Abstract
Coronary heart disease (CHD) remains the leading cause of death in the USA. CHD accounts for 48 % of all cardiovascular mortality or approximately one of every seven deaths. Disruption of atherosclerotic plaques—usually by rupture or erosion—and superimposed thrombosis can result in acute coronary syndromes and sudden cardiac death. Silent plaque disruption may also occur and result in coronary plaque progression and ultimately the symptomatic manifestations of stable CHD. Antiplatelet agents remain the cornerstone therapy for acute thrombotic coronary syndromes and are essential for thromboprophylaxis against these events in patients with stable CHD. Antiplatelet drugs are also important adjunct therapies during percutaneous coronary intervention (PCI) as they mitigate equipment-associated thrombotic complications that are partially induced by iatrogenic plaque rupture by interventionalists during balloon angioplasty in the cardiac catheterization laboratory. Since the introduction of clopidogrel, there has been considerable development in this field with at least three novel P2Y12 antagonists approved by the Food and Drug Administration (FDA) over the past decade. Rapidly accumulating evidence is helping to guide the optimal duration of treatment with dual antiplatelet therapy after stenting, especially with the newer drug-eluting stents. More data are also emerging on the hazards and long-term safety of these agents. It is therefore prudent for clinicians to remain current on treatment options and recent advances in this area. We herein review current and emerging antiplatelet therapies and summarize their characteristics and indications of use as well as challenges and areas of ongoing research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although deaths from coronary heart disease (CHD) have been steadily decreasing over the past several years, it still accounts for one out of every seven deaths in the USA or nearly 376,000 fatalities in the current era [1]. The estimated direct and indirect costs for heart disease exceed 200 billion dollars and are expected to rise through 2030 [1]. The number of patients undergoing percutaneous coronary interventions (PCIs) annually remains elevated with nearly half a million patients with drug-eluting stents (DESs) deployed in more than three quarters of all PCIs.
Notable advances in antiplatelet therapy have been made since the release of the CAPRIE and the CURE trials showing beneficial effects of clopidogrel in patients with stable CHD and acute coronary syndromes (ACSs), respectively [2, 3••]. Since then, additional P2Y12 receptor inhibitors (i.e., prasugrel, ticagrelor) have been shown to further reduce cardiovascular events compared to clopidogrel. Prasugrel, an oral thienopyridine with a more efficient metabolic pathway, was the first to show a greater reduction in reinfarction compared with clopidogrel but with increased bleeding events in the TRITON-TIMI 38 trial [4••, 5]. The PLATO trial subsequently demonstrated the superiority of ticagrelor, a direct oral inhibitor of the P2Y12 receptor, over clopidogrel in reducing not only reinfarction but also vascular mortality [6, 7••]. Additional antiplatelet agents such as vorapaxar and cangrelor, the first intravenous P2Y12 antagonist, were thereafter approved by the FDA [8, 9].
Pathophysiology of Coronary Heart Disease and Treatment Targets
Atherosclerosis in CHD typically occurs over decades and is influenced and perpetuated by many risk factors (e.g., hyperlipidemia, diabetes, and hypertension). As coronary atherosclerosis progresses, plaques may grow outward across the length of the coronary vessel or inward encroaching on the coronary lumen (negative remodeling). Vulnerable plaques (or at-risk plaques) are characterized by a large necrotic core, rich in cholesterol esters and debris, and by the presence of inflammatory cells such macrophages which are usually at the plaque shoulders, along with a thin fibrous cap. Plaques with thin fibrous caps (<65 μ), or thin cap fibroatheromas (TCFAs), represent an example of vulnerable plaques that have higher propensity to rupture. When this occurs spontaneously or following iatrogenic vascular injury (e.g., during PCI), platelet adhesion, activation, and aggregation occur (see Fig. 1) and activation of the coagulation cascade takes place [11]. The severity of the acute coronary obstruction and blood flow interruption will determine whether the ensuing ACS is an ST-segment elevation myocardial infarction (STEMI) or non-ST-elevation ACS (NSTE-ACS). Complete obstruction usually results in a STEMI and subsequent Q-wave formation on the electrocardiogram (ECG; >80 % of the time), while severely obstructive but <100 % occlusive plaque + thrombus complexes usually result in NSTE-ACS, often with no subsequent Q-wave development on the ECG. NSTE-ACS is further divided into non-ST-elevation MI (NSTEMI) and unstable angina (UA) depending on the occurrence of myonecrosis as evidenced by a characteristic rise and fall in cardiac troponins.
