Abstract
Constrictive pericarditis is a disorder of cardiac filling caused by an inelastic pericardium. This treatable cause of heart failure should be considered in all patients with unexplained right heart failure symptoms or signs, especially when the left ventricular ejection fraction is preserved. Diagnosing constrictive pericarditis remains challenging, and the most effective tools are designed to identify its unique pathophysiologic mechanisms: dissociation of intrathoracic and intracardiac pressures and enhanced ventricular interaction. The cornerstone of the diagnostic work-up remains comprehensive echocardiography with Doppler, but cross-sectional imaging and invasive hemodynamic assessment may be necessary in some cases. Cardiac MRI is particularly helpful in identifying those patients who may have inflammatory constriction that would resolve with anti-inflammatory therapy. Complete surgical pericardiectomy remains the only definitive treatment for patients with chronic constriction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Constrictive pericarditis is a disorder of diastolic filling caused by an inelastic pericardium. Correct diagnosis of this treatable cause of heart failure requires understanding of its complex pathophysiology and detection of its unique hemodynamics with echocardiography. Cross-sectional imaging and cardiac catheterization may be required in some cases. The following is a review of the important aspects of diagnosis and management for this challenging disease.
Etiology
Historically, tuberculosis was a dominant cause of constrictive pericarditis in North America, constituting 48 % of cases in a report published in 1962 [1]. Tuberculous constrictive pericarditis remains common in parts of the world with high prevalence of human immunodeficiency virus and the acquired immune deficiency syndrome. In a single-center review of 121 cases over 22 years (1990–2012) in South Africa, constrictive pericarditis was confirmed in 29.8 % and suspected in an additional 61.2 % of cases [2].
Constrictive pericarditis due to tuberculosis is now rare in North America. A review of 135 cases over 10 years (1985–1995) at the Mayo Clinic classified 80 % of cases as being idiopathic or due to prior cardiac surgery, acute pericarditis, or radiation therapy [3]. An increase in frequency of cases due to prior cardiac surgery or radiation therapy was noted in comparison to a historic cohort. The remaining cases were due to rheumatologic disease, infection, malignancy, trauma, asbestosis, or other miscellaneous causes. Other tertiary centers have published similar findings [4–6].
Epidemiology
The prevalence of constrictive pericarditis is unknown. Incidence has been determined only in patients who have had an episode of acute pericarditis and appears to be low. In a prospective study of 500 patients followed for a median of 6 years after an episode of acute pericarditis, 1.8 % developed constrictive pericarditis [7]. The incidence was lower in patients with idiopathic or viral pericarditis when compared to patients with pericarditis due to connective tissue disease, pericardial injury syndrome, neoplasm, or bacterial infection.
Pathology and Pathophysiology
Typically, the pericardium is fibrotic, calcified, and thickened in constrictive pericarditis. However, pericardial thickening is not required for the diagnosis and the thickness of the surgically removed pericardium has been reported to be normal in 18 % of cases [8]. In some cases, the dominant pericardial abnormality is inflammation, which has important treatment implications.
Constrictive pericarditis may develop acutely within days or chronically over years. Cardiac volume is limited by the inelastic pericardium, and ventricular diastolic filling is progressively restricted, leading to elevation and equalization of filling pressures in all cardiac chambers. Elevated atrial pressures drive rapid early diastolic ventricular filling, which then abruptly stops as volumes reach the limit imposed by the pericardium. Venous pressure increases, while stroke volume and cardiac output decrease.
The right and left heart chambers interact abnormally within the constrained cardiac space, with filling of one side at the expense of the other. This “enhanced” ventricular interaction is most evident during the respiratory cycle because the diseased pericardium insulates the cardiac chambers from changes in intrathoracic pressure. Therefore, intrathoracic and intracardiac pressures become relatively “dissociated.” Inspiration causes a decrease in intrathoracic and pulmonary venous pressures, while the left heart chamber pressures are affected less, if at all. The pressure gradient for left heart filling is therefore reduced and leads to lower diastolic filling and subsequent stroke volume. The reduction in left heart filling leads to a shift in the position of the interventricular septum toward the left ventricle and allows increased inspiratory right heart filling. Expiration reverses these changes and leads to increased left heart filling, a shift in the position of the interventricular septum back toward the right ventricle, and decreased right heart filling. The principles of dissociated intrathoracic and intracardiac pressures and enhanced ventricular interaction were originally described by Hatle et al. and underlie the most important diagnostic tests for constrictive pericarditis [9].
