a known negative cardiac depressant and thought to mediate these effects through βARs [114, 115]. As discussed, TNFα is thought to mediate these effects by engaging immediate and delayed signaling mediators. The immediate effects include alterations in Ca2+ [93], sphingolipid mediators [75] and nitric oxide synthase [74]. However, the mechanistic underpinnings of the delayed signaling mediators are still an area of intense study. Despite the knowledge that TNFα drives negative inotropy through βARs, less has been known on whether traditional pathways that mediate βAR dysfunction are engaged by the TNFα-driven mechanisms. Sympathetic hormones (catecholamines) mediate cardiac contractility through βARs [24, 125]. Diminution in βAR signaling to catecholamines occurs through βAR desensitization contributing to the pathogenesis of heart failure. βAR desensitization is mediated by phosphorylation of the receptor by G-protein coupled receptor kinases (GRK 2, 3, 5, and 6), protein kinase C (PKC), and protein kinase A (PKA). Among them, GRK2 is a predominant player [126,127,128] as blocking recruitment of GRK2 to the βARs through a strategy of deploying C-terminal of GRK2 (GRK2-Ct) results in beneficial cardiac remodeling [127]. In this context, GRK2 is consistently upregulated in response to proinflammatory cytokine TNFα in various tissues [129], and our studies identified that cardiac GRK2 is upregulated in mice with cardiac overexpression of TNFα, which occurs before cardiac dysfunction [22].
GRK2 upregulation in response to TNFα in these hearts indicates that GRK2 could be the proximal link in mediating cardio-depressant effects of TNFα through βAR dysfunction [113,114,115]. Studies showed that GRK2 is key in mediating β2AR desensitization following TNFα as cardiomyocyte conditional GRK2 knockout mice had preserved β2AR function despite elevated TNFα. In contrast to the known benefits afforded by cardiomyocyte-specific expression of GRK2-Ct, expression of GRK2-Ct expression was not able to ameliorate cardiac dysfunction in response to TNFα. This indicates the presence of a yet unknown mechanism by which GRK2 can be recruited to phosphorylate βAR instead of the traditional G-protein beta-gamma (Gβγ) subunits [126, 130, 131]. Furthermore, recruitment of GRK2 to the βAR complex is mediated by TNFR2 as there was marked reduction in GRK2 recruitment to βARs in TNFR2 knockout cells [22], potentially indicating mechanisms that could be independent of Gβγ subunits. However, such a role for TNFR2 is counterintuitive as TNFR2 is considered to play a beneficial role compared to TNFR1 signaling [22]. In conditions of heart failure with significant pre-existing sympathetic overdrive, TNFR2 signaling could provide beneficial effects by dampening cardiac function in presence of TNFα by mediating non-canonical βAR desensitization. Such mechanisms could underlie the beneficial role TNFR2 may play in presence of chronic βAR agonist as studies have shown that absence of TNFR2 (TNFR2 knockout mice) results in deleterious cardiac remodeling to β-agonist isoproterenol [21, 132]. These observations indicate a dynamic relationship exists between the sympathetic βAR activation and proinflammatory cytokine TNFα.
Sympathetic signals modulate immune response
Given the dynamic “quid-pro-quo” relationship, wherein TNFα mediates βAR dysfunction, thereby reducing the ability of βARs to respond to sympathetic stimulation. While, simultaneously, sympathetic overdrive in turn elevates inflammatory cytokine TNFα setting up a deleterious feed-forward and feed-back loop. A biochemical feature observed in many pathologies including obesity, rheumatoid arthritis, myocardial infarction, and kidney renal injury [60, 133, 134]. The sympathetic nervous system (SNS) is integral to the overdrive that mediates increased proinflammatory cytokines profile. SNS innervates both primary organs and lymphoids like bone marrow, thymus, and secondary lymphoid tissues, including the spleen and lymph nodes [135,136,137,138,139]. This innervation of noradrenergic fibers provide direct communication between the nervous system and cells of the immune system through catecholamine neurotransmission. In addition, circulating catecholamines released from the adrenal medulla also influences immunocompetent cell activity [140,141,142]. In this context, it is important to note that the functional response displayed by the immune cells in part is mediated by the βARs on these cells in response to catecholamines [139, 143,144,145,146].
