Abstract
Recent clinical trials directed at imaging of coronary artery disease (CAD) have demonstrated a paradigm shift away from endpoints related to detection of CAD in favor of those related to clinical outcomes. The objective of such trials has been to determine whether physiological metrics are superior to anatomical ones for guiding therapy and improving outcomes in patients with known or suspected CAD. The present review focuses on selected trials in this area in particular DEFER, FAME 1 and 2, a meta-analysis comparing FFR to anatomically guided treatment outcomes and COURAGE SPECT MPI sub study. The rationale for using physiological as opposed to anatomical endpoints to optimize patient management, in particular coronary revascularization decisions, is emphasized. The results of the FFR-based trials are concordant and indicate physiological metrics are superior to anatomical ones for guiding therapy and improving clinical outcomes in patients with known or suspected CAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Though it has been appreciated for many years that apparent anatomical stenosis severity generally correlates poorly with its physiological severity [1, 2••, 3••], only in the past 10–15 years have the combination of advances in technology (e.g., intra-coronary pressure and velocity sensor-tipped guide wires and more recently thermodilution capable volumetric flow wires [4]) and application of basic physiological principles governing coronary pressure flow relations, previously confined to study in the animal lab, been more routinely adopted for assessment of human subjects in the cardiac catheterization laboratory [5]. The ability to make such measurements, in particular fractional flow reserve (FFR) [6–8], has fostered clinical trials designed to test the hypothesis that the physiological index of stenosis severity would prove superior to anatomical assessment in guiding the decision for coronary revascularization and result in improved clinical outcomes. It is important to note in this regard that PET measurements of absolute myocardial blood flow (MBF) are equally, perhaps better, suited to physiological characterization of coronary stenosis severity and of course do so in noninvasive fashion [8, 9, 10••]. PET measurements of absolute MBF also are superior to PET quantitative measurements of tracer uptake or myocardial flow reserve for coronary stenosis detection [11, 12••, 13, 14••] but do not yet have randomized clinical trial data to demonstrate improved outcomes re: decision making for coronary revascularization. A brief review of pertinent coronary physiology related to FFR and related concepts is important before discussing the results of clinical trials based on this metric and is provided below.
FFR and Related Concepts: a Brief Review
In a simple hydraulic system, the pressure loss across a stenotic segment of pipe (dP) is given by the equation [15, 16]:
Where:
- A:
-
constant related to Pousille resistance
- B:
-
constant related to stenosis geometry
- Q:
-
fluid flow (ml/min) or velocity (cm/s)
What is important to note from the view of understanding FFR is that flow rate contributes heavily to the pressure drop across any given stenosis and absent any stenosis the constant “A” and “B” are nil and thus dP also is zero. FFR is measured under conditions of maximal coronary vasodilation, typically with adenosine though other primary dilators (e.g., dipyridamole, regadenoson, and papaverine) have been used, to null as much as possible impedance to flow distal to the stenosis and thereby assess dP across the stenosis independent of downstream influences. Absent a stenosis, there is no pressure drop across the epicardial, conduit coronary vessels until the microvascular level is reached (~200 μ) [17]. Thus, the ratio of mean aortic to distal coronary pressure is one. In a simple system, interposition of a stenosis between the aortic and conduit segment of a coronary vessel will result in a pressure drop across the stenosis as described by Eq. (1). In practice, it has been shown that the resulting ratio of mean distal coronary to mean aortic pressure provides a functional measure of the hemodynamic severity of the stenosis. The ratio is termed the FFR [6, 7] since the value of the fraction reflects the relative loss of flow reserve attributable to the stenosis in comparison with a normal vessel in which the ratio (distal/aortic pressure) is one. It is understood that a variety of physiological factors are known to impact the ratio [18] and have been considered in detail by others [19]. Suffice it to say, the list is long and includes prevailing hemodynamic conditions, presence of diffuse coronary, or microvascular disease, as well as the presence of serial stenoses in a given vessel [20] (Table 1). Nonetheless, despite known limitations, the simple ratio has proved very useful, as will be reviewed below, in helping to guide clinical decision making regarding coronary revascularization.
