Abstract
Purpose of Review
Current guidelines for primary and secondary prevention of cardiovascular events in adults up to age 75 years are well-established. However, recommendations for lipid-lowering therapies (LLT), particularly for primary prevention, are inconclusive after age 75. In this review, we focus on adults ≥ 75 years to assess low-density lipoprotein-cholesterol (LDL-C) as a marker for predicting atherosclerotic cardiovascular disease (ASCVD) risk, review risk assessment tools, highlight guidelines for LLT, and discuss benefits, risks, and deprescribing strategies.
Recent Findings
The relationship between LDL-C and all-cause mortality and cardiovascular outcomes in older adults is complex and confounded. Current ASCVD risk estimators heavily depend on age and lack geriatric-specific variables. Emerging tools may reclassify individuals based on biologic rather than chronologic age, with coronary artery calcium scores gaining popularity. After initiating LLT for primary or secondary prevention, target LDL-C levels for older adults are lacking, and non-statin therapy thresholds remain unknown, relying on evidence from younger populations. Shared decision-making is crucial, considering therapy's time to benefit, life expectancy, adverse events, and geriatric syndromes. Deprescribing is recommended in end-of-life care but remains unclear in fit or frail older adults.
Summary
After an ASCVD event, LLT is appropriate for most older adults, and deprescribing can be considered for those approaching the last months of life. Ongoing trials will guide statin prescription and deprescribing among older adults free of ASCVD. In the interim, for adults ≥ 75 years without a limited life expectancy who are free of ASCVD, an LLT approach that includes both lifestyle and medications, specifically statins, may be considered after shared decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The population aged 80 and older is rapidly growing, expected to reach 426 million worldwide by 2050 [1]. Older adults experience high rates of atherosclerotic cardiovascular disease (ASCVD) [2], with as many as 1/3 men and 1/5 women experiencing coronary artery disease (CAD) and more than 1/10 men and women experiencing strokes [3]. In the context of geriatric syndromes, ASCVD in older adults often results in reduced quality of life, financial burden, functional decline, polypharmacy, hospitalizations, and mortality [2, 4].
Lipid-lowering therapies (LLT) are effective for secondary ASCVD prevention, but uncertainty remains for benefits in primary prevention in older adults [5]. Factors such as time to benefit, limited life expectancy, competing mortality risks, concerns for adverse effects, polypharmacy, drug interactions, and prevalent geriatric syndromes such as frailty and cognitive impairment complicate decision-making [6,7,8]. Furthermore, a scarcity of age-specific evidence contributes to weak recommendations for LLT in national guidelines for primary prevention [9, 10] and corresponding low use of statins for primary prevention among older adults [11,12,13], particularly women, underweight individuals, and those with heart failure and dementia [14,15,16].
In this review we 1) assess the utility of low-density lipoprotein-cholesterol (LDL-C) as a marker for predicting ASCVD risk in older adults; 2) review existing ASCVD risk assessment tools in the context of the geriatric population, 3) highlight current guidelines and recommendations for LLT, with a focus on primary prevention, and 4) discuss benefits and risks of LLT (statin and non-statin therapies) in older adults, including strategies for deprescribing.
Primary Prevention of ASCVD in Older Adults
LDL-C as a Marker for Future ASCVD
For primary prevention, the association between LDL-C levels, cardiovascular events and all-cause mortality among older adults has been mixed [17,18,19,20]. In a systematic review of cohort studies with 68,094 participants, LDL-C was inversely associated with all-cause mortality among adults ≥ 60 years, with a U-shaped association with cardiovascular mortality, suggesting low levels of LDL-C may be harmful [21]. A US cohort of 2,667 adults ≥ 75 years free of ASCVD reported no association between LDL-C and ASCVD, even among those with risk factors such as smoking, diabetes, and hypertension [22]. On the other hand, the Copenhagen General Population study included 91,131 individuals followed for a median 7-year period. Among those without prior LLT, CVD, or diabetes, each 1 mmol/L increase in LDL-C was associated with an elevated risk of myocardial infarction (MI) (HR 1.34, 95% CI 1.27–1.41), even among those aged 70–100 years. Moreover, among those aged 70–100, moderate-intensity statins provided the greatest absolute risk reduction in ASCVD events and the lowest number needed to treat [23].
The paradox of low LDL-C and increased mortality in older adults could reflect changes in cholesterol metabolism, terminal decline, catabolic states, subclinical disease markers, or confounding conditions such as frailty and malnutrition [22, 24]. However, the lack of association in some studies could reflect a survivor effect. Long-term observational studies are needed to better understand LDL-C as a risk factor for ASCVD in older adults.
Life-expectancy and Biological Aging
Differentiating between chronologic age (time elapsed since birth) and biologic age (physiologic age) by incorporating geriatric assessments (frailty, cognition, function, mental health, multimorbidity, etc.) allows for better phenotypic differentiation of older adults [25]. For example, median survival for a non-frail ≥ 85-year-old is 7.4 years while a 66 year old with severe frailty has an estimated survival of 4.6 years, highlighting the importance of refining life expectancy beyond chronologic age alone, particularly when considering time to benefit from a given therapy [26]. For primary prevention, at least up to age 75 years, 2.5 years are needed to prevent one MACE for every 100 patients treated with a statin [27]. Online tools, such as ePrognosis.com, can assist clinicians in estimating time to benefit from treatment to aid decision-making [28].
Considering life expectancy and geriatric syndromes during shared decision-making may refine statin selection for primary prevention. This would ensure patients have sufficient time to accrue benefits while minimizing potential risks in those with limited life expectancy or advanced geriatric conditions.
Risk Score Tools & their Flexibility
Multiple ASCVD risk scores exist, highlighted in Table 1. All current risk scores heavily weight age, making them less relevant for stratifying risk at older ages. Tools such as PREVENT in the US have a maximum age of 79, while the UK QRISK3 has a maximal age of 84 [29]. The SCORE2-OP score was developed in Europe for adults ≥ 70 years and demonstrated improved accuracy stratifying risk in older adults, though it also heavily weights chronologic age [30]. Non-standard risk factors such as carotid intima-media thickness, malignancy, albuminuria, or education level have been considered with some improvement in prediction [31]. Biomarkers such as high-sensitivity C-reactive protein, only incrementally improve CVD risk prediction [32,33,34]. To date, geriatric domains, such as frailty and cognitive function, have not been incorporated into the existing risk scores despite evidence that frailty is a modifiable risk factor [35]. Future research must consider these factors to help stratify health outcomes in older adults.