The SNS driven catecholamine effects are mediated by the most commonly expressed member of adrenergic receptor family (composed of α1, α2, β1, β2, and β3 [147]), the β2ARs, that are found in the majority of immune cells except T-helper 2 cells [148,149,150,151]. However, studies have shown that the expression of the repertoire of the adrenergic receptor members can be altered in pathology, like upregulation of α1- and α2ARs in certain lymphoid compartments [152]. Though a speculation, the differences in receptor subtype expression in various immune cells may underlie the apparent controversial effects of catecholamines with regard to their pro- and anti-inflammatory effects that could be dependent on cell type, activation state, and other circumstances [151,152,153]. For example, αAR stimulation enhances, while βAR stimulation inhibits lymphocyte proliferation, antibody secretion, and proinflammatory cytokine production [148,149,150]. Further, β2AR activation is known to increase NK cell number and activity [142], whereas αAR drives bone marrow-derived lymphocyte production [154, 155] mimicking acute psychological stress and exercise [142, 154, 155]. In contrast to acute catecholamine release, chronic elevation of catecholamine levels decreases lymphocyte and NK cell numbers in the peripheral blood without altering immune cell distribution [156]. Despite these differential effects, accumulating evidence indicates that sympathetic overdrive leads to an increase in proinflammatory cytokine response resulting in neuroinflammation and pain in fibromyalgia [157], while causing negative inotropy in heart leading to heart failure.
An important aspect that needs recognition is the parallels observed between the sympathetic and immune systems. It is well-known that the “fight-or-flight” response triggers SNS and hypothalamic-pituitary-adrenal (HPA) axis activation in an attempt to overcome the effects of the stressors with an aim to return homeostasis [154, 155]. Similarly, during acute stress the innate immune system is activated to release proinflammatory cytokines that prepares the body to fight infections or limit injury [158]. However, in the context of inflammation, chronic stress is believed to induce a shift from an adaptive response to immunosuppression, potentially through receptor desensitization or downregulation, with detrimental outcomes for the host [159] that are associated with low-grade inflammation. This low-grade inflammation due to chronic stress has a negative impact in the context of cardiac function, as evidenced in the co-morbidities of numerous pathologies like cardiovascular disease, metabolic syndrome, and arthritis among others [160, 161]. Given the dynamic cross-talk between SNS and inflammatory response, it is possible that the stress-induced immunomodulation is mediated by the SNS activation, which maintains a low-grade inflammation. This in turn causes negative inotropy, leading to increase in epinephrine/norepinephrine levels and keeping this SNS-inflammatory cytokine cycle active. At a cellular level, epinephrine/norepinephrine released from the nerve endings or adrenal medulla results in adrenergic activation and GRK2 upregulation in lymphocytes, leukocytes, and other immune cells [162], which in turn underlies the chronic immune response that seems to occur in the absence of active infection. However, it is important to note that the immunomodulatory effects by epinephrine/norepinephrine could be different depending on pathology like rheumatoid arthritis and multiple sclerosis, which are associated with decreased PBMC GRK2 levels [163, 164] in contrast to heart failure [165].