In some cases of subacute constrictive pericarditis, a pericardial effusion may be present along with fibrous contracture of the visceral pericardium. This has been termed effusive-constrictive pericarditis and may be recognized by the persistence of elevated central venous pressure and constrictive hemodynamics despite the removal of pericardial fluid.
Clinical Manifestations
Approximately two thirds of patients with constrictive pericarditis present in heart failure, with dyspnea on exertion and edema being the most commonly reported symptoms [3]. The remainder may present with chest discomfort, fatigue, abdominal symptoms, tamponade, an atrial arrhythmia, or frank liver disease.
The most important aspect of the physical examination is assessment of the jugular venous pressure and contour. Nearly all patients with constrictive pericarditis have elevated jugular venous pressure. Exceptions may include those with early or mild constriction and also those with “occult” constriction in the setting of hypovolemia who require volume loading to reveal constrictive hemodynamics [10]. In addition to being elevated, the jugular venous pressure waveform in constrictive pericarditis is notable for a steep, deep y-descent that signifies rapid but brief early diastolic ventricular filling. An increase in venous pressure with inspiration (Kussmaul sign) may also be present. Although pulmonary edema is not common, a pleural effusion is often present. Cardiac auscultation may reveal an early diastolic sound (pericardial knock) due to the abrupt termination of ventricular diastolic filling; this sound corresponds to the nadir of the y-descent, which is slightly earlier than a typical third heart sound (S3). Significant variation in the systolic blood pressure with respiration (pulsus paradoxus) may be noted in constrictive pericarditis and is due to the dissociation of intrathoracic and intracardiac pressures and enhanced ventricular interaction described previously. The abdominal examination may reveal a pulsatile, enlarged liver and ascites. In advanced cases of constrictive pericarditis, cachexia may also be noted.
Diagnosis
Constrictive pericarditis may mimic other causes of predominantly right-sided heart failure signs and symptoms, which makes the diagnosis challenging. Many patients are misdiagnosed for an extended period of time with liver disease, idiopathic pleural effusion, or unexplained ankle swelling. Constriction should be considered in all patients presenting with unexplained jugular venous pressure elevation, particularly in the setting of prior cardiac surgery or chest radiation. A comprehensive diagnostic work-up is required to correctly identify constrictive pericarditis and exclude conditions that present similarly, such as restrictive cardiomyopathy. Evaluation should begin with an electrocardiogram (which usually shows nonspecific findings), chest radiograph, and echocardiogram.
Chest Radiography
Pericardial calcification on a chest radiograph strongly suggests constrictive pericarditis in the appropriate clinical setting, but is seen in only 27 % of cases involving surgically proven constriction [11]. Calcification of the pericardium suggests chronicity and appears to be most common in cases of idiopathic constrictive pericarditis. Chest radiography may therefore be helpful, but a definitive diagnosis of constrictive pericarditis should be based on detection of the characteristic hemodynamic features of constriction.
Comprehensive Echocardiography with Doppler Assessment
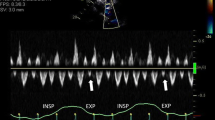
A standard echocardiographic examination allows exclusion of other more common causes of heart failure, such as right or left ventricular systolic dysfunction, valvular disease, or pulmonary hypertension. Clues to the presence of constrictive pericarditis may be present, but a dedicated “constriction-protocol” echocardiogram with simultaneous recording of respiration is usually necessary to confirm constrictive pericarditis and exclude restrictive cardiomyopathy. The examination should focus on the motion of the ventricular septum, variation in the mitral inflow velocity, variation in the hepatic vein profile, and tissue Doppler assessment of mitral annular velocities (Fig. 1).