Recognition that FFR has its limitations, not least that it is invasive, a ratio susceptible to multiple influences and in the case of multi-vessel CAD may require instrumentation of more than one coronary artery for decision-making purposes, has provided the impetus to find a more comprehensive, direct method for determining coronary vasodilator capacity. PET measurement of absolute MBF provides a non-invasive method for determining maximal MBF in all coronary territories and subterritories simultaneously [2••, 11, 12••, 13, 14••, 21]. Since the myocardium is dependent on augmenting MBF to meet the demands of increased work and since reasonable estimates of that quantity are known [21–23], the argument can and has been made for measuring the real quantity of interest (i.e., maximal MBF) and not a surrogate ratio such as FFR or coronary flow reserve (CFR), which themselves may provide discordant information for reasons discussed in detail by others [24••, 25]. As noted, clinical outcome data for such an approach is still lacking but preliminary work suggesting feasibility will be discussed.
Clinical Trials
-
A)
The DEFER study was the first relatively large-scale, randomized clinical trial designed to compare clinical outcomes following the decision to perform PCI based on anatomical versus functional coronary stenosis severity. The outcomes, defined as a composite of adverse events including all cause mortality, myocardial infarction, CABG, PCI, and CCS angina status were tallied at 2 (primary end point) and 5 years (secondary end point) after patient entry.
The patients enrolled and treated in the study between 1997 and 1998 all had been referred for elective PCI of chronic stable angina and had single-vessel disease, defined by visual analysis as ≥50 % lumen diameter narrowing. QCA of the target lesion also was performed as was FFR measurement. Patients were randomized either to “defer” or “perform” PCI groups (bare metal stents only available at the time) after which they had cardiac catheterization and FFR determination. If FFR was <0.75, then randomization assignment was ignored and PCI performed in what was designated the “reference” group. If FFR was ≥0.75, then PCI either was deferred or performed based on prior randomization assignment. The authors indicated the randomization and treatment protocol adopted was designed to determine if clinical outcomes would be improved by performing PCI of anatomically “intermediate” severity stenoses.
The most important results of the study were as follows. First, performance of PCI on what the authors referred to as non “reversible ischemia” causing lesions (i.e., FFR ≥0.75) failed at 5 years to improve clinical outcomes defined either as (1) event-free survival or (2) combined cardiac mortality and myocardial infarction or (3) percent free of angina. Especially noteworthy was the fact that QCA-determined anatomical stenosis severity overlapped entirely (range ~30–70 % diameter reduction) between the “defer” and “perform” groups and almost completely for the “reference” group as well, which had only a small minority (<10 %) in the 70–90 % range.
Second, and equally important, PCI in the “reference” group (i.e., FFR <0.75) not only failed to prevent cardiac death or acute MI but in fact was associated with a 5× increase incidence of these outcomes compared to medical therapy alone of similar anatomical appearing lesions (~15 vs ~3 %) [27]. The authors opined that use of bare metal stents (BMS) could not explain the results since prior studies had shown that drug-eluting stents (DES) also failed to reduce the incidence of cardiac death or MI (3–5 % in the initial year after placement) [27]. They simply concluded the presence of a functionally significant coronary stenosis even if treated successfully with PCI nonetheless was a marker for increased risk of subsequent cardiac death or MI especially in comparison with medical treatment of non-ischemia-inducing lesions.
-
B)
Both FAME 1 and FAME 2 focused on chronic stable angina patients with multi-vessel CAD who had been referred for clinically indicated cardiac catheterization and consideration of PCI. In FAME 1 [28], patients were randomized to FFR-guided PCI, performed only if FFR ≤0.80, versus angiographic visual assessment only (≥50 % lumen diameter reduction). Clinical outcomes included a composite endpoint of “death, non fatal MI, or repeat revascularization at 1 year” [28]. PCI when performed was with DES. In brief, the combined event rate at 1 year was 18 % in the angiography group and 13 % in the PCI-guided group (P = 0.02). While none of the individual components of the combined event rate differed significantly between groups, likely a function of sample size, the combined rate for death or non-fatal MI was significantly less in the FFR-guided group compared to the angiography only group (7 % versus 11 %, respectively, P = 0.04) [28]. Functional status did not differ between groups at the end of 1 year though fewer DES were used in the FFR group (mean 2 versus 3, P < 0.001) and cost of materials was less ($5.3 versus $6.0 K, P < 0.001). Accordingly, the functional approach to assessment of stenosis severity and decision for PCI again proved superior to that of anatomical assessment alone.