In conditions of heart failure, it is well-known that sympathetic overdrive is coupled with elevated proinflammatory cytokine profile. Studies have found a persistent relationship between circulating TNFα and soluble TNF receptors and mortality in patients with heart failure [51]. Given the evidence, it is perhaps easy to speculate that sympathetic overdrive induces TNFα, which mediates cellular/physiological responses that initially may be beneficial but becomes deleterious with time. In terms of the molecular signaling, TNFα produced in response to βAR activation is synthesized as a 26 kDa transmembrane protein, which can also be cleaved into a soluble 17 kDa TNFα by TNFα-converting enzyme (TACE) [133, 166]. As detailed previously, TNFα mediates its effects through TNFR1 or R2 via NF-κB “fine-tuning” the cellular response. TNFR1- and R2-mediated responses are complex as they engage multiple pathways like TNFR1 interaction with the death domain TNFR1 protein (TRADD) and TRAF2 activates NF-κB, while its engagement with TRADD-death domain containing Fas protein complex mediates apoptosis [133, 167]. While TNFR2 activation leads to beneficial effects through its interaction with TRAF2 that activates NF-κB, MAPK, and protein kinase B (AKT) that are pro-survival signals [133, 168, 169]. TRAF2 is the nodal molecule that mediates beneficial signals downstream of both TNFR1 and R2, and thus, its expression could be a key determinant of TNFα response in conditions of heart failure. Interestingly, high levels of myocyte-specific overexpression of TRAF2 lead to deleterious cardiac remodeling [20], while lower levels of TRAF2 provides beneficial remodeling in ischemia reperfusion injury [105]. Thus, expression levels of TRAF2 in cells may produce counterintuitive phenotypes in pathology. This is critical as sympathetic overdrive leads to βAR stimulation, increasing TNFα whose downstream effects would be determined by the ratio of TNFR1 and R2 expression and driven by levels of TRAF2 in various cells. In addition to this paradigm of cross-talk mediated by SNS, the pathophysiological phenotype is also further determined by whether TNFα is membrane bound or is in the soluble format. It is thought that membrane bound TNFα activates TNFR2 [106, 170, 171] initiating beneficial responses to TNFα. Correspondingly, studies show that cardiomyocyte-specific expression of non-cleavable TNFα leads to concentric hypertrophy, while expression of secreted TNFα results in dilated cardiomyopathy [110, 111]. More importantly, the key role of TNFα in cardiac pathophysiology is documented by findings showing reduction in apoptosis and amelioration in deleterious cardiac remodeling in TNFα knockout mice (TNF KO) upon myocardial infarction (MI) [172]. It is known that MI is associated with upregulation of sympathetic hormones elevating TNFα, and thus, the absence of TNFα in TNFα KO mice ameliorates the major βAR agonist epinephrine driven deleterious signals via TNFα. These exciting observations led to the idea of targeting elevated TNFα in humans with a premise to sequester the circulating TNFα, thus reducing the deleterious effects in heart failure. To accomplish the sequestration of elevated TNFα two approaches were employed, wherein (a) genetically engineered TNF receptor was used as decoy to bind and potentially clear circulating TNFα and (b) to use a chimeric monoclonal antibody that would bind and neutralize TNFα. The clinical trial using the genetically engineered humanized TNF receptor etanercept named RENAISSANCE and RECOVER were terminated prematurely due lack of accrued benefits [50]. Critically, a greater portion of patients on etanercept had worsened prognosis compared to placebos [7••, 50]. Similar to the observation with etanercept, use of monoclonal antibody infliximab neutralizing TNFα also resulted in early termination of the clinical trial due worsening heart failure [49]. The failure of the clinical trial resulted in significant sojourn of studies pertaining to this area. However, given that the underlying mechanisms for worsening in heart failure following anti-TNFα treatment in not well understood, it is imperative that more effort and resources need to be dedicated to gain insights as elevated TNFα is consistently associated with heart failure. This is all the more important given that anti-TNFα antibody infliximab provides beneficial effects in Crohn’s disease and rheumatoid arthritis [7••] reflecting the complexity of the disease etiologies. Finally, it argues to the idea that unique engagement of the certain pathways could provide beneficial effects in certain pathologies, while resulting in deleterious outcomes in others. Such an observation with anti-TNFα treatment suggests that better understanding of the mechanisms would allow for selectively leveraging the beneficial components of the anti-TNFα treatment in the complex etiology of heart failure.
Conclusions
A key focus of our review was to bring-to-fore the current state of understanding regarding the dynamic cross-talk between the proinflammatory cytokines and SNS, which have independent and interdependent effects on cardiac function and pathology. Our discussion was to focus on how the active feed-forward and feed-back between SNS and inflammatory cytokines effects cardiac function and pathology (Fig. 1). This is all the more critical given the failure of anti-TNFα, therapy, indicating that an in-depth understanding of the complexity of TNFα activity in context of TNFα-βAR signaling axis may shed light on deleterious manifestations in heart failure. On the same note, it is also important to understand the conundrum of upregulation of proinflammatory pathways following sympathetic overdrive, a hallmark feature of heart failure. Thus, molecular delineation of these bi-directional pathways will pave the way for comprehensive understanding of the mechanism, while simultaneously providing insights into potentially novel therapeutic strategies/approaches.
