Abstract
Purpose of Review
Prediabetes, or dysglycemia in the absence of diabetes, is a prevalent condition typically defined by a glycated hemoglobin (HgbA1c) of 5.7– < 6.5%. This article reviews current contemporary data examining the association between prediabetes and cardiovascular disease (CVD) as well as HgbA1c as a continuous measure of cardiovascular risk across the glycemic spectrum.
Recent Findings
Dysglycemia in the prediabetic range is associated with an increased risk of both subclinical and clinical CVD, including atherosclerotic CVD, chronic kidney disease, and heart failure. Several recent large, prospective studies demonstrate roughly linear risk with increasing HgbA1c, even below the threshold for prediabetes. “High-risk” patients with prediabetes have similar CVD risk as those with diabetes.
Summary
HgbA1c below the threshold for diabetes stratifies CVD risk. Use of HgbA1c as a continuous measure, rather than simply dichotomized, may inform current and future prevention strategies. Given the high population attributable risk associated with prediabetes, targeted prevention strategies in this population warrant dedicated study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As described in early prospective studies [1,2,3,4,5] and reaffirmed in contemporary analyses [6,7,8,9], diabetes mellitus (DM) is strongly and independently associated with the development of both microvascular complications (i.e., retinopathy, neuropathy, and nephropathy) and macrovascular atherosclerotic cardiovascular disease (ASCVD). Type 2 diabetes (T2D) represents > 90% of DM cases [10]. Although T2D commonly co-occurs with other conventional CVD risk factors, complicating causal inference [11], Mendelian randomization analyses indicate causal effects of T2D on the development of ASCVD [12]. Today, use of the glycated hemoglobin assay, commonly known as hemoglobin A1c (HgbA1c), is the standard for both diagnosis of DM (defined as HgbA1c ≥ 6.5%) and for monitoring treatment response of patients with DM, as endorsed by the American Diabetes Association (ADA) [13]. Dysglycemia below the threshold for DM, so-called prediabetes (defined as HgbA1c 5.7– < 6.5%), is increasing in prevalence, now affecting more than 1 in 3 US adults and 1 in 5 adolescents [14,15,16]. While patients with prediabetes have lower average risk for developing CVD than those with DM, a greater total number of CVD events likely occurs among prediabetic individuals in western countries [17••]. As such, recognizing and addressing the CVD risk associated with prediabetes represent an important component of comprehensive CVD prevention.
In this review, we explore HgbA1c as a continuous measure of risk from normoglycemia to prediabetes to DM as it relates to CVD. We highlight contemporary work which establishes prediabetes as a risk factor for development of ASCVD, heart failure (HF), and chronic kidney disease (CKD) and discuss potential translational implications for clinical care and future research.
The HgbA1c Assay and Diagnosis of Diabetes and Prediabetes
Abnormally high levels of glycated hemoglobin, the non-enzymatic linkage of sugar molecules to the hemoglobin protein, were first incidentally discovered in the serum of patients with DM in the late 1960s [18]. This finding led to the development of the HgbA1c assay, which is utilized clinically as a surrogate for a patient’s 3-month average serum blood glucose, roughly the lifespan of a red blood cell carrying glycated hemoglobin. The threshold of HgbA1c ≥ 6.5% for the diagnosis of DM was established in 1997 based on three cross-sectional studies demonstrating increased risk of diabetic retinopathy beyond this level [19]. Prediabetes was later defined as HgbA1c 5.7– < 6.5% based on an observed increased risk for developing frank T2D among individuals in this range [20]. A recent meta-analysis affirmed that a threshold of HgbA1c ≥ 6.5% was a robust inflection point for increased risk of diabetic retinopathy, with less clear evidence for risk demarcation for other microvascular complications (diabetic nephropathy and/or neuropathy) above this level [21].