FAME 2 [29••, 30] addressed the issue of whether or not FFR-guided PCI plus best medical therapy could improve clinical outcomes compared to best medical therapy alone in patients with clinically stable CAD. Data from the COURAGE trial [31] indicated in patients with stable angina that initial optimal medical therapy for angiographically significant CAD (≥70 % stenosis), with subsequent PCI if medical therapy failed, was safe and associated with similar event rates as initial PCI (plus optimal medical therapy) based on anatomical stenosis severity [31]. Initial PCI failed to reduce the incidence of death or non-fatal MI compared to medical therapy alone during the course of median follow-up of 4.6 years [31].
Since anatomical stenosis severity was the guiding decision-making tool in COURAGE, the FAME 2 investigators sought to determine if FFR-guided PCI would improve patient outcomes [30]. Thus, patients with stable angina who were being considered for PCI were randomized to a strategy of FFR-guided PCI (FFR ≤0.80) plus best medical therapy versus best medical therapy alone [29••, 30]. All patients had coronary angiography with visual assessment of stenosis severity and designation of potential target lesions at the time of cardiac catheterization. Subsequently, all potential targets had FFR determination, and those assigned to PCI had the procedure performed if FFR ≤0.80. Those assigned to medical therapy only did not. Those with FFR >0.80 of all potential targets were treated medically and assigned to a registry for follow-up purposes. Primary endpoint was a combination of all cause mortality, non-fatal MI, or urgent revascularization during the initial 2 years of follow-up.
The study was stopped early by the DSMB roughly 19 months after beginning enrollment (n = 1230 patients at the time) and an average follow-up in each group of approximately 7 months [30]. The reason for closing entry to the study was a statistically significant excess of urgent revascularization in the medical therapy only group (12.7 % versus 4.3 % PCI group, hazard ratio (PCI versus medical) = 0.32, 95 % CI = 0.19–0.53; P < 0.001). Noteworthy was the fact that (1) event rate in registry patients (~3 %) did not differ significantly versus PCI; (2) the incidence of death or non-fatal MI did not differ between PCI and medical therapy groups; (3) only 1300/1600 (81 %) angiographically significant stenoses (≥50 % lumen dia) were functionally significant by FFR; and (4) the authors noted the clinical outcomes of PCI were “more pronounced” for patients having lesions with FFR <0.65 compared to those having only lesion with “larger FFR “(P = 0.01) [30].
A separate paper reported the results of recruited and registry patients at 2 years of follow-up at which time the major findings of the study were unchanged though exact percentages as would be expected, had [29••]. Thus, the key difference in PCI plus medical therapy versus medical therapy alone remained urgent revascularization (4.0 % versus 16.3 %, hazard ratio 0.23, 95 % CI = 0.14–0.38, P < 0.001). Again, the incidence of death or non-fatal MI did not differ between PCI plus medical therapy and medical therapy only groups. The incidence of the primary combined endpoint in registry patients, however, had increased from ~3 % at the time recruitment was halted to 9 % at the completion of 2-year follow-up. Though not without controversy [32•], primarily because of the nature of the endpoint driving the conclusion in favor of PCI plus optimal medical therapy over medical therapy alone, the outcome, nonetheless, reinforced the notion of the importance of FFR determination in PCI decision making. This is particularly so given the fact that registry patients all had coronary stenoses which appeared angiographically significant but with FFR >0.80 and had primary endpoint risk comparable to that of PCI plus medical therapy (hazard ratio, PCI registry = 0.90, 95 % CI 0.49–1.64, P = 0.72) and clearly superior to that of medical therapy only (hazard ratio, medical therapy registry = 2.34, 95 % CI 1.35–4.05, P = 0.002). Thus, FFR measurement was effective in identifying a relatively low-risk population (FFR >0.80) which did well on medical therapy alone despite the presence of angiographically significant CAD.