Platelet adhesion is characterized by platelets (shown in orange) binding through specific receptors on their outer surface (i.e., P2Y12, TxA2, glycoprotein IIb/IIIa, etc.). Figure courtesy of Jneid [10]
The platelet cascade, consisting of platelet adhesion, activation, and aggregation, is vital to the understanding and management of antiplatelet pharmacotherapy (Figs. 1 and 2) [11]. Thrombin and other substances such as adenosine diphosphate (ADP) can stimulate platelet activation. Serotonin and thromboxane A2 as well as more ADP are thereafter released, which further promotes platelet activation and aggregation. ADP can activate the purinergic P2Y12 receptors on the surface of the platelet resulting in activation of the glycoprotein IIb/IIIa complex, a receptor for fibrinogen, and the final major common pathway controlling platelet aggregation. Other receptors such as protease-activated receptor-1 (PAR-1) are also expressed on the surface of platelets and can induce aggregation through thrombin and thrombin receptor agonist peptides (TRAPs). The existing oral antiplatelet agents have predominantly targeted ADP-induced platelet aggregation through antagonism of the P2Y12 receptor. An example of a P2Y12 receptor inhibitor is clopidogrel, whose active metabolites impair platelet aggregation for the remainder of the platelet lifespan (approximately 7 to 10 days) [12].
Treatment targets for antiplatelet agents in coronary heart disease (antagonism of the PAR-1 receptor with vorapaxar not pictured). Figure courtesy of Jneid [10]
Aspirin
Acetylsalicylic acid irreversibly inhibits both cyclooxygenase 1 and 2 (COX-1, COX-2) through acetylation of specific serine moieties. Through COX inhibition, production of thromboxane A2—a dual vasoconstrictor and platelet agonist—is also halted. The antiplatelet effects of aspirin persist for the entire lifetime of the platelet. Ibuprofen and most non-steroidal anti-inflammatory drugs (NSAIDs) also inhibit COX-1 activity which may diminish the cardiovascular benefits of aspirin when administered jointly. NSAIDs should therefore be avoided in patients with CHD. The antithrombotic effects of aspirin have long been established, making aspirin one of the most commonly prescribed medications for CHD. It is inexpensive and has longstanding evidence to support its use [13•, 14–16]. Thus, the American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) Guidelines support the immediate and prehospital loading of aspirin (162–325 mg) in ACS patients followed by preferably lower maintenance doses (81 mg daily). Aspirin is used in conjunction with a P2Y12 antagonist in ACS patients and in all patients undergoing PCI with stent placement [17–19]. The large, randomized, multi-centered CURRENT-OASIS 7 trial found no difference in the primary outcome of cardiovascular death, nonfatal myocardial infraction, or stroke but a small increase in the incidence of gastrointestinal bleeding (0.4 vs. 0.2 %, p = 0.04) with higher aspirin doses (300 to 325 mg daily) compared to a lower dose (75 to 100 mg daily) [20]. Enteric-coated formulations should be avoided in ACS patients because of delayed absorption. In an ACS setting, patients should be advised to chew aspirin for quicker absorption through the sublingual plexus and faster onset of antiplatelet effects. Abdominal pain and gastrointestinal upset are common complaints associated with aspirin. They are dose-dependent and may be minimized when given with food. Gastrointestinal bleeding (e.g., peptic ulcer disease, erosive gastritis/esophagitis) is a dreadful complication of aspirin but can be minimized by the concomitant use of proton pump inhibitors (PPIs). Rarely, patients may report hypersensitivity reactions such as angioedema, bronchospasm, or skin rash. Aspirin desensitization protocols are available and should be considered in suitable candidates who require PCI with stent placement.