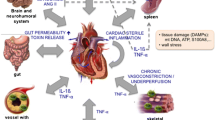
The schematic illustration describes the current understanding on the cross-regulation of proinflammatory cytokines and the neurohormonal-beta-adrenergic receptor (β-adrenergic receptors) axis. Cardiac stressors like changes in hemodynamic load or hypoxia leads to cardiac injury and in response proinflammatory cytokines, including tumor necrosis factor-α (TNFα), are employed as first step in the defense mechanism. This is followed by cardiac tissue repair and injury resolution phase primarily mediated by anti-inflammatory cytokines. However, as TNFα inhibits β-adrenergic receptor function, it results in the inability of these receptors to respond to sympathetic drive fundamentally impeding cardiac responses to changes in mechanical demand. Reduced β-adrenergic receptor response leads to a feed-back increase in sympathetic hormones that in turn results in feed-forward elevation in proinflammatory cytokine TNFα, which leads to self-perpetuating cycle that is now independent of pro- and anti-inflammatory response, which was initiated due to injury. This cycle of increased TNFα now inhibits β-adrenergic receptors, thereby leading to feed-back and feed-forward cycle resulting in deleterious signaling mechanisms that underlies cardiac hypertrophy, deleterious remodeling, and heart failure.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major Importance
Chung MW, Tsoutsman T, Semsarian C. Hypertrophic cardiomyopathy: from gene defect to clinical disease. Cell Res. 2003;13:9–20.
Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–41.
Francis GS, Tang WH. Pathophysiology of congestive heart failure. Rev Cardiovasc Med. 2003;4(Suppl 2):S14–20.
Lefkowitz RJ, Willerson JT. Prospects for cardiovascular research. JAMA. 2001;285:581–7.
Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9.
Valen G. Innate immunity and remodelling. Heart Fail Rev. 2011;16:71–8.
•• Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–68 This article provides discussion on the mechanisms that underlie the initial response to cardiac injury and how an unchecked inflammatory response could overwhelm the anti-inflammatory resolution processes.
Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35.
•• Bartekova M, Radosinska J, Jelemensky M and Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018. The review details the signlaing mechanism involved in mediating the effects of cytokines in cells with a focus on NF-κB, a key central transcirption factor that is activated in response to proinflammatory cytokines.
Ionita MG, Arslan F, de Kleijn DP, Pasterkamp G. Endogenous inflammatory molecules engage Toll-like receptors in cardiovascular disease. J Innate Immun. 2010;2:307–15.
Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45.
Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103:1718–20.
Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10.
Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101.
Ramadori G, Van Damme J, Rieder H, Meyer zum Buschenfelde KH. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988;18:1259–64.
•• Mohan ML, Vasudevan NT, Naga Prasad SV. Proinflammatory cytokines mediate GPCR dysfunction. J Cardiovasc Pharmacol. 2017;70:61–73 This manusript provides in-depth discussion on the cross-talk between the proinflammatory cytokines and G-protein coupled receptors describing how these cytokines inhibit receptor function.
Mommersteeg PM, Kupper N, Schoormans D, Emons W, Pedersen SS. Health-related quality of life is related to cytokine levels at 12 months in patients with chronic heart failure. Brain Behav Immun. 2010;24:615–22.
Hartupee J, Mann DL. Positioning of inflammatory biomarkers in the heart failure landscape. J Cardiovasc Transl Res. 2013;6:485–92.
Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta. 2015;443:71–7.
Divakaran VG, Evans S, Topkara VK, Diwan A, Burchfield J, Gao F, et al. Tumor necrosis factor receptor-associated factor 2 signaling provokes adverse cardiac remodeling in the adult Mammalian heart. Circulation Heart Failure. 2013;6:535–43.
Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits beta-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic Research in Cardiology. 2011;106:1193–205.