Coronary Artery Calcium Score
Coronary artery calcium (CAC) scores can re-stratify ASCVD risk among adults up to age 80 with a low burden of risk factors for whom the benefit of LLT is unclear [36]. While subclinical ASCVD rises with age, the Multi-Ethnic Study of Atherosclerosis found that 16% of adults ≥ 75 years had a CAC score of zero, indicating very low ASCVD risk [39]. In an analysis of 3 pooled US population-based studies including 1,478 participants (mean age 70), adults with a CAC score of zero had a 90% probability of remaining ASCVD event–free over 12 years [40]. Notably, risk is associated with coronary calcium burden; for example, in 1,795 individuals without pre-existing ASCVD (mean age 71), the relative risk of coronary events was 3.1 (95% CI, 1.2 to 7.9) for CAC scores 101–400, 4.6 (95% CI, 1.8 to 11.8) for CAC scores 401–1000, and 8.3 (95% CI, 3.3 to 21.1) for CAC scores > 1000 compared to CAC scores of 0–100 [41]. Additionally, among 2,290 participants in the Atherosclerosis Risk in Communities study aged ≥ 75 years free of ASCVD, CAC ≥ 1000 was associated with an increased risk of impaired physical function, dementia, and hearing loss compared to those with CAC scores of zero [42]. Finally, among 13,644 adults without ASCVD or malignancy followed for 9 years, those with CAC scores of 0 taking statins did not have lower MACE risk (HR: 1.00; 95% CI: 0.79–1.27) compared to no statin [43]. The ongoing CAC-PREVENTABLE (Pragmatic Evaluation of Events And Benefits of Lipid-lowering in Older Adults) study will evaluate the role of CAC in adults aged 75 and older, free of clinical ASCVD to guide statin recommendations [44].
Lipid Lowering Therapy for Primary Prevention in Older Adults
Lifestyle interventions remain the first line strategy, followed by lipid-lowering therapies, including statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and bempedoic acid (Table 2).
Up to age 75, the ACC/AHA guidelines report clear LDL-C targets following LLT for primary prevention, with an approach to intensification including adding non-statin therapy. For adults ≥ 75 years, the guidelines recommend an approach of shared decision-making, highlighting possible benefits while balancing the risk of adverse events and incorporating comorbidities and life expectancy. For adults ≥ 75 years at high or very high cardiovascular risk, ESC/EAS recommends statin use be individualized through shared decision-making, without specific LDL-C cut-offs. Even among older adults with diabetes, the 2023 American Diabetes Association relies on functional and cognitive status when considering LLT for primary prevention [56]. Below, we review the data for these strategies in adults aged ≥ 75 years, recognizing that few have been included in randomized controlled trials.
Lifestyle Interventions
Comprehensive lifestyle modifications, before introducing LLT medications are the cornerstone of primary ASCVD prevention. However, evidence for primary prevention is limited among older adults, especially for those ≥ 75 years. Nevertheless, lifestyle interventions include diet, physical activity, weight management, moderate alcohol intake, and smoking cessation [9, 36]. Dietary interventions demonstrate a consistent pattern of improved cardiovascular outcomes, emphasizing reduced saturated and trans fats and increased fiber intake through fruits, vegetables, whole grains, foods rich in phytosterols, and 2–3 portions of fish per week [9]. The 2021 AHA Scientific Statement notes that DASH/DASH-style diets are particularly effective for LDL-C reduction [57]. Exercise and weight loss are also recommended, targeting 30 min/day or ≥ 150 min/week of moderate-intensity or 75 min/week of vigorous-intensity physical activity. A 2023 meta-analysis of 20 randomized trials demonstrated significant reductions in total cholesterol, triglycerides, and LDL-C with aerobic and resistance exercise in older adults, with combined training offering the most significant LDL-C reduction [57, 58].
Statins
Evidence for Statins
Evidence for statins for primary ASCVD prevention for adults ≥ 75 years is sparse. In a 2019 meta-analysis from the Cholesterol Treatment Trialists' Collaboration, only 8% of the 186,854 participants across 28 trials were ≥ 75 years at randomization. The overall risk reduction among statin users (both primary and secondary prevention) and the effects on MACE per 1 mmol/L reduction in LDL-C was non-significant for those ≥ 75 years in the primary prevention subgroup (0.92, 95% CI [0.73–1.16]), reflecting the small sample size included [59].
To date, six randomized controlled trials included adults ≥ 75 to test the effect of statins on ASCVD outcomes for primary prevention (Table 2).
-
1.
Medical Research Council/British Heart Foundation (MRC/BHF) Heart Protection Study (2002) randomized 20,546 participants with and without ASCVD to 40 mg simvastatin vs placebo; 5,806 were ≥ 70 years old. There was a significant reduction in all-cause mortality (12.9% vs. 14.7%), CHD death (5.7% vs. 6.9%), MI or coronary death (8.7% vs. 11.8%), first occurrence of any major vascular events (19.8% vs. 25.2%), and stroke rates [45]. However, this study did not report subgroups by age or history of ASCVD.
-
2.
PROSPER (A Prospective Study of Pravastatin in the Elderly at Risk) (2002) enrolled 5,804 adults aged 70–82 years, with and without ASCVD, randomized to pravastatin 40 mg daily or a placebo. There was a significant reduction in the combined end-points of CHD death, MI, and CVA (Hazard Ratio (HR): 0.85, 95% CI 0.74–0.97) and no differences in cognitive function, disability, or stroke. For primary prevention specifically, there was no reduction in all-cause mortality, stroke risk, or composite cardiovascular outcomes in the statin group vs placebo [46].
-
3.
ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm) (2003) included 19,342 hypertensive participants aged 40–79 years (6,570 > 60 years) with at least three other cardiovascular risk factors randomized to atorvastatin 10 mg or placebo. After a median of 3.3 years, the primary endpoint (nonfatal MI and fatal CHD) was significantly lower in the atorvastatin group, HR of 0.64 (95% CI 0.50–0.83, p = 0.0005), as was fatal and non-fatal stroke [60] Importantly, among all participants > 60 years, HR for the primary endpoint was 0.64 (0.47–0.86) p = 0.0027.
-
4.