-
C)
FFR META ANALYSIS [33•]:
A recent meta-analysis has been reported of 51 studies pertaining to use of FFR versus coronary angiography alone for revascularization decision-making assistance. Patient outcomes (MACE = death, MI, revascularization) were the primary endpoints of the studies which included >9000 (study level) and almost 7000 (patient level) coronary lesions with clinical follow up over a median of roughly 15 months [33•]. The authors recognized a major limitation of the study, even with appropriate statistical adjustment, was “confounding by indication” [33•] since revascularization was included in the definition of MACE, and the studies encompassed a period in which FFR <0.75–0.80 was viewed, in the appropriate clinical circumstances, as an indication for revascularization of the target lesion(s). Other important limitations noted by the authors included incomplete data concerning (1) cause of death (cardiac versus non cardiac), (2) location of MI with respect to target lesion(s) and (3) whether or not revascularization during follow-up involved target or non-target lesions.
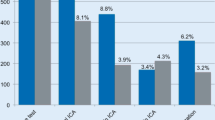
Notwithstanding its limitations, the study provided several interesting observations at least two of which were subsequently confirmed by the FAME 2 follow-up study [29••]. Thus, using a Cox proportional hazard model for 1-year incidence of MACE (patient level data), Figure 3B (from [33]) [33•] demonstrated medical therapy was associated with an inverse curvilinear relationship to FFR, increasing from ~7 %/year at FFR = 1.0 to ~23 %/year at FFR = 0.40. In contrast, revascularization (PCI/CABG) was associated with an almost flat relationship (MACE ~13–17 %/year) across the full range of FFR studied (0.40 to 1.0, Fig. 1). The curves crossed over at FFR = 0.67 [33•], almost precisely the level shown to provide the greatest benefit in terms of clinical outcomes for FAME 2 [30]. The meta-analysis [33•] also was confirmed by the more general finding of FAME 2 that FFR-guided revascularization therapy was associated with improved clinical outcomes compared to medical therapy alone for “ischemia causing lesions” (i.e., FFR <0.80).
Fig. 1 The FFR meta-analysis derived relationship between 1-year MACE and FFR. Note approximate cross over point (FFR ~0.65) where MACE rate for revascularization (REVASC) exceeds that for medical therapy. (Redrawn and modified from Fig 3B with permission; This figure was published in J Am Coll Cardiol, 64 (16), Johnson NP, Toth GG, Lai D, et al., Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes, 1641–1654, Copyright Elsevier [2014]) [33]
Figure 3B (from [33]) [33•], it should be noted, also indicates that revascularization for lesions with FFR >0.80 are likely to be associated with greater incidence of MACE compared with medical therapy alone; a prediction supported at least in part by the DEFER trial [27] in which patients with FFR ≥0.75 who were treated with medical therapy only (“defer”) had comparable overall clinical outcomes to those treated with PCI (“perform”) but a lesser incidence of death or MI (3.3 versus 7.9 %, respectively) which failed to reach statistical significance [27] likely for lack of power. As noted above, in DEFER angiographic stenosis, severity by QCA demonstrated complete overlap of the two groups (i.e., “defer” and “perform”) over the full range encountered (30–70 %) [27]. Similarly, in the meta-analysis, QCA-determined stenosis severity could account for only 31 % of the variance in FFR [33•]. Accordingly, the notion put forward in the meta-analysis that medical therapy very likely will be superior to revascularization at FFR >0.75–0.80 is further supported by results, taken together, of FAME 1 [28] and 2 [29••]. FAME 1 demonstrated improved clinical outcomes for the FFR-guided strategy versus anatomy notwithstanding comparable anatomy in both groups. Similarly, in FAME 2 registry patients (FFR >0.80) treated medically had overall clinical outcomes comparable to those treated with revascularization for functionally significant lesions and superior to that in the medical treatment arm with FFR ≤0.80 despite anatomically comparable stenosis severity.
-
D)
COURAGE SPECT MPI SUB-STUDY FOLLOW-UP [34•]:
This recently reported post hoc analysis of patients (n = 621) enrolled in the COURAGE clinical trial compared baseline reversible ischemic burden as determined by SPECT myocardial perfusion imaging (MPI) with baseline angiographic CAD burden (≥50 % stenosis by QCA) to predict a combined primary clinical endpoint of death, MI, or NSTEMI-ACS. Left ventricular ejection fraction at baseline also was considered as a predictor. The mean duration of follow-up was 4.7 ± 1.7 years.