Vasudevan NT, Mohan ML, Gupta MK, Martelli EE, Hussain AK, Qin Y, et al. Gbetagamma-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor alpha-induced cardiac beta-adrenergic receptor dysfunction. Circulation. 2013;128:377–87.
Prabhu SD. Cytokine-induced modulation of cardiac function. Circulation research. 2004;95:1140–53.
Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12.
Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402.
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–30.
Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–3.
Mann DL, Topkara VK, Evans S, Barger PM. Innate immunity in the adult mammalian heart: for whom the cell tolls. Trans Am Clin Climatol Assoc. 2010;121:34–50 discussion 50–1.
Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5.
Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44.
Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–10.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–33.
Erickson B, Sperber K, Frishman WH. Toll-like receptors: new therapeutic targets for the treatment of atherosclerosis, acute coronary syndromes, and myocardial failure. Cardiol Rev. 2008;16:273–9.
Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–54.
Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8:465–79.
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–92.
Lech M, Susanti HE, Rommele C, Grobmayr R, Gunthner R, Anders HJ. Quantitative expression of C-type lectin receptors in humans and mice. Int J Mol Sci. 2012;13:10113–31.
Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35.
Fildes JE, Shaw SM, Yonan N, Williams SG. The immune system and chronic heart failure: is the heart in control? J Am Coll Cardiol. 2009;53:1013–20.
Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, Mullen AJ, et al. Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation. 2001;104:I233–40.
Tang H, Zhong Y, Zhu Y, Zhao F, Cui X, Wang Z. Low responder T cell susceptibility to the suppressive function of regulatory T cells in patients with dilated cardiomyopathy. Heart. 2010;96:765–71.
Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, et al. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem. 2010;25:451–8.
Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–12.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40.
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–602.
Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–98.
Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42:519–23.
Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2006;41:580–91.
Jones WK, Brown M, Wilhide M, He S, Ren X. NF-kappaB in cardiovascular disease: diverse and specific effects of a “general” transcription factor? Cardiovasc Toxicol. 2005;5:183–202.
Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–52.
Li C, Kao RL, Ha T, Kelley J, Browder IW, Williams DL. Early activation of IKKbeta during in vivo myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H1264–71.
Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–40.
Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32.
Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2:243–9.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112.
Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–98.
Prabhu SD. Nitric oxide protects against pathological ventricular remodeling: reconsideration of the role of NO in the failing heart. Circ Res. 2004;94:1155–7.
Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovasc Res. 1999;41:514–23.
Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9.
McGowan FX Jr, Takeuchi K, del Nido PJ, Davis PJ, Lancaster JR Jr, Hattler BG. Myocardial effects of interleukin-2. Transplant Proc. 1994;26:209–10.
Kinugawa K, Takahashi T, Kohmoto O, Yao A, Aoyagi T, Momomura S, et al. Nitric oxide-mediated effects of interleukin-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994;75:285–95.
Sugishita K, Kinugawa K, Shimizu T, Harada K, Matsui H, Takahashi T, et al. Cellular basis for the acute inhibitory effects of IL-6 and TNF- alpha on excitation-contraction coupling. J Mol Cell Cardiol. 1999;31:1457–67.
Goldhaber JI, Kim KH, Natterson PD, Lawrence T, Yang P, Weiss JN. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am J Physiol. 1996;271:H1449–55.
Alloatti G, Penna C, De Martino A, Montrucchio G, Camussi G. Role of nitric oxide and platelet-activating factor in cardiac alterations induced by tumor necrosis factor-alpha in the guinea-pig papillary muscle. Cardiovasc Res. 1999;41:611–9.
Hofmann U, Domeier E, Frantz S, Laser M, Weckler B, Kuhlencordt P, et al. Increased myocardial oxygen consumption by TNF-alpha is mediated by a sphingosine signaling pathway. Am J Physiol Heart Circ Physiol. 2003;284:H2100–5.
Panas D, Khadour FH, Szabo C, Schulz R. Proinflammatory cytokines depress cardiac efficiency by a nitric oxide-dependent mechanism. Am J Physiol. 1998;275:H1016–23.
Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–18.
Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 1999;276:R265–76.