Principal echocardiographic findings in surgically confirmed constrictive pericarditis. a Mid-ventricular septal M-mode recording (parasternal long axis). Note leftward ventricular septal shift in inspiration. b Pulsed-wave Doppler recording (apical window) at the level of the open mitral leaflet tips. Note inspiratory decrease and expiratory increase in early (E) inflow velocity. c Medial and d lateral mitral annular tissue Doppler recordings (apical window). Arrows mark e’ velocities. Note normal to increased early relaxation velocity (e’), with medial velocity greater than lateral. e Pulsed-wave Doppler recording (subcostal window) within the hepatic vein. Note prominent diastolic flow reversals in expiration, with the reversal ratio defined as reversal velocity divided by forward velocity. Arrows mark forward and reversal velocities in expiration. Insp inspiration, Exp expiration. (Adapted with permission from [12••])
Respiration-related variation in ventricular septal motion, mitral inflow velocity, and hepatic vein reversals all arise from the dissociation of intrathoracic and intracardiac pressure changes and enhanced ventricular interaction in constrictive pericarditis. Inspiration reduces intrathoracic pressure, and normally, this pressure decrease is fully transmitted to the cardiac chambers. In constriction, however, the intracardiac pressure falls much less than intrathoracic pressure because of the pericardial barrier. This difference in pressure change with inspiration results in reduced filling of the left ventricle. The reduction in left heart filling during inspiration causes a reduction in mitral inflow velocity and a shift of the interventricular septum toward the left ventricle. The increase in left heart filling during expiration increases the mitral inflow velocity and shifts the interventricular septum back toward the right ventricle, leading to a late diastolic reversal of flow in the hepatic veins.
Because filling pressures are elevated in constrictive pericarditis, the ratio of early (E) to late (A) trans-mitral filling velocities is usually pseudo-normal or restrictive (E/A > 0.8) [13]. The use of Doppler to measure tissue velocity provides additional non-invasive evaluation of diastolic function. The early diastolic mitral annular relaxation velocity (e’) is a measure of left ventricular myocardial relaxation and is reduced in most forms of heart failure related to myocardial disease, including restrictive cardiomyopathy. In contrast, e’ is usually preserved or even increased in constrictive pericarditis. The medial mitral annular e’ velocity is usually greater than the lateral mitral annular e’. This again stands in contrast to what is expected in other forms of heart failure and may reflect tethering of the lateral annulus by the constrictive process (“annulus reversus”).
Our group studied the test performance characteristics of these echocardiographic findings in a group of 130 patients with surgically confirmed constrictive pericarditis compared to 36 patients with restrictive cardiomyopathy or severe tricuspid regurgitation [12••]. Three variables were independently associated with constrictive pericarditis: (1) the presence of ventricular septal shift, (2) medial mitral e’ velocity, and (3) the hepatic vein expiratory diastolic reversal ratio. Each of these criteria was also significantly associated with constrictive pericarditis in the subset of patients with atrial fibrillation or flutter. The presence of ventricular septal shift in combination with either medial e’ ≥ 9 cm/s or hepatic vein expiratory diastolic reversal ratio ≥ 0.79 was 87 % sensitive and 91 % specific for the diagnosis of constrictive pericarditis. These echocardiographic findings have been proposed as the Mayo Clinic criteria for the echocardiographic diagnosis of constrictive pericarditis. A suggested diagnostic algorithm is presented in Fig. 2.
Suggested diagnostic algorithm for the echocardiographic diagnosis of constrictive pericarditis. A late mitral inflow velocity, E early mitral inflow velocity, e’ early diastolic mitral annular relaxation velocity, SVC superior vena cava. (Adapted with permission from [14])
Two other echocardiographic findings are expected in constrictive pericarditis as well as in restrictive cardiomyopathy. The first is a plethoric inferior vena cava, which may appear dilated or collapse insufficiently during inspiration. This is the echocardiographic marker for increased venous pressure. The second is a relatively “flat” Doppler profile in the superior vena cava. In contrast to normal patients and those with obstructive lung physiology, patients with constrictive pericarditis have restricted cardiac filling and exhibit little variation in the superior vena caval inflow velocity during the respiratory cycle. This finding is clinically useful because severe obstructive lung disease or other conditions associated with exaggerated respiratory effort may sometimes cause echocardiographic findings that mimic those of constrictive pericarditis [15].