Ticlopidine and Clopidogrel [11, 12]
Ticlopidine was the original thienopyridine used for ADP antagonism during PCI and stenting to prevent stent thrombosis. Due to its poor safety profile, which includes life-threatening blood dyscrasias (i.e., neutropenia), ticlopidine is rarely used by clinicians. In addition to its improved safety profile, clopidogrel is a more potent antiplatelet agent, has a quicker onset of action, convenient once daily dosing, and has been studied for a broad range of indications and in a variety of clinical settings [2, 3••, 12, 21]. It was initially approved by the FDA in November 1997. The CURE study, a randomized controlled trial (RCT) of more than 12,000 patients with NSTE-ACS, demonstrated that clopidogrel confers (on the background of aspirin therapy) a 20 % reduction in the composite of cardiovascular (CV) death, nonfatal MI or stroke over aspirin alone after a mean follow-up of 9 months [3••]. In the CAPRIE trial, clopidogrel has shown superior effects to aspirin in the primary prevention setting. CAPRIE demonstrated an 8.7 % relative reduction in the composite of vascular death, MI, or ischemic stroke with clopidogrel compared to aspirin after nearly 2 years of therapy in patients with established atherosclerotic disease [2]. Clopidogrel has also shown benefits in STEMI patients following fibrinolytic therapy and after PCI [21–23] and is currently the most commonly used antiplatelet therapy after aspirin, and the agents which newer oral antiplatelet agents are compared to in clinical trials. The generic availability of clopidogrel makes it even more readily accessible worldwide.
Clopidogrel has however several shortcomings. Its metabolism requires a two-step enzymatic transformation to an active metabolite. This involves several cytochrome P450 enzymes and notably the CYP2C19 isoenzyme. Several loss-of-function alleles for the CYP2C19 isoenzyme were shown to affect the metabolism of clopidogrel leading to concerns about its real-world effectiveness and prompting a black box warning by the FDA. Genotyping is offered but there is a lack of consensus regarding its usefulness and cost-effectiveness. Because of the multiple cytochrome enzymes involved in its activation, there are concerns of drug interactions and how they could alter its effects. Common interactions involve the CYP2C19 inhibitors and include frequently used medications such as omeprazole and esomeprazole. The COGENT RCT did not, however, show adverse CV effects when clopidogrel was combined in one tablet with 20-mg omeprazole [24]. The ACCF/AHA guidelines have recognized the inconsistent evidence associating the use of certain PPIs with adverse CV events in clopidogrel-treated patients [25]. Nevertheless, it may not be unreasonable to consider other alternative PPIs such as pantoprazole when prescribing clopidogrel.
Table 1 highlights pertinent clinical characteristics of clopidogrel. Loading doses of 600 mg are typically used in PCI-treated patients, while medically treated ACS patients and those <75-year-old who are receiving fibrinolytic therapy are usually loaded with a 300-mg regimen. The maintenance dose is usually 75 mg daily. A double-dose clopidogrel regimen after ACS (600 mg on day 1 followed by 150 mg daily for the first 6 days) has been tested in the CURRENT-OASIS 7 trial and showed a numerical but non-statistically significant reduction in the composite of CV death, MI, or stroke (4.2 vs. 4.4 %, p = 0.3) but a higher incidence of major bleeding (2.5 vs. 2 %, p = 0.01) compared to a standard dose (300 mg on day 1 then 75 mg daily) [20]. The double-dose clopidogrel regimen was also associated with lower numbers of definite stent thrombosis in ACS patients undergoing PCI. A repeat loading dose of clopidogrel may be beneficial in ACS patients undergoing PCI who are already on chronic therapy [26, 27]. Clopidogrel is generally well tolerated although patients may experience gastrointestinal bleeding as well as a vexing rash, which can occur within the first few days of therapy and may become clinically significant if not addressed. Thrombotic thrombocytopenic purpura has been reported rarely with clopidogrel, although it is much more common with ticlopidine. Desensitization protocols are available in the literature; however, it may be more practical to switch to an alternative P2Y12 receptor inhibitor such as ticagrelor or prasugrel in the absence of contraindications (i.e., stroke) to these agents.