Bartel S, Stein B, Eschenhagen T, Mende U, Neumann J, Schmitz W, et al. Protein phosphorylation in isolated trabeculae from nonfailing and failing human hearts. Mol Cell Biochem. 1996;157:171–9.
Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, et al. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–64.
Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303–12.
Edmunds NJ, Lal H, Woodward B. Effects of tumour necrosis factor-alpha on left ventricular function in the rat isolated perfused heart: possible mechanisms for a decline in cardiac function. Br J Pharmacol. 1999;126:189–96.
Oral H, Dorn GW 2nd, Mann DL. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian cardiac myocyte. J Biol Chem. 1997;272:4836–42.
Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8.
Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–20.
Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994;269:5757–63.
Friedrichs GS, Swillo RE, Jow B, Bridal T, Numann R, Warner LM, et al. Sphingosine modulates myocyte electrophysiology, induces negative inotropy, and decreases survival after myocardial ischemia. J Cardiovasc Pharmacol. 2002;39:18–28.
Dettbarn CA, Betto R, Salviati G, Palade P, Jenkins GM, Sabbadini RA. Modulation of cardiac sarcoplasmic reticulum ryanodine receptor by sphingosine. J Mol Cell Cardiol. 1994;26:229–42.
Schreur KD, Liu S. Involvement of ceramide in inhibitory effect of IL-1 beta on L-type Ca2+ current in adult rat ventricular myocytes. Am J Phys. 1997;272:H2591–8.
Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–93.
Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGowan FX Jr. Rapid endotoxin-induced alterations in myocardial calcium handling: obligatory role of cardiac TNF-alpha. Anesthesiology. 2001;95:1396–405.
Edmunds NJ, Woodward B. Effects of tumour necrosis factor-alpha on the coronary circulation of the rat isolated perfused heart: a potential role for thromboxane A2 and sphingosine. Br J Pharmacol. 1998;124:493–8.
Stamm C, Friehs I, Cowan DB, Moran AM, Cao-Danh H, Duebener LF, et al. Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation. 2001;104:I350–5.
Cailleret M, Amadou A, Andrieu-Abadie N, Nawrocki A, Adamy C, Ait-Mamar B, et al. N-acetylcysteine prevents the deleterious effect of tumor necrosis factor-(alpha) on calcium transients and contraction in adult rat cardiomyocytes. Circulation. 2004;109:406–11.
Robinson BS, Hii CS, Poulos A, Ferrante A. Activation of neutral sphingomyelinase in human neutrophils by polyunsaturated fatty acids. Immunology. 1997;91:274–80.
Mazurais D, Robert P, Gout B, Berrebi-Bertrand I, Laville MP, Calmels T. Cell type-specific localization of human cardiac S1P receptors. J Histochem Cytochem. 2002;50:661–70.
Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–7.
Amadou A, Nawrocki A, Best-Belpomme M, Pavoine C, Pecker F. Arachidonic acid mediates dual effect of TNF-alpha on Ca2+ transients and contraction of adult rat cardiomyocytes. Am J Physiol Cell Physiol. 2002;282:C1339–47.
Liu SJ, McHowat J. Stimulation of different phospholipase A2 isoforms by TNF-alpha and IL-1beta in adult rat ventricular myocytes. Am J Physiol. 1998;275:H1462–72.
Damron DS, Summers BA. Arachidonic acid enhances contraction and intracellular Ca2+ transients in individual rat ventricular myocytes. Am J Physiol. 1997;272:H350–9.
de Bracco MM, Fink SB, Finiasz MR, Borda ES, Sterin-Borda L. Positive inotropic effect of interleukin-2. Role of phospholipases and protein kinase C. Int J Immunopharmacol. 1991;13:509–15.
Fink SB, Finiasz M, Sterin-Borda L, Borda E, de Bracco MM. Stimulation of heart contractility by supernatants from lectin-activated lymphocytes. Role of IL-2. Int J Immunopharmacol. 1989;11:367–70.
Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994;91:9886–90.
Pavoine C, Magne S, Sauvadet A, Pecker F. Evidence for a beta2-adrenergic/arachidonic acid pathway in ventricular cardiomyocytes. Regulation by the beta1-adrenergic/camp pathway. J Biol Chem. 1999;274:628–37.