Early trials examining DM and CVD utilized several methods to diagnose and monitor hyperglycemia in patients with DM including fasting blood glucose, glucose tolerance tests, and the HgbA1c. Since its widespread clinical availability, the HgbA1c assay has undergone serial efforts to standardize results to account for differences in technique (e.g., high performance liquid chromatography versus immunoassays) and across labs around the world [22, 23], and today it is the most common diagnostic assay for both diagnosis and monitoring the effect of treatment in both DM and prediabetes as endorsed by specialty guidelines [13]. HgbA1c has further been shown to be more strongly associated with CVD in patients with DM than other measures of dysglycemia, including fasting blood glucose [24••, 25, 26].
One limitation of the HgbA1c assay is that levels may less accurately reflect average blood glucose in non-White patients. In one study of patients with DM who were monitored with both serial HgbA1c and continuous glucose monitors (CGM), HgbA1c was 0.4% higher in Black patients compared with White patients with the same average CGM-ascertained glucose concentration [27]. When compared to other surrogate markers of average blood glucose present in serum but not carried in erythrocytes, such as glycated albumin and fructosamine, no such racial differences were observed. Nonetheless, HgbA1c remains the contemporary standard for quantification of glycemic burden.
Prediabetes and Atherosclerotic Cardiovascular Disease
Growing evidence links prediabetes and development of both subclinical and clinical ASCVD.
Subclinical ASCVD
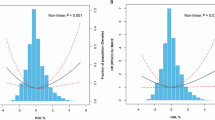
A recent prospective study by Rossello et al. of 3973 non-diabetic individuals aged 40–54 years observed a linear association of with HgbA1c with subclinical atherosclerosis ascertained by vascular ultrasound (carotid arteries, infrarenal abdominal aorta, and iliofemoral arteries) and cardiac computed tomography imaging [28••]. After adjustment for confounders, HgbA1c was associated with subclinical atherosclerosis in multiple vascular territories in non-diabetic patients at both low and moderate risk by conventional risk calculators (ACC/AHA Pooled Cohort Equations [29] and the European SCORE calculator [30]). This association was present even at HgbA1c levels below the traditional ADA-defined HgbA1c threshold of 5.7% for prediabetes—specifically, down to HgbA1c of 5.5%. Interestingly, fasting blood glucose was not associated with subclinical atherosclerosis in non-diabetic patients, indicating that HgbA1c may be a more useful biomarker in this population. The authors found that inclusion of HgbA1c had additive value to the traditional SCORE risk calculator in identifying patients with higher likelihood of having subclinical atherosclerosis (area under the curve 0.751 vs. 0.732, P < 0.001); of note, the SCORE calculator, unlike the Pooled Cohort Equations used widely in the USA, does not incorporate an indicator for diabetes status. The authors concluded that the significance of this association lies in the high number of patients with prediabetes who are at risk for developing subclinical atherosclerosis, highlighting the need for interventions both to prevent progression to DM and prevent and stabilize subclinical atherosclerosis.
Clinical ASCVD
Multiple recent analyses have linked prediabetes with clinical ASCVD events (Table 1). In one analysis of over 11,000 subjects in the prospective Atherosclerosis in Communities (ARIC) study, HgbA1c was associated in a linear fashion with CAD and ischemic stroke [24••]. In both minimally and fully adjusted models, any HgbA1c ≥ 5.5% was associated with incident CAD (e.g., HgbA1c 5.5–6%: adjusted HR 1.25 95% CI 1.09–1.44), and HgbA1c ≥ 6% was associated with incident ischemic stroke (HgbA1c 6.0–6.5%: adjusted HR 2.19 95% CI 1.58–3.05). In fully adjusted models including glucose, each percentage point increase in HgbA1c was associated with 50% and 55% increased risk for CAD and ischemic stroke, respectively (HR 1.50 95% CI 1.33–1.68, and HR 1.55, 95% CI 1.28–1.88). In this study, HgbA1c was significantly associated with CVD outcomes, whereas fasting glucose was not.