Several other echocardiographic findings may be helpful when present. First, distortion of the left or right ventricular contour by a constrictive pericardium is a highly specific finding found in approximately one third of patients with constrictive pericarditis [12••]. Second, in a majority of patients with constrictive pericarditis, the right ventricular free wall appears to be “tethered” at the interface with the liver, rather than exhibiting the expected independent “sliding” motion during the cardiac cycle. However, this finding is insufficiently specific for the diagnosis of constrictive pericarditis. Third, there is a growing evidence base for myocardial strain imaging as a reliable means for identifying constrictive pericarditis, and this will likely be a useful addition to “constriction-protocol” echocardiographic studies in the future [16].
In many cases, a diagnosis of constrictive pericarditis may be established on the basis of clinical history, physical examination, chest radiograph, and echocardiogram. Additional studies may, however, be necessary to confirm the diagnosis and help determine appropriate treatment. Options include computed tomography (CT) scanning, cardiac magnetic resonance imaging (MRI), and invasive hemodynamic assessment.
CT Scanning
CT scanning provides a more accurate assessment of pericardial thickness and calcification than is possible by chest radiography or echocardiography, although it should be remembered that neither thickening nor calcification is necessary for a diagnosis of constrictive pericarditis. In addition, CT scanning may reveal abnormalities in ventricular contour caused by a constrictive pericardium. Defining the relationship of the pericardium to the coronary arteries and other thoracic structures may also be helpful in determining candidacy for and risk of surgical pericardiectomy, particularly in patients who have undergone prior cardiothoracic surgery [17••].
Cardiac MRI
Gated cardiac MRI offers anatomic detail, hemodynamic information, and an assessment of pericardial inflammation and is therefore considered to be very helpful in the work-up and management of patients suspected to have constrictive pericarditis. MRI allows detection of pericardial thickening and the presence of pericardial fluid. Free-breathing sequences may demonstrate the abnormal ventricular septal motion and respiration-related variation in mitral inflow that may be seen on the echocardiogram [18]. Perhaps most importantly, cardiac MRI may reveal delayed gadolinium enhancement of the pericardium, which suggests inflammation and the possibility of reversal with anti-inflammatory therapy [19, 20].
Invasive Hemodynamic Assessment
Cardiac catheterization with hemodynamic assessment is still considered the “gold standard” for the diagnosis of constrictive pericarditis and may be necessary when the non-invasive evaluation is indeterminate. “Classic” hemodynamic features of constrictive pericarditis include increased central venous pressure, near-equalization of right and left heart filling pressures, modest elevation in right ventricular systolic pressure (<50 mmHg), and a right ventricular end-diastolic pressure that is at least one third of the right ventricular systolic pressure. These features, however, may be seen in restrictive cardiomyopathy and are therefore not sufficiently specific for constrictive pericarditis [21]. The “modern” approach to the hemodynamic diagnosis of constrictive pericarditis is based on the principle of enhanced ventricular interaction previously discussed. This interaction or interdependence of the ventricles may be detected by simultaneous evaluation of the right and left ventricular systolic pressure waveforms, which vary discordantly during the respiratory cycle, as opposed to the concordant variation expected in conditions other than constrictive pericarditis. An increased ratio of right ventricular to left ventricular systolic area in inspiration versus expiration (systolic area index) has been shown to have a sensitivity of 97 % and a predictive accuracy of 100 % for the identification of patients with surgically proven constrictive pericarditis [22••]. Simultaneous evaluation of the pulmonary capillary wedge pressure and left ventricular diastolic pressure waveforms also allows detection of the dissociated intrathoracic and intracardiac pressures expected in constrictive pericarditis, as previously discussed.