JUPITER (Justification for the Use of Statins in Primary Prevention) enrolled 17,802 participants (5,695 ≥ 70 years) free of ASCVD or risk factors with elevated high-sensitivity C-reactive protein > 2.0 mg/L randomized to rosuvastatin 20 mg. After a median follow-up of 1.9 years, and among older adults ≥ 70 years, there was a significant reduction for primary endpoint (MI, CVA, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes) (0.61 (0.46–0.82)), CVA (0.55 (0.33–0.93)), revascularization or hospitalization for unstable angina (0.51 (0.33–0.80)), but not MI, cardiovascular death, or all-cause mortality [47].
-
5.
HOPE-3 (Heart Outcomes Prevention Evaluation) randomized 12,705 participants (3,086 ≥ 70 years) with no known ASCVD but with risk factors such as smoking, elevated blood glucose, and a family history of premature coronary disease to rosuvastatin 10 mg versus placebo. In sub-group analysis, among older adults (mean age 70 years), there was a reduction in co-primary outcome (cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) by 25%. Furthermore, the second coprimary outcome (cardiac arrest, heart failure, and revascularization) was reduced by 26% [48].
When the JUPITER and HOPE-3 were meta-analyzed together to focus on adults ≥70 years, there was a 26% relative risk reduction in endpoints of nonfatal MI, nonfatal CVA, or cardiovascular death (HR, 0.74; 0.61-0.91) for rosuvastatin 10-20mg vs placebo [61].
-
6.
ALLHAT-LLT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial—Lipid-Lowering Trial) trial randomized 10,355 participants with hypertension and one additional ASCVD risk factor to pravastatin 40 mg vs placebo. In a subgroup analysis focused on 1,467 participants ≥ 65 years (mean age: 71) stratified by age (65–74 y and ≥ 75 y), there was no significant benefit for ASCVD outcomes or mortality in both age groups [62].
Given the limited trial data, observational studies using real-world data have been conducted to examine the role of statins in older adults. A recent target trial emulation study using electronic health records and propensity score matching (1:1 initiators and non-initiators; 42,680 matched patients aged 75 to 84 years) evaluated the risk/benefit of statins among older adults (≥ 75 years) with no known ASCVD in Hong Kong. Statins use was associated with reduced cardiovascular events (intention-to-treat analysis: HR 0.94 [CI, 0.90–0.98] and a 5-year standardized absolute risk reduction of 1.20% [CI, 0.57%-1.82%] in the 75–84 year group) and lower risk of all-cause mortality, even among the very old group (≥ 85 years), without an increase in adverse events [63]. A 2021 systematic review and meta-analysis of 10 observational studies (n = 815,667) found that using statin among adults ≥ 65 years for primary prevention was associated with reduced all-cause mortality, CVD death, and CVA, but not MI. However, the association with reduced all-cause mortality was not evident in the absence of diabetes [64]. Furthermore, among 326,981 US veterans ≥ 75 years with no known ASCVD, new statin use was associated with a 20% lower risk of death from any cause and an 8% lower risk of an ASCVD event [65]. The protective association of statins extended to high-risk populations, such as those with dementia, frailty, and even those over the age of 90 years [66, 67, 68]. These observational studies have to be interpreted with caution due to multiple limitations and cannot be considered causal. A few of these limitations revolve around the variability in LDL-C measurements, residual risk, and adherence. There is variability in LDL-C measurements such as non-protocol driven time of measurement, whether initial or repeat. Another limitation includes baseline differences among participants. Third, there may be residual risk, including unmeasured confounders, population heterogeneity, and incomplete risk factor assessment or control, which may obscure residual cardiovascular risk after lowering LDL-C. Finally, adherence to therapy in observational studies is often unknown. For example, in patients with cognitive impairment, adherence rates might be lower than those with normal cognition, impacting the observed clinical outcomes between LLT, LDL-C levels, and cardiovascular outcomes.
The exclusion of older adults and those with geriatric syndromes from clinical trials has resulted in limited data on the efficacy and safety of statins for primary prevention in this population. Importantly, this lack of evidence does not imply benefit or harm but highlights the need for further research. Observational data suggest that older adults are at high risk for ASCVD events and might benefit the most from preventive interventions, supported by the JUPITER and HOPE-3 meta-analysis. However, few individuals over age 75 were included. Therefore, rather than a ‘whole or none’ approach, a more tempered risk-based approach may be the preferred method for using statins as primary prevention among high-risk older adults while balancing the risk of adverse events.
Nevertheless, older adults are less likely to receive optimal statin intensity, have low tolerance, and adherence remains suboptimal, with rates as low as 45% in one year (in those aged ≥ 65 years who initiated statin therapy). These challenges highlight the complexities of statin use in this age group [69, 70].
Challenges with Statins
Drug-Drug Interactions
Commonly reported drug/drug interactions have been reported with statins and cardiovascular medications, including antiarrhythmics (amiodarone), blood pressure, and rate control medications (calcium channel blockers), antiplatelets, anticoagulants [71]. Most interactions are seen with statins that exhibit CYP3A4 metabolism. The interactions are of importance since the prevalence of atrial fibrillation increases with aging, requiring rate and rhythm control in addition to anticoagulation [72]. Therefore, this raises the importance of utilizing non-statin LLT, such as ezetimibe, which may lower the risk of drug-drug interactions [73,74,75].
Adverse Events
Muscle-related (myopathy, rhabdomyolysis, elevated creatine kinase, skeletal muscle dysfunction), functional dependence, diabetes mellitus, and cognitive impairment are the most commonly reported events with statins [6]. However, not all adverse events have been well replicated in studies.
-
1-
Muscle-related adverse events: In a meta-analysis from Cholesterol Treatment Trialists' Collaboration, (n = 154,664) with a mean age of 63 years, with close to 50% receiving statin for primary prevention, there was a small excess risk of muscle symptoms (absolute excess rate of 11 events per 1000 person-years) with statins. However, when analyzed by age (≥ 75 years), the rate ratio for any muscle pain or weakness was 1.04 (0.95–1.13) vs placebo. Additionally, after 1 year, there was no significant excess in first reports of muscle pain or weakness [76]. Furthermore, in older adults (> 75 years) from the Provider Assessment of Lipid Management (PALM) Registry, older individuals were less likely to report any adverse symptoms (41.3% vs 46.6%; P = 0.003) or myalgias specifically (27.3% vs 33.3%; P < 0.001) [77]. Another phenomenon related to myalgia is the nocebo effect, or an individual’s awareness and concerns of an adverse effect may have a significant impact on their expectations and lead to a negative outcome [78]. In the SAMSON trial (Self-Assessment Method for Statin Side-effects Or Nocebo), randomized participants were given a 12-month prescription of 20 mg of atorvastatin, 4 placebo, and 4 empty. Although statin and placebo prescriptions had higher mean symptom scores, they were not statistically significant [79]. In summary, the impact of statin-associated muscle symptoms in older adults remains unclear, however a strategy of rechallenging a statin, either at a lower dose or another statin, could be considered when the decision is made to pursue treatment.