In a prospective study of > 450,000 subjects in the UK Biobank without CVD, de Jong et al. found that HgbA1c was associated in a linear fashion with incident myocardial infarction (MI) along the spectrum from no DM, prediabetes, undiagnosed DM, and (highest risk) previously diagnosed DM, in both women and men [31•]. Each 1% increase in HgbA1c was associated with 18% increased risk of MI in both sexes.
Another UK Biobank-based study by Welsh et al. of 370,000 subjects without previously diagnosed DM similarly found increasing risk for ASCVD in patients who went on to develop prediabetes and DM [32•]. Patients in this cohort with prediabetes had an unadjusted 83% increased risk (HR 1.83 95% CI 1.69–1.97) for development of ASCVD (including MI, stroke, and transient ischemic attack). When adjusted for commonly comorbid classical CVD risk factors in this population (including hypertension, obesity, dyslipidemia, smoking, and family history of CVD), associated risk attenuated to 11% but remained significantly increased (HR 1.11, 95% CI 1.03–1.20). The authors concluded that while this association demonstrates patients with prediabetes have about 80% increased CVD risk compared to individuals with normal HgbA1c, the risk is largely captured by conventional CVD risk factors present in the prediabetic population. These authors further incorporated the HgbA1c into existing ASCVD risk calculators. There was a modest increase in discrimination when HgbA1c was added to the ACC/AHA ASCVD risk calculator, which already includes diabetes status (C-index increased by 0.0007). In a subgroup of patients in the ARIC study who did not have DM at the time of enrollment but later developed prediabetes, HgbA1c was superior to fasting glucose for risk discrimination for a variety of clinical outcomes, including ASCVD [33].
Another recent, large, prospective study from the UK Biobank similarly found that risk for ASCVD increased in a linear fashion with respect to HgbA1c. Honigberg et al. found that adjusting for age, sex, and race, prediabetes compared to normoglycemia was associated with 44% increased risk of ASCVD (defined as a composite of incident CAD, ischemic stroke, and peripheral arterial disease, HR 1.44, 95% CI 1.39–1.49, P > 0.001) in a primary prevention population [17••]. Risk of ASCVD associated with prediabetes attenuated but remained significant after comprehensive covariate adjustment (HR 1.11, 95% CI 1.08–1.15, P < 0.001). In demographic-adjusted cubic spline models, CVD risk nadired at HgbA1c of 5% for incident ASCVD. In sensitivity analyses that incorporated incident T2D as a time-varying covariate, ASCVD risk increased significantly at HgbA1c greater than 5.4%. While CAD was the most commonly diagnosed ASCVD subtype included in the composite outcome, incident peripheral arterial disease was most strongly associated per unit change in HgbA1c (patients without T2D: HR per HgbA1c % increase 2.32, 95% CI 2.06–2.61; after full covariate adjustment: HR 1.37, 95% CI 1.21–1.54). Importantly, population attributable risk was larger for baseline prediabetes compared to T2D status for ASCVD (8.1% vs 5.9%), which was consistent across ASCVD subtypes (CAD: 7.9% vs 5.9%; ischemic stroke: 6.8% vs 5.3%; peripheral arterial disease 12.8% vs 9.3%).
The authors further isolated a cohort of patients with so-called “high-risk prediabetes,” which included patients who were current or former smokers, and had medication-adjusted systolic blood pressure, non-HDL cholesterol, and C-reactive protein each in the top tertile of the study sample. This group of patients had a nearly superimposable risk for ASCVD compared to patients with DM in the study period (16.7% vs 16.8%) and had nearly twofold risk of developing any of the primary outcomes measured (ASCVD, CKD, and heart failure) compared to other patients with prediabetes.
A recently updated meta-analysis confirms the association with prediabetes and ASCVD. Cai et al. reviewed 129 studies involving over 10 million individuals for analysis [34•]. Prediabetes was associated with 15% increased risk for composite endpoint of CVD (HR 1.15, 95% CI 1.11–1.18), 16% increased risk for CAD (HR 1.16, 95% CI 1.11–1.21), and 14% increased risk of stroke (HR 1.14, 95% CI 1.08–1.20).