Prasugrel
Prasugrel is a newer generation thienopyridine that is more potent and faster acting than clopidogrel. It was approved by the FDA in July 2009. Unlike clopidogrel, it undergoes a one-step metabolism for conversion to its active metabolite. It is indicated for the reduction of thrombotic CV events, including stent thrombosis, in ACS patients undergoing PCI. Its efficacy was demonstrated in the large TRITON-TIMI 38 trial, where it was compared head-to-head with clopidogrel (on the background of aspirin therapy) and demonstrated a greater reduction in the combined endpoint of CV death, nonfatal MI, or stroke [4••]. The benefit from prasugrel in the primary composite endpoint was mainly driven by a reduction in nonfatal MI, and a significant reduction was also noted in the incidence of stent thrombosis but not in CV mortality [4••, 5]. On subgroup analyses, diabetics and patients with previous MI had greater benefits from prasugrel use, while those with a previous history of stroke or transient ischemic attack (TIA) experienced net clinical harm. Thus, prasugrel carries a black box warning against its use in patients with a prior history of stroke or TIA [5]. Patients who were 75 years and older or who weighed ˂60 kg reported no clinical benefit from prasugrel.
Unlike clopidogrel and ticagrelor, prasugrel may be administered with inducers (e.g., phenytoin, carbamazepine, barbiturates) or inhibitors (e.g., protease inhibitors) of the cytochrome P450 system. The ACCOAST trial findings confirmed that upstream treatment with prasugrel is not beneficial in NSTE-ACS [28]. Therefore, it is recommended to delineate the coronary anatomy and ascertain that the patient is committed to undergo PCI before loading with prasugrel. In the TRILOGY ACS trial, patients with NSTE-ACS who were undergoing medical management only had no increased benefit from the use of prasugrel over clopidogrel, so this agent should be avoided in medically treated ACS patients [29]. The FDA granted approval for use of the 5-mg dose as a maintenance dose in patients >75 years of age or who weigh less than 60 kg, although this dose has no definitive data supporting its efficacy. Table 1 summarizes pertinent clinical and pharmacologic characteristics of prasugrel.
Ticagrelor
Ticagrelor is an FDA-approved (2011) reversible direct-acting inhibitor of the P2Y12 receptor that is also more potent and faster acting than clopidogrel. It has an indication for reduction of thrombotic CV events in patients with ACS regardless of the management strategy (i.e., both PCI- and medically treated patients). Superiority of ticagrelor over clopidogrel, on the background of aspirin therapy, with respect to ischemic outcomes was demonstrated in the PLATO trial at 12 months (in the setting of an acute MI) and subsequently at a median of 33 months in the PEGASUS-TIMI 54 trial (in patients with a history of MI more than 1 year earlier) [7••, 30••]. In the PLATO trial, ticagrelor reduced the composite of vascular death, MI, or stroke in all ACS patients [7••]. Unlike prasugrel, the primary endpoint in PLATO was driven not only by a reduction in MI but also by a 1.1 % statistically significant absolute risk reduction in vascular mortality [4••, 7••]. Overall, ticagrelor also significantly reduced mortality by 1.4 % [7••].