The results of the analysis may be summarized as follows. Ischemic burden as determined by SPECT MPI failed to emerge as an independent predictor of clinical outcome. In contrast, angiographic burden of disease and LVEF both were found to be independent predictors of clinical outcome.
The authors recognized a number of study limitations including technical ones such as (1) its post hoc nature, (2) small sample size, (3) absence of more detailed CAD burden score (e.g., SYNTAX [35, 36]), (4) lack of physiological data such as FFR, and (5) use of bare metal stents. They also acknowledged their results were discordant with that of prior SPECT MPI studies, which indicate that extent of ischemia is an excellent risk predictor for clinical events such a death and MI [37–40]. The results of the study also are at odds with the data from the PROMISE clinical trial [41] (see below).
-
E)
PROMISE CLINICAL TRIAL [41, 42]:
The recently reported PROMISE trial [41] randomized over 10,000 symptomatic patients who were being evaluated for known or suspected CAD (pre-test probability of CAD ~50 %). An initial strategy of anatomical testing with CT coronary angiography (CTCA) was compared with that of functional testing, which included stand-alone exercise treadmill testing, SPECT MPI, or stress echocardiography. Results of initial testing were then used to guide further clinical decision making related to additional diagnostic and therapeutic interventions. Clinical outcomes, primary endpoint of “death, myocardial infarction, hospitalization for unstable angina, or major procedural complication” [41], were compared after slightly more than 2 years of follow-up.
The results of the study failed to demonstrate superiority of one diagnostic approach over the other. Thus, frequency of the primary endpoint (~3 % over median 2-year follow-up) did not differ significantly between groups [41].
More detailed analysis revealed mixed results. While more patients in the CTCA group went on to invasive coronary angiography (ICA) compared to the functional testing group (12 versus 8 %, respectively), fewer in the CTCA group were found to have “non obstructive” CAD by ICA compared to that of the functional testing (3 versus 4 %, respectively, p = 0.02). Overall, ionizing radiation exposure ultimately was greater in the CTA group versus functional testing since roughly one third of those in the later group was not exposed at all (12 versus 10 mSv, respectively, P < 0.001). Importantly, there was no difference between groups in the combined endpoints either of death or non-fatal MI (hazard ratio—CTA, Functional = 0.88 (0.67–1.05, p = 0.35); or death or non-fatal MI or hospitalization for unstable angina (hazard ratio—CTA functional = 1.04 (0.84–1.31, p = 0.70). The authors concluded, therefore, an initial strategy of CTA failed to improve clinical outcomes compared to that of functional testing in symptomatic patients with intermediate prior probability of CAD.
An accompanying editorial took note of several limitations of the study including (1) the overall low event rate which in turn was dominated by a “soft end-point”, namely, hospitalization for unstable angina, (2) the high rate of appropriate medical therapy including statin use in both groups, which likely contributed to the low overall event rate in each, (3) the fact that more advance imaging modalities such as PET and cardiac MR were not employed for functional testing, and (4) absence of a third cohort treated medically on presentation without further evaluation [42]. Finally, the editorial noted the ISCHEMIA trial, which currently is recruiting patients with baseline moderate to severe ischemia and employing the most up to date PCI revascularization methods, may help answer the question whether outcomes are improved when the results of a positive functional test are used to guide subsequent clinical decision making regarding best medical therapy alone, initially, versus initial ICA and revascularization plus best medical therapy.