Prediabetes and Heart Failure
Several recent studies associate prediabetes with cardiac structural and functional changes as well as clinical HF. A subgroup of the ARIC study population without CAD or HF was examined for evidence of structural heart disease when classified into groups based on HgbA1c cutoffs (normal, prediabetes, and DM). This cross-sectional analysis of about 4400 individuals demonstrated that both prediabetes and DM were associated with increased LV mass, worse diastolic function, and subtle reduction in LV systolic function compared to those with normoglycemia [35].
Adding to these findings, Selvin et al. examined a group of more than 9000 subjects in the ARIC study without evident CVD at baseline, including normal high sensitivity cardiac troponin (hs-TnT) [36••]. At 6 years of follow-up, 6.4% of those with prediabetes at baseline had developed myocardial damage as reflected by newly elevated hs-TnT ≥ 14 ng/L (risk ratio 1.38), which was accentuated in the DM group of whom 10.8% had newly elevated hs-TnT (risk ratio 2.46). In subgroup analysis after an additional 6 years of follow-up, subjects with prediabetes and DM were found to have increased risk of incident HF with hazard ratios of 4.30 (95% CI 3.01–6.14) among those with pre-diabetes and 6.37 (95% CI 4.27–9.51) among those with DM. Elevated hs-TnT was strongly associated with microvascular risk factors (e.g., hypertension, prediabetes, DM) but not with classic macrovascular, or atherosclerotic, risk factors such as low-density lipoprotein cholesterol, suggesting that the myocardial damage may be mediated through small vessel ischemia or myocardial hypertrophy.
Building on these findings, Honigberg et al. found observed increased risk for HF among prediabetic individuals in the UK Biobank [17••]. In demographic-adjusted models, prediabetes was associated with 46% increased risk for HF (HR 1.46, 95% CI 1.38–1.56, P < 0.001) and DM with 148% increased risk. Risk began to increase starting at an HgbA1c ≥ 5.4% and increased progressively with higher HgbA1c. However, after complete covariate adjustment, hazard ratios for HgbA1c categories lower than 7% were non-significant, suggesting that HgbA1c below this range is more accurately described as a risk signal as opposed to an independent risk factor for HF. Similar to ASCVD, population attributable risk for HF was larger for baseline prediabetes vs T2D (9.9% vs 7.1%). As seen with ASCVD, those with “high-risk prediabetes” had similar cumulative incidence of HF vs. those with DM.
Prediabetes and Chronic Kidney Disease
CKD is associated with increased risk for CVD [37], but individuals with CKD and DM are known to have even higher risk for CVD. Growing evidence links prediabetes with development of CKD. In the Chronic Renal Insufficiency Cohort, a prospective study of more than 3700 subjects, prediabetes comorbid with CKD was associated with increased risk of composite CVD (HF, MI, or stroke) and progression of proteinuria, but not associated with a composite CKD outcome (progression to end-stage renal disease, decline of glomerular filtration rate by > 50% and less than 15 mL/min/1.73 m2, and/or doubling of urine protein-creatinine ratio) when compared to normoglycemia [38]. In this cohort, after adjustment for other comorbidities, prediabetes with CKD was associated with 38% increased risk for CVD, while DM was associated with 63% increased risk for CVD.
Similar to the risk associated with development of ASCVD and HF, Honigberg et al. found that elevation of HgbA1c in the prediabetic range is also associated with increased risk for development of CKD [17••]. In models with sparse and complete covariate adjustment, respectively, prediabetes was associated with 48% (HR 1.48, 95% CI 1.40–1.56, P < 0.001) and 8% (HR 1.08, 95% CI 1.02–1.14, P 0.009) increased risk for CKD. Risk for CKD was significantly increased at HgbA1c > 6.2% in fully adjusted models. As with the other outcomes studied, continuously increased risk for CKD was observed with increasing HgbA1c. However, unlike ASCVD and HF, the population attributable risk for CKD was slightly larger for baseline T2D vs. prediabetes (10.5% vs. 9.8%).