Ticagrelor requires twice daily dosing and is metabolized through the CYP3A4 system which predisposes it to numerous drug–drug interactions (i.e., carbamazepine, phenytoin, etc.) [6]. Providers should be especially cautious to avoid its use with CYP3A4 inducers due to the potential for decreased concentrations, which may theoretically increase the risk for a thrombotic event. Conversely, CYP3A4 inhibitors (e.g., protease inhibitors) can potentially increase ticagrelor concentrations and augment the risk of bleeding. Aspirin maintenance dose with ticagrelor should not exceed 100 mg daily (i.e., 81 mg once daily in the USA) because of the potential for worse outcomes with higher aspirin maintenance doses. The manufacturer also cautions against doses greater than 40 mg daily for simvastatin and lovastatin because of the heightened risk for myalgia arising from CYP3A4 competition. This is unlikely to be clinically important given that high-intensity statins are now recommended to all patients with established atherosclerotic CVD [31]. Due to structural similarities to adenosine, ticagrelor may be associated with ventricular pauses and dyspnea. Ticagrelor should also be avoided in those with a prior history of intracranial hemorrhage. The loading dose of ticagrelor is 180 mg followed by 90 mg twice daily which can be administered either upstream or following successful PCI. The ATLANTIC trial failed to demonstrate benefits from prehospital administration (i.e., during ambulance transport) of ticagrelor [32]. Lastly, the PEGASUS-TIMI 54 demonstrated prolonged benefits of ticagrelor beyond the typical 12-month duration in patients with a prior MI (albeit at an increased risk for major bleeding) and validated the efficacy of chronic ticagrelor therapy with the 60 mg twice daily regimen currently approved by the FDA [30••] (see Tables 1, 2, and 3).
Vorapaxar
As opposed to the P2Y12 receptor inhibitors, vorapaxar inhibits platelet aggregation via a unique mechanism. It acts through competitive and selective antagonism of the PAR-1 receptor [8]. The PAR-1 is the major thrombin receptor in the platelet but can also be found in other cell types. Vorapaxar inhibits platelet aggregation promoted through thrombin and thrombin receptor agonist peptide-1. Although this blockade is reversible, its long half-life makes it essentially irreversible. Vorapaxar was approved by the FDA in May 2014 following the results of the TRA 2P-TIMI 50 study [33]. In this study, vorapaxar reduced the primary efficacy composite endpoint of CV death, MI, or stroke at 3 years in patients with stable atherosclerosis who were receiving standard therapy [33]. However, it increased the risk of moderate or severe bleeding including intracranial hemorrhage, and the trial was terminated prematurely by the DSMB in patients with a prior history of stroke [33].
The FDA approved vorapaxar for reduction of thrombotic CV events in patients with a history of MI or with established PAD. One unusual aspect of vorapaxar is the fact that 2.5 mg daily was studied in the TRA 2P-TIMI 50 trial; however, the approved dose is 2.08 mg once daily which is a revised formulation designed to be dose equivalent to the dose that was studied. Vorapaxar should be avoided in patients with a prior history of stroke (black box warning by the FDA). Vorapaxar was also studied in the TRACER trial, a study of nearly 13,000 ACS patients, which failed to demonstrate a benefit and was terminated early (see Table 2) [34]. In TRACER, vorapaxar was used in conjunction with guideline recommended medications including dual antiplatelet therapy (DAPT) which primarily consisted of clopidogrel and aspirin. It is difficult to identify the role that vorapaxar plays in the management of ACS based on the discrepancy in dose, findings from TRACER, and the balance of its risk and benefit profile (Table 2). As of now, the ACCF/AHA guidelines for NSTE-ACS, STEMI, or stable ischemic heart disease (SIHD) did not incorporate vorapaxar as a treatment option [17–19, 35] (Table 4).