Kumar A, Kosuri R, Kandula P, Dimou C, Allen J, Parrillo JE. Effects of epinephrine and amrinone on contractility and cyclic adenosine monophosphate generation of tumor necrosis factor alpha-exposed cardiac myocytes. Crit Care Med. 1999;27:286–92.
Liu SJ, Zhou W, Kennedy RH. Suppression of beta-adrenergic responsiveness of L-type Ca2+ current by IL-1beta in rat ventricular myocytes. Am J Physiol. 1999;276:H141–8.
De Paepe B, Creus KK, De Bleecker JL. The tumor necrosis factor superfamily of cytokines in the inflammatory myopathies: potential targets for therapy. Clin Dev Immunol. 2012;2012:369432.
Yndestad A, Damas JK, Geir Eiken H, Holm T, Haug T, Simonsen S, et al. Increased gene expression of tumor necrosis factor superfamily ligands in peripheral blood mononuclear cells during chronic heart failure. Cardiovasc Res. 2002;54:175–82.
Loncar G, Bozic B, Cvorovic V, Radojicic Z, Dimkovic S, Markovic N, et al. Relationship between RANKL and neuroendocrine activation in elderly males with heart failure. Endocrine. 2010;37:148–56.
Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, et al. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail. 2010;3:157–64.
Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–305.
Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005;19:1637–45.
Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front Immunol. 2013;4:478.
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–11.
Dibbs ZI, Diwan A, Nemoto S, DeFreitas G, Abdellatif M, Carabello BA, et al. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation. 2003;108:1002–8.
Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, et al. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004;109:262–8.
Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–6.
Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–35.
Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989;86:6753–7.
Chung MK, Gulick TS, Rotondo RE, Schreiner GF, Lange LG. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ Res. 1990;67:753–63.
Muller-Werdan U, Schumann H, Fuchs R, Reithmann C, Loppnow H, Koch S, et al. Tumor necrosis factor alpha (TNF alpha) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(NO)-synthase (iNOS) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–23.
Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–60.
Verhoeckx KC, Doornbos RP, Witkamp RF, van der Greef J, Rodenburg RJ. Beta-adrenergic receptor agonists induce the release of granulocyte chemotactic protein-2, oncostatin M, and vascular endothelial growth factor from macrophages. Int Immunopharmacol. 2006;6:1–7.
Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J Investig Dermatol. 2014;134:809–17.
Roth Flach RJ, Matevossian A, Akie TE, Negrin KA, Paul MT, Czech MP. beta3-Adrenergic receptor stimulation induces E-selectin-mediated adipose tissue inflammation. J Biol Chem. 2013;288:2882–92.
Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101:2103–9.
Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. Beta-adrenergic stimulation induces interleukin-18 expression via beta2-AR, PI3K, Akt, IKK, and NF-kappaB. Biochem Biophys Res Commun. 2004;319:304–11.
Chandrasekar B, Vemula K, Surabhi RM, Li-Weber M, Owen-Schaub LB, Jensen LE, et al. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J Biol Chem. 2004;279:20221–33.
Grisanti LA, Repas AA, Talarico JA, Gold JI, Carter RL, Koch WJ, et al. Temporal and gefitinib-sensitive regulation of cardiac cytokine expression via chronic beta-adrenergic receptor stimulation. Am J Physiol Heart Circ Physiol. 2015;308:H316–30.
Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacol Ther. 2016;164:82–96.
Koch WJ, Lefkowitz RJ, Rockman HA. Functional consequences of altering myocardial adrenergic receptor signaling. Annu Rev Physiol. 2000;62:237–60.
Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–3.
Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–80.
Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, et al. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–17.
Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–7.
White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–33.
Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, et al. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–97.
Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62.
Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–5.
Felten SY, Madden KS, Bellinger DL, Kruszewska B, Moynihan JA, Felten DL. The role of the sympathetic nervous system in the modulation of immune responses. Adv Pharmacol. 1998;42:583–7.
Felten DL, Felten SY, Bellinger DL, Lorton D. Noradrenergic and peptidergic innervation of secondary lymphoid organs: role in experimental rheumatoid arthritis. Eur J Clin Invest. 1992;22(Suppl 1):37–41.