All-cause Mortality
Mounting evidence associates increasing HgbA1c, even in the prediabetic range, with all-cause mortality. Brewer et al. discovered a U shape mortality curve with respect to HgbA1c, with all-cause mortality increasing for all HgbA1c values ≥ 5% [39]. When analyzed as a continuous variable, each % increase in HgbA1c was associated with 16% increased risk of all-cause mortality (HR 1.16 95% CI 1.11–1.22). In particular, deaths from cardiovascular causes were increased at HgbA1c ≥ 6% in a lightly adjusted model (HR 2.46 95% CI 1.44–4.19). A similar J-shaped all-cause mortality curve was observed with respect to HgbA1c by Silbernagel et al. [40] and Selvin et al. [36••]. In comprehensively adjusted models, association with all-cause mortality became significant for HgbA1c ≥ 6.5%, but cardiovascular mortality trended toward significance for the HgbA1c category of 6.0– < 6.5%. Similar to prior studies, these trends were not observed with fasting glucose.
In the UK Biobank study by Honigberg et al., prediabetes compared to normoglycemia was an independent risk factor for all-cause mortality (HR 1.10 95% CI 1.05–1.14) [17••]. In a fully adjusted model, risk for all-cause mortality began to rise at HgbA1c of 6.2%, with each percentage increase in HgbA1c associated with 11% increased risk (HR 1.11 95% CI 1.09–1.14). Another prospective study by Selvin et al. included stratification of patients by HgbA1c and incidental detection of elevated hs-TnT > 14 ng/L after 6 years of follow-up [36••]. Patients with prediabetes and without pre-existing CVD in this cohort had increased all-cause mortality which was significantly higher when elevated hs-TnT was detected during follow-up (HR 1.71 95% CI 1.43–2.06 vs HR 2.61 95% CI 1.97–345), which in and of itself was more likely to be identified in prediabetic and diabetic patients.
A recent meta-analysis of almost 130 studies containing data from more than 10 million patients confirms these associations [34•]. In the general population, prediabetes was associated with 13% increased risk for all-cause mortality (HR 1.13, 95% CI 1.10–1.17). As expected, the association was greater in a smaller subset of patients with prediabetes and established ASCVD, with 36% increased risk for all-cause mortality.
Potential Mechanisms Linking Dysglycemia to Cardiovascular Disease
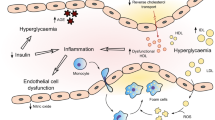
The association of dysglycemia with CVD across the spectrum from prediabetes to overt DM raises the question as to the mechanism(s) by which dysglycemia is deleterious to the cardiovascular system. It is well known that DM is associated with calcification of the media of coronary arteries and with atherosclerotic plaques found to have larger number of macrophage-containing necrotic cores [41]. Interestingly, in type I DM, patients with concomitant insulin resistance have been found to have increased risk of coronary artery calcification, independent of degree of hyperglycemia [42]. This suggests insulin resistance, a hallmark of most patients with prediabetes and a defining feature of T2D, is an independent risk factor for CAD. Hyperglycemia can also induce direct damage to blood vessels through the accumulation of free radicals (in particular the superoxide anion) which has been shown to further trigger downstream cellular pathways mediated by advanced glycation end products (AGEs) [43], protein kinase C (PKC), and NFkB, which are associated with vascular inflammation [44]. AGEs are known to be elevated in patients with DM and are associated with insulin resistance [45,46,47,48], and mechanistically are linked to medial calcification through multiple mechanisms which share a common pathway via activation of receptors for advanced glycation end products (RAGE) that supports the transformation of vascular smooth muscle cells to an osteoblast-like phenotype [49].