Conclusions
A large body of data has emerged in recent years from randomized clinical trials, several of which are discussed above, which clearly indicate physiological assessment of CAD severity results in improved clinical outcomes in comparison with that of decision making based on angiographic severity of disease. To date, these studies have required invasive assessment of physiological severity principally in the form of FFR measurements. While the COURAGE sub-study of SPECT MPI may appear to contradict the notion that function trumps anatomy [34•], the study’s many limitations require considerable caution in accepting that conclusion. This is especially so in face of the large body of data reviewed above indicating the opposite is the case as well as a substantial number of SPECT-MPI studies which also indicate clinical outcomes generally correlate well with extent of reversible ischemia and that a normal SPECT-MPI is associated with a very low risk of adverse cardiac events [38–40]. Further, the recently reported PROMISE trial [41], notwithstanding the known weaknesses of its multiple methods for assessing functional severity of CAD, also does not support the notion that anatomy trumps physiology. Thus, a better noninvasive method (as alluded to in the editorial on the PROMISE trail [42]) is required and presently is potentially widely available in the form of quantitative PET measurements of absolute myocardial blood flow [2••, 10••, 11, 12••, 13, 14••, 21, 43]. A randomized clinical trial focusing on clinical outcomes similar to those reviewed herein but with PET methodology used for physiological assessment of CAD severity has been proposed previously2 and deserves to be performed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2(8):1009–23.
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–53. A comprehensive state of the art paper which critically reviews both invasive and quantitative PET myocardial blood flow methods for assessing coronary stenosis severity. The authors also make the cases for a randomized clinical trial focusing on PET assessment of stenosis severity for guiding decision making for coronary revascularization and resulting clinical outcomes.
Johnson NP, Kirkeeide RL, Gould KL. Coronary anatomy to predict physiology: fundamental limits. Circ Cardiovasc Imaging. 2013;6(5):817–32. An essential, comprehensive review of basic coronary physiology which renders highly problematic efforts to deduce hemodynamic stenosis severity based on apparent angiographic severity.
Aarnoudse W, Van’t Veer M, Pijls NH, Ter Woorst J, Vercauteren S, Tonino P, et al. Direct volumetric blood flow measurement in coronary arteries by thermodilution. J Am Coll Cardiol. 2007;50(24):2294–304.
De Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94(8):1842–9.
Pijls NH, De Bruyne B. Coronary pressure measurement and fractional flow reserve. Heart. 1998;80(6):539–42.
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–8.
Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122(6):603–13.
De Bruyne B, Baudhum T, Melin JA, Pijls NHJ, Sys SU, Bol A, et al. Coronary flow reserve calculated from pressure measurements in humans: validation with positron emission tomography. Circulation. 1994;89:1013–22.
Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5(4):430–40. This study reports important observations linking absolute level of PET determined myocardial blood flow with clinical evidence of myocardial ischemia during dipyridamole stress testing in humans.
Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. JACC Cardiovasc Imaging. 2009;2(6):751–8.
Danad I, Raijmakers PG, Appelman YE, Harms HJ, de Haan S, van den Oever ML, et al. Hybrid imaging using quantitative H215O PET and CT-based coronary angiography for the detection of coronary artery disease. J Nucl Med. 2013;54(1):55–63. An important study confirming earlier work which indicates absolute maximal myocardial blood flow measurement by PET is superior to myocardial flow reserve ratio for detection of hemodynamically significant coronary stenoses.
Kajander SA, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, et al. Clinical value of absolute quantification of myocardial perfusion with 15O-water in coronary artery disease. Circ Cardiovasc Imaging. 2011;4(6):678–84.
Danad I, Uusitalo V, Kero T, Saraste A, Raijmakers PG, Lammertsma AA, et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J Am Coll Cardiol. 2014;64(14):1464–75. This study extends earlier work by this group and others by providing guidelines for interpreting PET measurements of absolute myocardial blood flow for assessment of coronary stenosis hemodynamic severity.
Young DF, Tsai FY. Flow characteristics in models of arterial stenosis. I. Steady flow. J Biomech. 1973;6:396–410.
Young DF, Tsai FY. Flow characteristics in models of arterial stenosis. II, Unsteady flow. J Biomech. 1973;6:547–59.
Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res. 1996;32(4):668–78.
Gewirtz H. Fractional flow reserve. Circulation. 1996;94(9):2306–7.
Siebes M, Chamuleau SA, Meuwissen M, Piek JJ, Spaan JA. Influence of hemodynamic conditions on fractional flow reserve: parametric analysis of underlying model. Am J Physiol Heart Circ Physiol. 2002;283(4):H1462–70.
De Bruyne B, Pijls NH, Heyndrickx GR, Hodeige D, Kirkeeide R, Gould KL. Pressure-derived fractional flow reserve to assess serial epicardial stenoses: theoretical basis and animal validation. Circulation. 2000;101(15):1840–7.