-
2-
Cognitive function: Despite the 2012 black box warning from the U.S. Food and Drug Administration for possible adverse effects of statins on cognitive function, there is no evidence that statins or very low LDL-C levels lead to cognitive impairment [80]. A systematic review and meta-analysis of 57 observational studies of statins reported a decreased risk of any dementia [OR 0.80 (CI 0.75–0.86)] and Alzheimer’s dementia [OR 0.68 (CI 0.56–0.81)] with high potency statins associated with higher risk reduction vs low potency statins [81]. High quality evidence from randomized trials does not support the association between statins or lowering LDL-C levels and adverse cognitive events or worsening cognitive test scores, events with potent agents such as PCSK9 inhibitors, and the benefit with ASCVD seems to overweight the observational evidence of cognitive impairment [82].
-
3-
Diabetes Mellitus: Multiple studies have reported an increased risk of new-onset diabetes. In a Cholesterol Treatment Trialists’ Collaboration meta-analysis, low-intensity or moderate-intensity statins vs. placebo resulted in a 10% relative increase in new-onset diabetes, with an absolute excess of 0.12% (95% CI 0.04–0.20) during each year of treatment, mainly among those with pre-diabetes [83]. When extrapolating to older adults, given the incidence risk is 0.12% per year, the risk of starting a statin at age 75 may be less of a concern than younger adults. Nevertheless, among statins, pravastatin was associated with the lowest risk for new-onset diabetes mellitus, while rosuvastatin carried the highest risk [84].
Non-statin Therapies for Primary Prevention
No specific guidelines for non-statin therapy in primary prevention exist for older adults (≥ 75 years) due to the lack of evidence [37]. Despite the release of the 2022 ACC Expert Consensus Decision Pathway on the Role of Non-statin Therapies, relevant cut-offs for this age group (≥ 75 years) are not defined due to the lack of data. In general, non-statin therapies are reserved for secondary prevention in patients who fail to achieve established LDL-C goals or for individuals with diabetes or elevated risk scores who fail to reach target LDL-C levels according to their risk scores despite the maximally tolerated statin dose.
Ezetimibe
Evidence suggests adding non-statin LLT benefits older adults by reducing adverse events from higher statin doses, but most trials focused on secondary prevention or those under 75. In the EWTOPIA 75 (Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older) [85], ezetimibe reduced the incidence of the primary outcome (sudden cardiac death, MI, coronary revascularization, or stroke and reduced cardiac events) (HR, 0.66; 95% CI, 0.50–0.86; P = 0.002) when prescribed for primary prevention. No differences were seen in the incidence of stroke, all-cause mortality, or adverse events. However, there were limitations related to design (open-label, early termination, and follow-up).
Proprotein Convertase Subtilisin/Kexin 9 (PCSK9)
PCSK9 increases LDL-receptor degradation, consequently reducing LDL-receptors and thus lowering LDL clearance from the circulation. PCSK9 inhibitors have been of growing use, effectively lowering LDL and apoB-lipoproteins by inhibiting the above mechanism. Multiple sites of action exist: (1) free plasma PCSK9 (alirocumab and evolocumab) and (2) small interfering RNA-altering the transcription of PCSK9 (Inclisiran) [86]. Most evidence for PCSK9 inhibitors and inclisiran in older adults comes from trials on secondary prevention rather than primary prevention [53, 55].
Bempedoic Acid
Works through adenosine triphosphate–citrate lyase inhibition, an earlier step in cholesterol synthesis than HMG-CoA reductase [87]. While it effectively reduces cholesterol levels and cardiovascular events, its use among older adults, especially those over 75, remains limited due to scarce evidence. The CLEAR (Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen Outcomes) trial randomized patients 18 to 85 years of age (mean age: 65.5 ± 9.0 years, with more than 50% ≥ 65 and 15% ≥ 75 years) for primary/secondary prevention to bempedoic acid vs. placebo showed a reduction in cardiovascular events with bempedoic acid, particularly benefiting people with diabetes. However, it did not significantly impact stroke or overall mortality [88, 89]. Adverse effects, including liver enzyme elevation, muscle-related symptoms, and tendon disorders, were more common in older adults, making the role of bempedoic acid unclear for older adults [90].
Secondary Prevention of ASCVD in Older Adults
The use of LLT (statin or non-statin) for LDL-C reduction among older adults following ASCVD has been well studied and supported in national guidelines (Table 2).
LDL-C Target Levels and Risk Scores
Similar to primary prevention, LDL-C targets are not specific to adults ≥ 75 years. According to ACC/AHA guidelines, among very high-risk adults with established ASCVD, clinicians should target an LDL-C reduction by ≥ 50% and LDL-C < 55 mg/dL, a high-intensity statin is recommended, while adding a non-statin (ezetimibe, PCSK9, bempedoic acid or Inclisiran) following LDL-C target failure. In contrast, LDL-C target is < 70 mg/dL for those not at very high risk. Specifically for adults ≥ 75 years, it is reasonable to resume moderate-high intensity statin if well tolerated and to individualize therapy when planning to initiate. The ESC/EAS recommends treating older adults (≥ 65 years) similarly to younger patients, adding a non-statin therapy when LDL-C ≥ 55 mg/dL despite maximally tolerated statin dosage. (Class I). However, they advise starting with a low dose and titrating up to reach LDL-C goals, particularly in the presence of renal impairment and drug-drug interactions.