Cangrelor
After an extensive and controversial review, the FDA approved cangrelor on June 2015 as the first intravenous P2Y12 receptor inhibitor. Its use is intended as an adjunct to PCI for reducing the risk of peri-procedural MI, repeat coronary revascularization, and stent thrombosis in patients who were not treated with a P2Y12 receptor inhibitor previously and who are not receiving a glycoprotein IIb/IIIa inhibitor [9]. Cangrelor has several advantages over current therapies, including reversible inhibition of the P2Y12 receptors similar to ticagrelor, rapid onset (platelet inhibition occurring within 2 min) and offset (platelet function returning to normal within an hour), short half-life (3–6 min), and less variable platelet inhibition compared to clopidogrel (see Table 1) [9, 36–40]. Based on its pharmacokinetics, an antidote may not be necessary to reverse cangelor’s antiplatelet effects, and it may be a viable option for patients requiring urgent surgery [36–38]. Additionally, its metabolic pathway predisposes it to significantly less drug–drug interactions [9]. The safety and efficacy of this agent were based on a few clinical trials including the CHAMPION, CHAMPION-PCI, and CHAMPION-PHOENIX trials which, due to conflicting results, led to a protracted approval process for this agent [39–41]. Cangrelor’s approval was ultimately based on the findings from the CHAMPION-PHOENIX trial [41] (Table 5).
The CHAMPION-PHOENIX trial randomized patients to cangrelor (bolus and infusion) versus clopidogrel (300–600 mg bolus), with an additional clopidogrel 600 mg bolus given post-PCI in the cangrelor arm [41]. The primary composite endpoint of death from any cause, MI, ischemia-driven revascularization, or stent thrombosis at 48 h was significantly lower in the cangrelor group (4.7 vs. 5.9 % in the clopidogrel group, p = 0.005) [41]. The number needed to treat for cangrelor to prevent one primary endpoint event was 84 (95 % CI, 49 to 285). The composite efficacy endpoint was significantly lower in the cangrelor group at 30 days (6 vs. 7 %, p = 0.03) [41]. The secondary efficacy endpoint of stent thrombosis was lower in the cangrelor group at 48 h and at 30 days, and the use of glycoprotein IIb/IIIa inhibitor rescue therapy as well as the rate of procedural complications were also lower in the cangrelor group [41]. The primary safety endpoint of GUSTO severe bleeding was comparable between both drugs. Steg and colleagues subsequently conducted a meta-analysis of three key clinical trials (CHAMPION-PCI, PLATFORM, and PHOENIX) and demonstrated a reduction in the primary composite endpoint of death, MI, ischemia-driven revascularization, or stent thrombosis at 48 h with cangrelor (3.8 vs. 4.7 % for control; OR 0.81, 95 % CI 0.71–0.91, p = 0.0007) [42].
Overall, cangrelor is useful as an adjunct to PCI in patients not pretreated with an oral P2Y12 receptor inhibitor and not receiving an intravenous GP IIb/IIIa inhibitor. Given its rapid onset and offset kinetics, it may be also useful as a bridge therapy in stented patients in whom oral P2Y12 receptor inhibitors are discontinued in anticipation of surgery. Its efficacy and usefulness need to be further corroborated by additional studies and clinical experience.
Duration of Oral Antiplatelet Therapy
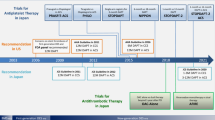
As newer generation stents become available, clinicians continue to explore the benefits of prolonged DAPT and balance its salubrious effects on ischemic events, including stent thrombosis, with its bleeding hazards [43, 44]. The ACCF/AHA clinical practice guidelines recommend at least 12 months of DAPT following either an ACS or PCI with DES placement [17–19]. Whether prolonging DAPT beyond a year is useful remains controversial but may be a reasonable strategy in patients at low bleeding risk who are at risk for very late stent thrombosis (i.e., >12 months after stent implantation). Anatomical and clinical predictors of stent thrombosis, at least in terms of older generation DES, include long stents, small vessel reference diameter, diabetes, kidney disease, and non-compliance. Some clinical studies failed to find conclusive evidence that extending DAPT beyond 1 year reduced major adverse cardiac events, although those are met with criticism about their trial design and other potential flaws [45–48]. Other studies have focused on shorter durations of DAPT and compared 12-month DAPT durations with shorter time periods, and of those many were non-inferiority trials that mainly enrolled low risk populations and failed to show significant differences in outcomes [46–55]. There are some studies to suggest that a shorter duration (≤6 months) of DAPT may have similar outcomes as opposed to longer periods (≤6 months) in those undergoing percutaneous coronary intervention with newer generation stents who are aspirin sensitive [52–56].