Felten SY, Felten DL, Bellinger DL, Olschowka JA. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1992;52:25–48.
Friedman EM, Irwin MR. Modulation of immune cell function by the autonomic nervous system. Pharmacol Ther. 1997;74:27–38.
Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56.
Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20–7.
Hatfield SM, Petersen BH, DiMicco JA. Beta-adrenergic agonists blocked the expression of IL-2 receptors on mitogen-stimulated lymphocytes and IL-2-dependent T cell lines. J Immunol. 1988;141:1418–20.
Oberbeck R. Catecholamines: physiological immunomodulators during health and illness. Curr Med Chem. 2006;13:1979–89.
Stevens-Felten SY, Bellinger DL. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1997;69:99–131.
Heijnen CJ, Kavelaars A. The importance of being receptive. J Neuroimmunol. 1999;100:197–202.
Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–104.
Sitkauskiene B, Sakalauskas R. The role of beta(2)-adrenergic receptors in inflammation and allergy. Curr Drug Targets Inflamm Allergy. 2005;4:157–62.
Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV. Regulation of beta-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle. 2011;10:3684–91.
Madden KS, Felten SY, Felten DL, Bellinger DL. Sympathetic nervous system--immune system interactions in young and old Fischer 344 rats. Ann N Y Acad Sci. 1995;771:523–34.
Madden KS, Felten DL. Experimental basis for neural-immune interactions. Physiol Rev. 1995;75:77–106.
Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–48.
Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun. 2007;21:736–45.
Link AA, Kino T, Worth JA, JL MG, Crane ML, Chrousos GP, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–42.
Benarroch EE. Autonomic-mediated immunomodulation and potential clinical relevance. Neurology. 2009;73:236–42.
Elenkov I. Neuroendocrine effects on immune system. In: L. J. De Groot, G. Chrousos, K. Dungan, K. R. Feingold, A. Grossman, J. M. Hershman, C. Koch, M. Korbonits, R. McLachlan, M. New, J. Purnell, R. Rebar, F. Singer and A. Vinik, eds. Endotext South Dartmouth (MA); 2000.
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638.
Imrich R, Tibenska E, Koska J, Ksinantova L, Kvetnansky R, Bergendiova-Sedlackova K, et al. Repeated stress-induced stimulation of catecholamine response is not followed by altered immune cell redistribution. Ann N Y Acad Sci. 2004;1018:266–72.
Zhang X, Hartung JE, Bortsov AV, Kim S, O'Buckley SC, Kozlowski J, et al. Sustained stimulation of beta2- and beta3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav Immun. 2018.
Bierhaus A, Humpert PM, Nawroth PP. Linking stress to inflammation. Anesthesiol Clin. 2006;24:325–40.
Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332.
Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–6.
Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–47.
Loudon RP, Perussia B, Benovic JL. Differentially regulated expression of the G-protein-coupled receptor kinases, betaARK and GRK6, during myelomonocytic cell development in vitro. Blood. 1996;88:4547–57.
Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999;13:715–25.
Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, et al. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4400–6.
Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, et al. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–8.
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33.
Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308.
Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–74.
Bianchi K, Meier P. A tangled web of ubiquitin chains: breaking news in TNF-R1 signaling. Mol Cell. 2009;36:736–42.
Al-Lamki RS, Brookes AP, Wang J, Reid MJ, Parameshwar J, Goddard MJ, et al. TNF receptors differentially signal and are differentially expressed and regulated in the human heart. Am J Transpl. 2009;9:2679–96.
Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802.
Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, et al. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–8.
Funding
The work in part is supported by NIH ROO HL132882 and Cleveland Clinic Startup funds (S.M.S) and by NIH RO1 HL089473 and HL128382 (S.V.NP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sarah M. Schumacher and Sathyamangla V. Naga Prasad declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Myocardial Disease
Rights and permissions
About this article
Cite this article
Schumacher, S.M., Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: an Updated Review. Curr Cardiol Rep 20, 117 (2018). https://doi.org/10.1007/s11886-018-1067-7
Published:
DOI: https://doi.org/10.1007/s11886-018-1067-7