Evidence on Lipid Lowering (Statin and Non-statin)
This reflects evidence from high-quality studies, such as a systematic review and meta-analysis of 29 randomized controlled trials for primary/secondary prevention (24 trials from the Cholesterol Treatment Trialists' Collaboration meta-analysis plus five individual trials). LDL-C lowering significantly reduced the risk of major vascular events in older patients by 26% per 1 mmol/L reduction in LDL-C (RR, 0.74; 95% CI 0.61–0.89). Other endpoints showing benefit included cardiovascular death (15% per 1 mmol/L reduction), MI, 20%, stroke by 27% (higher benefit with non-statin), and coronary revascularization by 20%, but no impact on all-cause death [7]. Importantly, irrespective of age, reduction in major vascular events was similar among those with prior ASCVD [59]. Several trials from non-statin LLT yielded positive results in reducing cardiovascular endpoints among older adults reduction in the primary endpoint including cardiovascular death, major coronary events, and stroke, and reduction in statin intolerance when combined with ezetimibe [51, 52, 55]. Medications like inclisiran offer several advantages that may be beneficial for older adults with polypharmacy and cognitive impairment. First, its extended dosing interval every six months may reduce the medication burden. Second, subcutaneous injection by a healthcare professional simplifies administration and allows for adherence monitoring, which can be a challenge for this population [37].
Nevertheless, despite the proven benefit of LLT for secondary prevention, older adults still face a pattern of under-prescription. In a multicenter retrospective cohort study from 14 commercial health plans geographically dispersed across the U.S. of older adults (≥ 75 years) with ASCVD, less than 50% were on statins, and very few received non-statin therapies (eg, ezetimibe) [8].
Deprescribing
Physicians caring for older adults often face the question of deprescribing. However, only 18 of 33 guidelines include recommendations for discontinuing statins, primarily due to side effects, with only three explicitly addressing older adults with poor health status [91]. Discontinuation of LLT therapy should be patient-centered, considering life expectancy, risk of harm, functional status, frailty, and ASCVD risk-enhancing factors. Observational studies show an increased risk of ASCVD following statin discontinuation, such as in a Danish study involving 67,418 adults aged ≥ 75 years on long-term statin treatment. This study found that discontinuation led to an adjusted HR of 1.32 (95% CI, 1.18–1.48), indicating one excess MACE per 112 persons who discontinued statins yearly. Other studies also noted a similar trend for primary prevention, with higher MACE associated with discontinuation [92, 93].
In contrast, a palliative care randomized trial of 189 adults, mean age of 74 years, with and without ASCVD and a life expectancy of less than one year, found no significant difference in 60-day mortality rates after statin discontinuation. However, these patients experienced a higher quality of life, as assessed by the McGill Quality of Life Questionnaire, particularly in the support domain. It is important to note that these findings apply specifically to a unique palliative population and may not be relevant to older adults with a life expectancy exceeding one year and who are free from cancer. Additionally, the study was unblinded and included more patients with cognitive impairment in the discontinuation arm, which could have influenced the outcomes, especially quality of life measures [94]. During end-of-life care, the ADA recommends reducing intensity and withdrawing LLT [56].
Two trials are expected to improve the knowledge of statin deprescription among older adults. The SITE (Statins In The Elderly) trial, an open-label randomized trial of older adults ≥ 75 years old investigating the quality-adjusted life years gained and mortality at 3 years following statin discontinuation, was initially prescribed for primary prevention [95]. STREAM (Statins in Multimorbid Older Adults Without Cardiovascular Disease) in Switzerland will randomly assign participants to continuation/discontinuation of statins prescribed for primary prevention. The primary outcome of composite endpoint of all-cause death and major non-fatal cardiovascular events (non-fatal myocardial infarction non-fatal ischemic stroke, and with secondary outcomes encompassing falls, strength, and quality of life changes [NCT05178420].
Conclusion/Future Directions
The question "Cholesterol Lowering in Older Adults: Should We Wait for Further Evidence?" presents a complex and ongoing challenge, particularly for primary prevention. While comprehensive evidence on the efficacy and safety of LLT is limited, available data (largely observational and from secondary analyses) increasingly suggests benefits for high-risk older adults, including those who are frail and without a very limited life expectancy (< 1 year).
Following a comprehensive cardiovascular risk assessment, patient-centered decisions should incorporate patient priorities and preferences while considering potential adverse effects and the complexities of geriatric care (functional status, cognitive status, polypharmacy) and competing mortality risks. Additionally, utilizing noninvasive assessments like CAC scoring can be considered to evaluate biological age beyond chronological age, potentially reclassifying patients with intermediate scores or those hesitant about therapy.
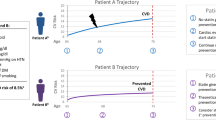
The ultimate goal for patients and their clinicians is to optimize healthy longevity while enhancing the quality of life, ideally through the maintenance of independence and cognitive function. Upcoming trials will refine the selection of ideal older adults for LLT. In the interim, for adults aged 75 and older without a life limiting illness, consideration of LLT for prevention of both ASCVD events and mortality can be included as part of a larger conversation of healthy aging (Fig. 1).
Data Availability
Data sharing is not applicable to this article as no datasets were generated to prepare this manuscript.
Code Availability
Not applicable.
References
Organization WH. World report on ageing and health: World Health Organization; 2015.
Aïdoud A, Gana W, Poitau F, Debacq C, Leroy V, Nkodo JA, et al. High Prevalence of Geriatric Conditions Among Older Adults With Cardiovascular Disease. J Am Heart Assoc. 2023;12(2):e026850.
Sheet SF. Older Americans & Cardiovascular Diseases. Dallas: American Stroke Association; 2013.
Xie J, Wu EQ, Zheng Z-J, Croft JB, Greenlund KJ, Mensah GA, et al. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke. 2006;37(10):2567–72.
Nanna MG, Abdullah A, Mortensen MB, Navar AM. Primary prevention statin therapy in older adults. Curr Opin Cardiol. 2023;38(1):11–20.
Mortensen MB, Falk E. Primary Prevention With Statins in the Elderly. J Am Coll Cardiol. 2018;71(1):85–94.
Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. The Lancet. 2020;396(10263):1637–43.
Nanna MG, Nelson AJ, Haynes K, Shambhu S, Eapen Z, Cziraky MJ, et al. Lipid-lowering treatment among older patients with atherosclerotic cardiovascular disease. J Am Geriatr Soc. 2023;71(4):1243–9.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the european society of cardiology (ESC) and european atherosclerosis society (EAS). Eur Heart J. 2019;41(1):111–88.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the american college of cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143.
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States From 1999–2012. JAMA. 2015;314(17):1818–31.
Odden MC, Pletcher MJ, Coxson PG, Thekkethala D, Guzman D, Heller D, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med. 2015;162(8):533–41.