The DAPT trial stands out as a large and well-conducted clinical trial in the field, which compared 12 versus 30 months of DAPT in 9961 patients undergoing PCI with DES implantation [57]. This trial showed significant reductions in major adverse cardiac and cerebrovascular events [MACCE] (4.3 vs. 5.9 %, p < 0.001) at the expense of a higher rate of moderate to severe bleeding (2.5 vs. 1.6 %, p = 0.001). There was a worrisome numerical increase in all-cause mortality (2 vs. 1.5 %, p = 0.05) in the extended (30 months) thienopyridine group which, however, appeared to be driven by non-CV events (predominantly cancer-related deaths). Importantly, a significant reduction in the occurrence of stent thrombosis (0.4 vs. 1.4 %, p < 0.001) was observed in the extended thienopyridine group, with a rebound increase in thrombotic events in the initial 3 months following discontinuation of the thienopyridine. On the other hand, the DAPT investigators demonstrated that extending thienopyridine for an additional 18 months in patients receiving a bare-metal stent (BMS) and who tolerated 12 months of thienopyridine did not improve stent thrombosis or MACCE rates [43].
Another study, the PEGASUS-TIMI 54 trial, examined patients with a history of MI in the preceding 1–3 years and randomized them in a 1:1:1 manner to ticagrelor 60 mg twice daily (n = 7045), 90 mg twice daily (n = 7050), or placebo (n = 7067) [30••]. Following a median of 33 months, the primary composite endpoint (CV death, MI, or stroke) occurred in 7.8, 7.9, and 9.0 % of patients receiving 60 mg twice daily, 90 mg twice daily, and placebo, respectively (HR for 90 mg vs. placebo, 0.85, 95 % CI, 0.75–0.96; HR for 60 mg vs. placebo 0.84, 95 % CI, 0.74–0.95). However, patients on ticagrelor 60 and 90 mg twice daily experienced high rates of TIMI major bleeding compared to placebo (2.3 and 2.6 %, respectively, vs. placebo 1.06 %; p < 0.001 for each dose comparison). A subsequent meta-analysis demonstrated reductions in CV death, recurrent MI, or stroke but increased major bleeding in those stable but high-risk patients who continue DAPT over a year for up to an average of 31 months [55].
Intravenous Glycoprotein IIb/IIIa Receptor Inhibitors
Studies supporting the use of glycoprotein (GP) IIb/IIIa receptor inhibitors predated the trials that established the benefits of P2Y12 receptor inhibitors, early invasive therapy in NSTE-ACS patients, and contemporary medical treatments. Three types of intravenous GP IIb/IIIa antagonists exist: abciximab and the small molecules, eptifibatide and tirofiban. The small molecules are beneficial as an adjunct to aspirin in NSTE-ACS patients receiving aspirin monotherapy.
Clinical trials supported the upstream use of a GP IIb/IIIa receptor inhibitor as a second agent in combination with aspirin for dual antiplatelet therapy in patients with NSTE-ACS and in all CHD patients undergoing PCI. The benefits appeared more pronounced in patients at highest risk, such as diabetic patients and those with elevated troponin enzymes.
In the current era, where patients are pretreated with a P2YY12 receptor inhibitor, the benefits of GP IIb/IIIa receptor inhibitors are less certain. In this instance, they are likely to be beneficial in high-risk patients (e.g., those with a thrombotic ACS event) and for bail-out during PCI (e.g., wire thrombosis, distal embolization, acute closure). In NSTE-ACS patients, the routine use of upstream intravenous GP IIb/IIIa receptor inhibitors in NSTE-ACS patients pretreated with DAPT increases the risk of bleeding and does not improve ischemic outcomes, as demonstrated in the EARLY-ACS trial [58].