Differentiating Constrictive Pericarditis from Restrictive Cardiomyopathy and Tamponade
Constrictive pericarditis and restrictive cardiomyopathy may be indistinguishable on initial presentation. The initial diagnostic step after a comprehensive history and physical examination should be a dedicated “constriction-protocol” echocardiogram with simultaneous recording of respiration. If constrictive pericarditis is present, there should be echocardiographic evidence of dissociated intrathoracic and intracardiac pressures and enhanced ventricular interaction, as well as higher-than-expected mitral annular diastolic relaxation velocities. None of these findings would be expected in the setting of restrictive cardiomyopathy. If the echocardiogram is indeterminate, additional investigation is required. This may involve cross-sectional imaging with CT or MRI, or a “gold-standard” invasive hemodynamic assessment. Measurement of the plasma brain natriuretic peptide level may be helpful, as it tends to be more elevated in restrictive cardiomyopathy, and may sometimes be low in constrictive pericarditis [23]. Even after all of these diagnostic steps, the diagnosis sometimes remains in question and a risk-benefit discussion is required to determine whether empiric intervention should be pursued. This diagnostic ambiguity is more common in patients who may have elements of both constrictive pericarditis and restrictive cardiomyopathy, a situation that may arise in the setting of prior chest radiation.
Differentiating constrictive pericarditis from pericardial tamponade can usually be accomplished based on the clinical history, examination, and echocardiogram. A pericardial effusion is required for the diagnosis of pericardial tamponade, but is observed in only 10 % of patients with constrictive pericarditis [12••]. The diastolic filling abnormalities also differ between the two disorders. In severe tamponade, cardiac filling is impaired throughout diastole due to extrinsic compression of the cardiac chambers by pericardial fluid under pressure. In contrast, a brief period of rapid diastolic filling is allowed in constrictive pericarditis. Accordingly, the jugular venous and right atrial pressure waveforms demonstrate blunting of the y-descent in pericardial tamponade, rather than the deep, steep y-descent expected in constrictive pericarditis. Echocardiographic imaging may also reveal diastolic chamber compression in severe pericardial tamponade.
Treatment
Transient Constrictive Pericarditis
Some cases of inflammatory subacute constrictive pericarditis may be transient and resolve spontaneously or with anti-inflammatory treatment. In a review of 212 cases of constrictive pericarditis, 36 (17 %) resolved spontaneously an average of 8.3 weeks after diagnosis [24]. These cases of transient constrictive pericarditis occurred most commonly after cardiac surgery; the remainder were idiopathic or due to infection, trauma, or malignancy. Treatment consisted of non-steroidal anti-inflammatory drugs or steroid therapy in most patients. To identify patients who may respond to such therapy, assessment of inflammatory markers and cardiac MRI may be helpful [19]. A greater degree of late gadolinium enhancement of the pericardium appears to suggest greater potential for reversibility with anti-inflammatory treatment. In stable patients without long-standing symptoms who have clinical features suggestive of transient constriction, a 2- to 3-month trial of anti-inflammatory therapy is therefore appropriate.
Chronic Constrictive Pericarditis
In most cases, the constrictive process is chronic and permanent, and progressive decline is expected. Diuretic therapy may palliate symptoms, but the only definitive treatment is complete surgical pericardiectomy. Outcomes are likely to be best in high-volume centers with expertise in pericardiectomy, as the procedure is challenging and constrictive pericarditis may recur if pericardial removal is incomplete. In such centers, pericardiectomy has an average perioperative mortality rate of 6 %, but varies significantly with etiology and patient characteristics [3, 4]. Patients with idiopathic constrictive pericarditis have the best outcomes, with reported 88 % survival at 7 years. Patients with constrictive pericarditis due to chest radiation have markedly poorer outcomes, with only approximately one third of patients surviving to 7 years. These disappointing results in patients with radiation-induced constrictive pericarditis are likely due to widespread damaging effects of radiation on the myocardium, coronary arteries, and cardiac valves, as well as radiation-induced lung disease. Other clinical characteristics that worsen the prognosis include advanced New York Heart Association functional class, older age, impaired renal function, pulmonary hypertension, and decreased left ventricular ejection fraction. Those patients who do reach long-term survival after pericardiectomy appear to demonstrate sustained benefit from the procedure, with over 80 % free of clinical symptoms.
Conclusions
Constrictive pericarditis should be considered in all patients with unexplained right heart failure symptoms or signs, especially when the left ventricular ejection fraction is preserved. Diagnosis remains challenging, and the most effective tools are designed to identify the unique pathophysiologic mechanisms underlying constrictive pericarditis: dissociation of intrathoracic and intracardiac pressures and enhanced ventricular interaction. The cornerstone of the diagnostic work-up remains comprehensive echocardiography with Doppler, but cross-sectional imaging and invasive hemodynamic assessment may be necessary in some cases. Cardiac MRI is particularly helpful in identifying those patients who may have inflammatory constriction that would resolve with anti-inflammatory therapy. Complete surgical pericardiectomy remains the only definitive treatment for patients with chronic constriction.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Robertson R, Arnold CR. Constrictive pericarditis with particular reference to etiology. Circulation. 1962;26:525–9.