Rhee TG, Kumar M, Ross JS, Coll PP. Age-related trajectories of cardiovascular risk and use of aspirin and statin among U.S. adults aged 50 or older, 2011–2018. J Am Geriatr Soc. 2021;69(5):1272–82.
Sarraju A, Spencer-Bonilla G, Chung S, Gomez S, Li J, Heidenreich P, et al. Statin use in older adults for primary cardiovascular disease prevention across a spectrum of cardiovascular risk. J Gen Intern Med. 2022;37(11):2642–9.
Spencer-Bonilla G, Chung S, Sarraju A, Heidenreich P, Palaniappan L, Rodriguez F. Statin use in older adults with stable atherosclerotic cardiovascular disease. J Am Geriatr Soc. 2021;69(4):979–85.
Cournot M, Cambou J-P, Quentzel S, Danchin N. Key factors associated with the under-prescription of statins in elderly coronary heart disease patients: Results from the ELIAGE and ELICOEUR surveys. Int J Cardiol. 2006;111(1):12–8.
Sittiwet C, Simonen P, Gylling H, Strandberg TE. Mortality and cholesterol metabolism in subjects aged 75 years and older: the helsinki businessmen study. J Am Geriatr Soc. 2020;68(2):281–7.
Rong S, Li B, Chen L, Sun Y, Du Y, Liu B, et al. Association of Low‐Density Lipoprotein Cholesterol Levels with More than 20‐Year Risk of Cardiovascular and All‐Cause Mortality in the General Population. J Am Heart Assoc. 2022;11(15):e023690.
Zhou Z, Tonkin AM, Curtis AJ, Murray A, Zhu C, Reid CM, et al. Low-density-lipoprotein cholesterol and mortality outcomes among healthy older adults: a post hoc analysis of ASPREE Trial. J Gerontol A Biol Sci Med Sci. 2024;79(4):glad268. https://doi.org/10.1093/gerona/glad268.
Newson RS, Felix JF, Heeringa J, Hofman A, Witteman JC, Tiemeier H. Association between serum cholesterol and noncardiovascular mortality in older age. J Am Geriatr Soc. 2011;59(10):1779–85.
Ravnskov U, Diamond DM, Hama R, Hamazaki T, Hammarskjöld B, Hynes N, et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open. 2016;6(6):e010401.
Nanna MG, Navar AM, Wojdyla D, Peterson ED. The association between low-density lipoprotein cholesterol and incident atherosclerotic cardiovascular disease in older adults: results from the national institutes of health pooled cohorts. J Am Geriatr Soc. 2019;67(12):2560–7.
Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. The Lancet. 2020;396(10263):1644–52.
Strandberg TE. Role of statin therapy in primary prevention of cardiovascular disease in elderly patients. Curr Atheroscler Rep. 2019;21(8):28.
Damluji AA, Nanna MG, Rymer J, Kochar A, Lowenstern A, Baron SJ, et al. Chronological vs biological age in interventional cardiology: a comprehensive approach to care for older adults: jacc family series. JACC Cardiovasc Interv. 2024;17(8):961–78.
Orkaby AR, Nussbaum L, Ho Y-L, Gagnon D, Quach L, Ward R, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002–2012. J Gerontol: Series A. 2018;74(8):1257–64.
Yourman LC, Cenzer IS, Boscardin WJ, Nguyen BT, Smith AK, Schonberg MA, et al. Evaluation of time to benefit of statins for the primary prevention of cardiovascular events in adults aged 50 to 75 years: a meta-analysis. JAMA Intern Med. 2021;181(2):179–85.
Lee S, Smith A, Widera E, Yourman L, Schonberg M, Ahalt C. Eprognosis. 2011. https://eprognosis.ucsf.edu/calculators.php. Accessed 26 June 2024.
Parsons RE, Liu X, Collister JA, et al. Independent external validation of the QRISK3 cardiovascular disease risk prediction model using UK BiobankHeart. 2023;109:1690–7.
Cooney MT, Selmer R, Lindman A, Tverdal A, Menotti A, Thomsen T, et al. Cardiovascular risk estimation in older persons: SCORE O.P. Eur J Prev Cardiol. 2016;23(10):1093–103.
Hageman SHJ, Petitjaen C, Pennells L, Kaptoge S, Pajouheshnia R, Tillmann T, et al. Improving 10-year cardiovascular risk prediction in apparently healthy people: flexible addition of risk modifiers on top of SCORE2. Eur J Prev Cardiol. 2023;30(15):1705–14.
Saeed A, Nambi V, Sun W, Virani SS, Taffet GE, Deswal A, et al. Short-term global cardiovascular disease risk prediction in older adults. J Am Coll Cardiol. 2018;71(22):2527–36.
Neumann JT, Twerenbold R, Weimann J, Ballantyne CM, Benjamin EJ, Costanzo S, et al. Prognostic value of cardiovascular biomarkers in the population. JAMA. 2024;331(22):1898–909. https://doi.org/10.1001/jama.2024.5596.
Gaziano TA, Gaziano JM. Can cardiovascular risk assessment be improved in the 21st century? JAMA. 2024;331(22):1891–3. https://doi.org/10.1001/jama.2024.7644.
He D, Wang Z, Li J, Yu K, He Y, He X, et al. Changes in frailty and incident cardiovascular disease in three prospective cohorts. Eur Heart J. 2024;45(12):1058–68. https://doi.org/10.1093/eurheartj/ehad885.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e563–95.
Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-Cholesterol lowering in the management of atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2022;80(14):1366–418.
Force UPST. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. 2022;328(8):746–53.
Tota-Maharaj R, Blaha MJ, McEvoy JW, Blumenthal RS, Muse ED, Budoff MJ, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33(23):2955–62.
Yano Y, O’Donnell CJ, Kuller L, Kavousi M, Erbel R, Ning H, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2(9):986–94.
Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112(4):572–7.
Obisesan OH, Boakye E, Wang FM, Dardari Z, Dzaye O, Cainzos-Achirica M, et al. Coronary artery calcium as a marker of healthy and unhealthy aging in adults aged 75 and older: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2024;392. https://doi.org/10.1016/j.atherosclerosis.2024.117475. An observational study from The Atherosclerosis Risk in Communities (ARIC), including adults aged ≥75 years with no known coronary disease, correlated coronary calcium score with biological aging factors such as hearing impairment, physical functioning, and grip strength.
Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72(25):3233–42.
Joseph J, Pajewski NM, Dolor RJ, Sellers MA, Perdue LH, Peeples SR, et al. Pragmatic evaluation of events and benefits of lipid lowering in older adults (PREVENTABLE): trial design and rationale. J Am Geriatr Soc. 2023;71(6):1701–13.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. https://doi.org/10.1016/S0140-6736(02)09327-3.
Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–30.
Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010;152(8):488–96, w174.
Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374(21):2021–31.
Zhou Z, Ofori-Asenso R, Curtis AJ, Breslin M, Wolfe R, McNeil JJ, et al. Association of statin use with disability-free survival and cardiovascular disease among healthy older adults. J Am Coll Cardiol. 2020;76(1):17–27.
Deedwania P, Stone PH, BaireyMerz CN, Cosin-Aguilar J, Koylan N, Luo D, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation. 2007;115(6):700–7.
Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalán R, Špinar J, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from improve-it (improved reduction of outcomes: vytorin efficacy international trial). Circulation. 2018;137(15):1571–82.
Lee SH, Lee YJ, Heo JH, Hur SH, Choi HH, Kim KJ, et al. Combination moderate-intensity statin and Ezetimibe therapy for elderly patients with atherosclerosis. J Am Coll Cardiol. 2023;81(14):1339–49.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Wright RSS, Ray KK, Raal FJ, Kallend D, Jaros M, Koenig W, et al. Abstract 16427: Efficacy and safety of inclisiran according to age: a pooled analysis of phase III Studies (ORION 9, 10 and 11). Circulation. 2020;142(Suppl_3):A16427-A.
Sinnaeve PR, Schwartz GG, Wojdyla DM, Alings M, Bhatt DL, Bittner VA, et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J. 2020;41(24):2248–58.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 13 Older Adults: Standards of Care in Diabetes—2023. Diabetes Care. 2022;46(Supplement_1):S216–29.
Gibbs BB, Hivert M-F, Jerome GJ, Kraus WE, Rosenkranz SK, Schorr EN, et al. Physical activity as a critical component of first-line treatment for elevated blood pressure or cholesterol: who, what, and how?: a scientific statement from the american heart association. Hypertension. 2021;78(2):e26–37.
Yun H, Su W, Zhao H, Li H, Wang Z, Cui X, et al. Effects of different exercise modalities on lipid profile in the elderly population: a meta-analysis. Medicine (Baltimore). 2023;102(29):e33854.
Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407–15.
Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58.
Ridker PM, Lonn E, Paynter NP, Glynn R, Yusuf S. Primary prevention with statin therapy in the elderly. Circulation. 2017;135(20):1979–81.
Han BH, Sutin D, Williamson JD, Davis BR, Piller LB, Pervin H, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med. 2017;177(7):955–65.
Wanchun Xu, Amanda Lauren Lee, Cindy Lo Kuen Lam, et al. Benefits and risks associated with statin therapy for primary prevention in old and very old adults: real-world evidence from a target trial emulation study. Ann Intern Med. https://doi.org/10.7326/M24-0004. A recently published target trial emulation study, including 42,680 matched person-trials aged 75 to 84 years, compared statin initiators to non-initiators for primary prevention, focusing on cardiovascular and safety outcomes.
Awad K, Mohammed M, Zaki MM, Abushouk AI, Lip GYH, Blaha MJ, et al. Association of statin use in older people primary prevention group with risk of cardiovascular events and mortality: a systematic review and meta-analysis of observational studies. BMC Med. 2021;19(1):139.
Orkaby AR, Driver JA, Ho Y-L, Lu B, Costa L, Honerlaw J, et al. Association of statin use with all-cause and cardiovascular mortality in US veterans 75 years and older. JAMA. 2020;324(1):68–78.
Pilotto A, Panza F, Copetti M, Simonato M, Sancarlo D, Gallina P, et al. Statin treatment and mortality in community-dwelling frail older patients with diabetes mellitus: a retrospective observational study. PLoS ONE. 2015;10(6):e0130946.
Orkaby AR, Lu B, Ho YL, Treu T, Galloway A, Wilson PWF, et al. New statin use, mortality, and first cardiovascular events in older US Veterans by frailty status. J Am Geriatr Soc. 2024;72(2):410-22. https://doi.org/10.1111/jgs.18700. An observational study classifying 710,313 older U.S. veterans by frailty status, who received new statins versus none for primary prevention, demonstrated a reduction in major adverse cardiovascular events in the former regardless of frailty status.
O’Sullivan JL, Kohl R, Lech S, Romanescu L, Schuster J, Kuhlmey A, et al. Statin use and all-cause mortality in nursing home residents with and without dementia: a retrospective cohort study using claims data. Neurology. 2024;102(6):e209189.
Ofori-Asenso R, Ilomäki J, Tacey M, Si S, Curtis AJ, Zomer E, et al. Predictors of first-year nonadherence and discontinuation of statins among older adults: a retrospective cohort study. Br J Clin Pharmacol. 2019;85(1):227–35.
Ofori-Asenso R, Ilomaki J, Tacey M, Curtis AJ, Zomer E, Bell JS, et al. Prevalence and incidence of statin use and 3-year adherence and discontinuation rates among older adults with dementia. Am J Alzheimers Dis Other Demen. 2018;33(8):527–34.
Karimi S, Hough A, Beckey C, Parra D. Results of a safety initiative for patients on concomitant amiodarone and simvastatin therapy in a Veterans Affairs medical center. J Manag Care Pharm. 2010;16(7):472–81.
Khurshid S, Ashburner JM, Ellinor PT, McManus DD, Atlas SJ, Singer DE, et al. Prevalence and Incidence of Atrial Fibrillation Among Older Primary Care Patients. JAMA Network Open. 2023;6(2):e2255838-e.
Bardolia C, Amin NS, Turgeon J. Emerging Non-statin Treatment Options for Lowering Low-Density Lipoprotein Cholesterol. Front Cardiovasc Med. 2021;8:789931.
Damiani I, Corsini A, Bellosta S. Potential statin drug interactions in elderly patients: a review. Expert Opin Drug Metab Toxicol. 2020;16(12):1133–45.
Wiggins BS, Saseen JJ, Page RL, Reed BN, Sneed K, Kostis JB, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468–95.
Reith C, Baigent C, Blackwell L, Emberson J, Spata E, Davies K, et al. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. The Lancet. 2022;400(10355):832–45.