Gewirtz H. PET measurement of adenosine stimulated absolute myocardial blood flow for physiological assessment of the coronary circulation. J Nucl Cardiol. 2012;19(2):347–54.
Skopicki HA, Abraham SA, Picard MH, Alpert NM, Fischman AJ, Gewirtz H. Effects of dobutamine at maximally tolerated dose on myocardial blood flow in humans with ischemic heart disease. Circulation. 1997;96(10):3346–52.
Laaksonen MS, Kalliokoski KK, Luotolahti M, Kemppainen J, Teras M, Kyrolainen H, et al. Myocardial perfusion during exercise in endurance-trained and untrained humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R837–43.
Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012;5(2):193–202. A very helpful review of physiological reasons for discordance between FFR and CFR measurements and underscores important limitations of each as indicators of maximal coronary vasodilator capacity.
van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7(3):301–11.
Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928–34.
Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–11.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24.
De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–17. An important report of the complete 2 year follow up results of FAME 2.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001.
Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16.
Boden WE. Which is more enduring—FAME or COURAGE? N Engl J Med. 2012;367(11):1059–61. An interesting rebuttal editorial from the PI of COURAGE regarding results of FAME 2.
Johnson NP, Toth GG, Lai D, Zhu H, Acar G, Agostoni P, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64(16):1641–54. An interesting meta-analysis of prior clinical trials in which FFR was used as a revascularization guide and clinical outcomes constituted the primary end point.
Mancini GB, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7(2):195–201. An interesting SPECT MPI sub study of the COURAGE clinical trial in which SPECT MPI assessed ischemia failed to predict clinical outcomes whereas angiographic severity of CAD did.
Garg S, Sarno G, Garcia-Garcia HM, Girasis C, Wykrzykowska J, Dawkins KD, et al. A new tool for the risk stratification of patients with complex coronary artery disease: the clinical SYNTAX score. Circ Cardiovasc Interv. 2010;3(4):317–26.
Wykrzykowska JJ, Garg S, Girasis C, de Vries T, Morel MA, van Es GA, et al. Value of the SYNTAX score for risk assessment in the all-comers population of the randomized multicenter LEADERS (Limus Eluted from A Durable versus ERodable Stent coating) trial. J Am Coll Cardiol. 2010;56(4):272–7.
Farzaneh-Far A, Phillips HR, Shaw LK, Starr AZ, Fiuzat M, O’Connor CM, et al. Ischemia change in stable coronary artery disease is an independent predictor of death and myocardial infarction. JACC Cardiovasc Imaging. 2012;5(7):715–24.
Nakazato R, Berman DS, Gransar H, Hyun M, Miranda-Peats R, Kite FC, et al. Prognostic value of quantitative high-speed myocardial perfusion imaging. J Nucl Cardiol. 2012;19(6):1113–23.
Berman DS, Shaw LJ, Hachamovitch R, Friedman JD, Polk DM, Hayes SW, et al. Comparative use of radionuclide stress testing, coronary artery calcium scanning, and noninvasive coronary angiography for diagnostic and prognostic cardiac assessment. Semin Nucl Med. 2007;37(1):2–16.
Berman DS, Shaw LJ, Min JK, Hachamovitch R, Abidov A, Germano G, et al. SPECT/PET myocardial perfusion imaging versus coronary CT angiography in patients with known or suspected CAD. Q J Nucl Med Mol Imaging. 2010;54(2):177–200.
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300.
Kramer CM. Cardiovascular imaging and outcomes—PROMISEs to keep. N Engl J Med. 2015;372(14):1366–7.
Johnson NP, Gould KL. Physiological basis for angina and ST-segment change PET-verified thresholds of quantitative stress myocardial perfusion and coronary flow reserve. JACC Cardiovasc Imaging. 2011;4(9):990–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Henry Gewirtz declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Gewirtz, H. Functional Versus Anatomic Imaging of CAD: Lessons Learned from Recent Clinical Trials. Curr Cardiol Rep 18, 4 (2016). https://doi.org/10.1007/s11886-015-0686-5
Published:
DOI: https://doi.org/10.1007/s11886-015-0686-5