Conclusions
HgbA1c below the threshold for the diagnosis of DM, both in the prediabetic range and even below in some cases, associates with subclinical and clinical ASCVD, CKD, HF, and all-cause mortality. The magnitude of this association partially attenuates after adjustment for other commonly comorbid risk factors for CVD, highlighting that HgbA1c in the prediabetic range is a powerful integrator of CVD risk. Clinical risk assessment particularly for primary prevention of CVD may benefit from reframing such that clinicians take notice and address even mild elevation of the HgbA1c, which previously may not have been recognized as a marker of elevated cardiovascular risk. At a minimum, diagnosis of prediabetes should prompt a clinician to re-evaluate a patient’s overall cardiometabolic risk status, addressing important comorbid classical CVD risk factors including hypertension, obesity, smoking, and dyslipidemia.
Furthermore, it appears feasible to isolate patients with prediabetes who have elevated cardiovascular risk similar to patients with DM [17••]. Cardiovascular outcome trials for glucagon-like peptide-1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors have consistently shown reduction in major adverse cardiovascular events in diabetic individuals [50, 51], with evidence that the benefits of SGLT2 inhibitors extend to those without DM as well [52,53,54]. Taken together, these findings call for future trials to test novel risk-reducing therapies specifically in high-risk pre-diabetic individuals. Indeed, trials are underway examining GLP-1 agonists [55] and metformin [56] in this population.
Overall, these data highlight the importance of HgbA1c as a powerful integrator of cardiovascular risk across the glycemic spectrum, including below the threshold for diagnosis of diabetes. Dedicated cardiovascular risk-reduction strategies warrant further study in this large and growing population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329:977–86. https://doi.org/10.1056/NEJM199309303291401.
Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. https://doi.org/10.1136/bmj.321.7258.405.
Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:405–12. https://doi.org/10.1161/01.CIR.59.1.8.
Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13 000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–6. https://doi.org/10.1001/archinte.164.13.1422.
Yusuf PS, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. https://doi.org/10.1016/S0140-6736(04)17018-9.
Dinesh Shah A, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1·9 million people. Lancet. 2015;385(Suppl 1):S86. https://doi.org/10.1016/s0140-6736(15)60401-9.
McAllister DA, Read SH, Kerssens J, Livingstone S, McGurnaghan S, Jhund P, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138:2774–86. https://doi.org/10.1161/CIRCULATIONAHA.118.034986.
Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson A-M, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. New Engl J Med. 2017;376:1407–18. https://doi.org/10.1056/nejmoa1608664.
Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson A-M, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. New Engl J Med. 2018;379:633–44. https://doi.org/10.1056/nejmoa1800256.
Kalyani RR. Glucose-lowering drugs to reduce cardiovascular risk in type 2 diabetes. New Engl J Med. 2021;384:1248–60. https://doi.org/10.1056/nejmcp2000280.
Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med. 2016;241:1323–31. https://doi.org/10.1177/1535370216654227.
Goodarzi MO, Rotter JI. Genetics insights in the relationship between type 2 diabetes and coronary heart disease. Circ Res. 2020:1526–48. https://doi.org/10.1161/CIRCRESAHA.119.316065.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S15-33. https://doi.org/10.2337/dc21-S002.
Rett K, Gottwald-Hostalek U. Understanding prediabetes: definition, prevalence, burden and treatment options for an emerging disease. Curr Med Res Opin. 2019;35:1529–34. https://doi.org/10.1080/03007995.2019.1601455.
Bullard KMK, Saydah SH, Imperatore G, Cowie CC, Gregg EW, Geiss LS, et al. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: national health and nutrition examination surveys, 1999–2010. Diabetes Care. 2013;36:2286–93. https://doi.org/10.2337/dc12-2563.
Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020;174:e194498. https://doi.org/10.1001/jamapediatrics.2019.4498.