Nanna MG, Navar AM, Wang TY, Mi X, Virani SS, Louie MJ, et al. Statin use and adverse effects among adults >75 years of age: insights from the patient and provider assessment of lipid management (PALM) Registry. J Am Heart Assoc. 2018;7(10):e008546. https://doi.org/10.1161/JAHA.118.008546.
Wood FA, Howard JP, Finegold JA, Nowbar AN, Thompson DM, Arnold AD, et al. N-of-1 Trial of a Statin, Placebo, or No Treatment to Assess Side Effects. N Engl J Med. 2020;383(22):2182–4.
Howard JP, Wood FA, Finegold JA, Nowbar AN, Thompson DM, Arnold AD, et al. Side Effect Patterns in a Crossover Trial of Statin, Placebo, and No Treatment. J Am Coll Cardiol. 2021;78(12):1210–22.
Adhikari A, Tripathy S, Chuzi S, Peterson J, Stone NJ. Association between statin use and cognitive function: A systematic review of randomized clinical trials and observational studies. J Clin Lipidol. 2021;15(1):22-32.e12.
Olmastroni E, Molari G, De Beni N, Colpani O, Galimberti F, Gazzotti M, et al. Statin use and risk of dementia or Alzheimer's disease: a systematic review and meta-analysis of observational studies. Eur J Prev Cardiol. 2022;29(5):804-14. https://doi.org/10.1093/eurjpc/zwab208. A 2022 systematic review and meta-analysis demonstrated the potential benefit of statins on neurocognitive function rather than the increased risk of dementia as previously thought.
Goldstein LB, Toth PP, Dearborn-Tomazos JL, Giugliano RP, Hirsh BJ, Peña JM, et al. Aggressive LDL-C lowering and the brain: impact on risk for dementia and hemorrhagic stroke: a scientific statement from the american heart association. Arterioscler Thromb Vasc Biol. 2023;43(10):e404–42.
Reith C, Preiss D, Blackwell L, Emberson J, Spata E, Davies K, et al. Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: an individual participant data meta-analysis. Lancet Diabetes Endocrinol. 2024;12(5):306–19.
Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111(8):1123–30.
Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140(12):992–1003.
Bao X, Liang Y, Chang H, Cai T, Feng B, Gordon K, et al. Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): from bench to bedside. Signal Transduct Target Ther. 2024;9(1):13.
MasanaMarín L, Plana Gil N. Bempedoic acid. Mechanism of action and pharmacokinetic and pharmacodynamic properties. Clin Investig Arterioscler. 2021;33(Suppl 1):53–7.
Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64.
Ray KK, Nicholls SJ, Li N, Louie MJ, Brennan D, Lincoff AM, et al. Efficacy and safety of bempedoic acid among patients with and without diabetes: prespecified analysis of the CLEAR Outcomes randomised trial. Lancet Diabetes Endocrinol. 2024;12(1):19–28.
Di Minno A, Lupoli R, Calcaterra I, Poggio P, Forte F, Spadarella G, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia: systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2020;9(15):e016262.
van der Ploeg MA, Floriani C, Achterberg WP, Bogaerts JMK, Gussekloo J, Mooijaart SP, et al. Recommendations for (discontinuation of) statin treatment in older adults: review of guidelines. J Am Geriatr Soc. 2020;68(2):417–25.
Thompson W, Morin L, Jarbøl DE, Andersen JH, Ernst MT, Nielsen JB, et al. Statin discontinuation and cardiovascular events among older people in Denmark. JAMA Netw Open. 2021;4(12):e2136802.
Giral P, Neumann A, Weill A, Coste J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population-based cohort study in France. Eur Heart J. 2019;40(43):3516–25.
Kutner JS, Blatchford PJ, Taylor DH Jr, Ritchie CS, Bull JH, Fairclough DL, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med. 2015;175(5):691–700.
Bonnet F, Bénard A, Poulizac P, Afonso M, Maillard A, Salvo F, et al. Discontinuing statins or not in the elderly? Study protocol for a randomized controlled trial. Trials. 2020;21(1):342.
Acknowledgements
The central illustration was created using Biorender.com
Funding
Dr. Orkaby reports funding from VA CSRD CDA-2 IK2CX001800, and NIA R01AG081287. Salil V Deo MD receives external funding support from Johnson and Johnson not related to this study.
Author information
Authors and Affiliations
Contributions
YJ and DA wrote the main manuscript. RC wrote the introduction and prepared Table 2. YJ finalized all tables and created the central illustration.MK and SVD edited and reviewed this paper to provide guidance and suggestions. ARO supervised, reviewed, edited, and finalized the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
All Authors have nothing to disclose except for the funding disclosures.
Human and Animal Rights and Informed Consent
No animal or human subjects by the authors were used in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Few ASCVD risk scores (SCORE2-OP and QRISK) have been validated in older adults after age 84, and all scores heavily weight age and lack considerations for life expectancy and time to benefit.
• LDL-C levels may not be reliable markers for future ASCVD in older adults without prior CVD.
• Limited data on using CAC in older adults suggests that those with a score of zero may be less likely to benefit from LLT and can aid in risk reclassification.
• Despite scarcity of trial evidence among older adults ≥ 75 years, there is some evidence that statins reduce ASCVD, even among high risk patients (dementia and frail).
• It is crucial to evaluate drug-drug interactions before initiating statins due to known interactions with common cardiovascular medications. While statins may increase myalgias and creatinine kinase levels, rates are generally low and do not appear higher among older adults. New onset diabetes mellitus has been reported, especially among those at risk for diabetes, but the rates are low per year and may be less relevant to older adults. No robust evidence exists linking statins to poor cognitive performance.
• Non-statin therapies are second-line agents and have been shown to lower LDL-C and improve cardiovascular outcomes, especially for secondary prevention. However, their role in primary prevention for older adults (≥ 75 years) remains unknown.
• Deprescribing remains challenging, and more evidence is needed to guide the approach (e.g., dose reduction and patient selection) since observational studies suggest a potential increase in ASCVD.
Rights and permissions
About this article
Cite this article
Jamil, Y.A., Cohen, R., Alameddine, D.K. et al. Cholesterol Lowering in Older Adults: Should We Wait for Further Evidence?. Curr Atheroscler Rep 26, 521–536 (2024). https://doi.org/10.1007/s11883-024-01224-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-024-01224-4