Mutyaba AK, Balkaran S, Cloete R et al. Constrictive pericarditis requiring pericardiectomy at Groote Schuur Hospital, Cape Town, South Africa: causes and perioperative outcomes in the HIV era (1990–2012). J Thorac Cardiovasc Surg 2014.
Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380–6.
Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445–52.
Szabo G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardio-Thoracic Surg: Off J Eur Assoc Cardio-Thoracic Surg. 2013;44:1023–8. discussion 1028.
George TJ, Arnaoutakis GJ, Beaty CA, Kilic A, Baumgartner WA, Conte JV. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012;94:445–51.
Imazio M, Brucato A, Maestroni S, et al. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124:1270–5.
Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852–7.
Hatle LK, Appleton CP, Popp RL. Differentiation of constrictive pericarditis and restrictive cardiomyopathy by Doppler echocardiography. Circulation. 1989;79:357–70.
Bush CA, Stang JM, Wooley CF, Kilman JW. Occult constrictive pericardial disease. Diagnosis by rapid volume expansion and correction by pericardiectomy. Circulation. 1977;56:924–30.
Ling LH, Oh JK, Breen JF, et al. Calcific constrictive pericarditis: is it still with us? Ann Intern Med. 2000;132:444–50.
Welch TD, Ling LH, Espinosa RE, et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovas Imaging. 2014;7:526–34. This study provides a blinded assessment of modern echocardiographic criteria for constrictive pericarditis.
Oh JK, Hatle LK, Seward JB, et al. Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol. 1994;23:154–62.
Syed FF, Schaff HV, Oh JK. Constrictive pericarditis—a curable diastolic heart failure. Nat Rev Cardiol. 2014;11:530–44.
Boonyaratavej S, Oh JK, Tajik AJ, Appleton CP, Seward JB. Comparison of mitral inflow and superior vena cava Doppler velocities in chronic obstructive pulmonary disease and constrictive pericarditis. J Am Coll Cardiol. 1998;32:2043–8.
Kusunose K, Dahiya A, Popovic ZB, et al. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovas Imaging. 2013;6:399–406.
Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26:965–1012 e15. This review summarizes the use of multi-modality imaging in constrictive pericarditis.
Thavendiranathan P, Verhaert D, Walls MC, et al. Simultaneous right and left heart real-time, free-breathing CMR flow quantification identifies constrictive physiology. J Am Coll Cardiol Img. 2012;5:15–24.
Feng D, Glockner J, Kim K, et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: a pilot study. Circulation. 2011;124:1830–7.
Zurick AO, Bolen MA, Kwon DH, et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. J Am Coll Cardiol Img. 2011;4:1180–91.
Vaitkus PT, Kussmaul WG. Constrictive pericarditis versus restrictive cardiomyopathy: a reappraisal and update of diagnostic criteria. Am Heart J. 1991;122:1431–41.
Talreja DR, Nishimura RA, Oh JK, Holmes DR. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol. 2008;51:315–9. This study defined the modern invasive hemodynamic criteria for constrictive pericarditis.
Babuin L, Alegria JR, Oh JK, Nishimura RA, Jaffe AS. Brain natriuretic peptide levels in constrictive pericarditis and restrictive cardiomyopathy. J Am Coll Cardiol. 2006;47:1489–91.
Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271–5.
Compliance with Ethics Guidelines
Conflict of Interest
Terrence D. Welch and Jae K. Oh declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pericardial Disease
Rights and permissions
About this article
Cite this article
Welch, T.D., Oh, J.K. Constrictive Pericarditis: Old Disease, New Approaches. Curr Cardiol Rep 17, 20 (2015). https://doi.org/10.1007/s11886-015-0576-x
Published:
DOI: https://doi.org/10.1007/s11886-015-0576-x