••Honigberg MC, Zekavat SM, Pirruccello JP, Natarajan P, Vaduganathan M. Cardiovascular and kidney outcomes across the glycemic spectrum: insights from the UK Biobank. J Am Coll Cardiol. 2021;78:453–64. https://doi.org/10.1016/j.jacc.2021.05.004. (Prospective study of the UK Biobank demonstrating significant association of nearly linear increased risk for ASCVD, HF, CKD, and all-cause mortality with HgbA1c and identified a high-risk population of prediabetes with similar CVD risk as T2D.)
Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Comm. 1969;36:838–43. https://doi.org/10.1016/0006-291x(69)90685-8.
Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. https://doi.org/10.2337/dc09-9033.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. https://doi.org/10.2337/dc11-S062.
Butler AE, English E, Kilpatrick ES, Östlundh L, Chemaitelly HS, Abu-Raddad LJ, et al. Diagnosing type 2 diabetes using Hemoglobin A1c: a systematic review and meta-analysis of the diagnostic cutpoint based on microvascular complications. Acta Diabetol. 2021;58:279–300. https://doi.org/10.1007/s00592-020-01606-5.
Little RR, Rohlfing CL, Sacks DB. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57:205–14. https://doi.org/10.1373/clinchem.2010.148841.
Committee C. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30:2399–400. https://doi.org/10.2337/dc07-9925.
••Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. New Engl J Med. 2010;362:800–11. https://doi.org/10.1056/NEJMoa0908359. (Prospective study demonstrates association of HgbA1c in the prediabetic range with increased risk for CAD and ischemic stroke.)
Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–20. https://doi.org/10.7326/0003-4819-141-6-200409210-00006.
Van’T Riet E, Rijkelijkhuizen JM, Alssema M, Nijpels G, Stehouwer CDA, Heine RJ, et al. HbA1c is an independent predictor of non-fatal cardiovascular disease in a Caucasian population without diabetes: a 10-year follow-up of the Hoorn Study. Eur J Cardiovasc Prev Rehabil. 2012;19:23–31. https://doi.org/10.1097/HJR.0b013e32833b0932.
Bergenstal RM, Gal RL, Connor CG, Gubitosi-Klug R, Kruger D, Olson BA, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167:95–102. https://doi.org/10.7326/M16-2596.
••Rossello X, Raposeiras-Roubin S, Oliva B, Sánchez-Cabo F, García-Ruíz JM, Caimari F, et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J Am Coll Cardiol. 2021;77:2777–91. https://doi.org/10.1016/j.jacc.2021.03.335. (Prospective study identifies increased risk for subclinical atherosclerosis in multiple vascular territories in individuals with HgbA1c below threshold for diagnosis of DM.)
Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49-73. https://doi.org/10.1161/01.cir.0000437741.48606.98.
Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, de Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. https://doi.org/10.1016/s0195-668x(03)00114-3.
•de Jong M, Woodward M, Peters SAE. Diabetes, glycated hemoglobin, and the risk of myocardial infarction in women and men: a prospective cohort study of the UK Biobank. Diabetes Care. 2020;43:2050–9. https://doi.org/10.2337/dc19-2363. (Prospective study demonstrates linear association of HgbA1c with incident MI, with each 1% increase in HgbA1c representing 18% increased risk for MI.)
•Welsh C, Welsh P, Celis-Morales CA, Mark PB, Mackay D, Ghouri N, et al. Glycated hemoglobin, prediabetes, and the links to cardiovascular disease: data from UK Biobank. Diabetes Care. 2020;43:440–5. https://doi.org/10.2337/dc19-1683. (Prospective study associates prediabetes with ASCVD.)
Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5:34–42. https://doi.org/10.1016/S2213-8587(16)30321-7.
•Cai X, Zhang Y, Li M, Wu JHY, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. https://doi.org/10.1136/bmj.m2297. (Meta-analysis of 129 studies including over 10 million subjects associates prediabetes with increased risk for ASCVD and all-cause mortality.)
Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail. 2015;8:448–54. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001990.
••Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130:1374–82. https://doi.org/10.1161/CIRCULATIONAHA.114.010815. (Prospective study demonstrates prediabetes is associated with myocardial damage in the form of increased incidence of elevated hs-TnT, HF, and all-cause mortality.)
Vallianou NG, Mitesh S, Gkogkou A, Geladari E. Chronic kidney disease and cardiovascular disease: is there any relationship? Curr Cardiol Rev. 2019;15:55–63. https://doi.org/10.2174/1573403X14666180711124825.
Neves JS, Correa S, Baeta Baptista R, Bigotte Vieira M, Waikar SS, Mc Causland FR. Association of prediabetes with CKD progression and adverse cardiovascular outcomes: an analysis of the CRIC study. J Clin Endocrinol Metabol. 2020;105:e1772–80. https://doi.org/10.1210/clinem/dgaa017.
Brewer N, Wright CS, Travier N, Cunningham CW, Hornell J, Pearce N, et al. A New Zealand linkage study examining the associations between A1C concentration and mortality. Diabetes Care. 2008;31:1144–9. https://doi.org/10.2337/dc07-2374.
Silbernagel G, Grammer TB, Winkelmann BR, Boehm BO, März W. Glycated hemoglobin predicts all-cause, cardiovascular, and cancer mortality in people without a history of diabetes undergoing coronary angiography. Diabetes Care. 2011;34:1355–61. https://doi.org/10.2337/dc10-2010.
Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191–204. https://doi.org/10.1161/ATVBAHA.116.306256.
Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, et al. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complicat. 2016;30:586–90. https://doi.org/10.1016/j.jdiacomp.2016.02.011.
Soman S, Raju R, Sandhya VK, Advani J, Khan AA, Harsha HC, et al. A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal. 2013;7:19–23. https://doi.org/10.1007/s12079-012-0181-3.
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. https://doi.org/10.1038/35008121.
Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150:523–31.
Stitt AW, Moore JE, Sharkey JA, Murphy G, Simpson DA, Bucala R, et al. Advanced glycation end products in vitreous: structural and functional implications for diabetic vitreopathy. Investig Ophthalmol Vis Sci. 1998;39:2517–23.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. https://doi.org/10.1161/CIRCRESAHA.110.223545.
Tan KCB, Shiu SWM, Wong Y, Tam X. Serum advanced glycation end products (AGEs) are associated with insulin resistance. Diabetes Metabol Res Rev. 2011;27:488–92. https://doi.org/10.1002/dmrr.1188.
Kay AM, Simpson CL, Stewart JA. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res. 2016;2016:6809703. https://doi.org/10.1155/2016/6809703.
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–58. https://doi.org/10.1001/jamacardio.2020.4511.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. https://doi.org/10.1016/S2213-8587(19)30249-9.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. New Engl J Med. 2019;381:1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. New Engl J Med. 2020;383:1436–46. https://doi.org/10.1056/NEJMoa2024816.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. New Engl J Med. 2020;383:1413–24. https://doi.org/10.1056/NEJMoa2022190.
ClinicalTrials.gov. Identifier NCT03574597: semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT) 2021. https://www.clinicaltrials.gov/ct2/show/NCT03574597 (accessed January 11, 2022).
ClinicalTrials.gov. Identifier NCT02915198: investigation of metformin in pre-diabetes on atherosclerotic cardiovascular outcomes (VA-IMPACT) 2021. https://www.clinicaltrials.gov/ct2/show/NCT02915198 (accessed January 11, 2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiometabolic Disease and Treatment
Rights and permissions
About this article
Cite this article
Hoffmann, A.P., Honigberg, M.C. Glycated Hemoglobin as an Integrator of Cardiovascular Risk in Individuals Without Diabetes: Lessons from Recent Epidemiologic Studies. Curr Atheroscler Rep 24, 435–442 (2022). https://doi.org/10.1007/s11883-022-01024-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-01024-8