Conclusions
Antiplatelet therapies remain the mainstay treatment for CHD. Every ACS patients and patients undergoing PCI should in general receive DAPT. The choice of a second agent to add to aspirin (preferably 81 mg dose) remains a matter of constant debate. There are no definitive head-to-head comparative studies examining the safety and efficacy of the newer P2Y12 antagonists, prasugrel and ticagrelor. The ISAR-REACT-5 trial (NCT01944800) is an ongoing, prospective, randomized multicenter trial aiming to compare ticagrelor versus prasugrel in 4000 ACS patients planned to undergo an invasive management strategy. This may ultimately guide the choice of the second P2Y12 inhibitor to be added to aspirin as a DAPT regimen in these patients. This trial, however, is not expected to be completed until October 2018. Notably, research is needed to inform clinicians on the optimal means to transition patients between the different P2Y12 inhibitors. Patients taking triple antithrombotic therapy are at high risk for bleeding and poor outcomes and also warrant further study. Extended durations (>12 months) of DAPT may be acceptable in those at high risk of ischemic events who tolerate at least 12 months of DAPT. While at least 12-month duration is currently the acceptable DAPT duration after DES implantation and after an ACS, shorter duration (6 months) may be sufficient in stable CHD patients undergoing elective PCI with DES.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mozaffarian D, Benjamin EJ, Go AS, et al on behalf of the American Heart Association Statistics Committee and Stroke Statistics Committee. Heart disease and stroke statistics—2015 update. Circulation. 2015;131(4):e29–322
Steering Committee CAPRIE. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE). Lancet. 1996;348(9038):1329–39.
Yusuf S, Zhao F, Mehta SR, on behalf of the CURE Investigators, et al. Effect of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. This was the first trial to report the benefits of clopidogrel on top of aspirin in reducing ischemic CV events after a non-STE-elevation ACS (NSTE-ACS). A separate substudy, the PCI-CURE trial, further demonstrated a reduction in recurrent ischemic CV events in NSTE-ACS patients undergoing percutaneous coronary intervention (PCI). Both reports established clopidogrel as a cornerstone in dual antiplatelet therapy after NSTE-ACS.
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. The TRITON-TIMI 38 trial proved that prasugrel, a more potent and predictable thienopyridine, was superior to clopidogrel in reducing recurrent ischemic events in those undergoing percutaneous coronary intervention for acute coronary syndrome at the expense of an increase in major and life threatening bleeding.
Effient (Prasugrel) Package insert. Indianapolis, IN: Eli Lilly and Company and Daiichi Sankyo, Inc.; 2015
Brilinta (Ticagrelor) Package insert. Wilmington, DE: AstraZeneca LP; 2015
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. The PLATO trial demonstrated that ticagrelor, a faster-acting and more potent P2Y12 receptor inhibitor, was superior to clopidogrel in reducing ischemic CV events (on the background of aspirin therapy) in patients with acute myocardial infarction (medically-treated or PCI-treated). Notably, ticagrelor benefits were driven by a reduction in both recurrent MI and mortality. Similar major bleeding risk (primary safety endpoint) was observed compared with clopidogrel.
Zontivity (Vorapaxar) Package insert. Whitehouse Station, NJ: Merck & Co., Inc.; 2015
Kangreal (cangrelor) Package insert. Parsippany, NJ: The Medicines Company; 2015
Jneid H. Pharmacotherapy in the modern interventional suite. In: Bhatt DL, editor. Cardiovascular intervention: a companion to Braunwald’s heart disease. Philadelphia: Elsevier; 2016. p. 4.1–4.22.
Jneid H, Bhatt DL, Corti R, et al. Aspirin and clopidogrel in acute coronary syndromes. Arch Intern Med. 2003;163:1145–53.
Plavix (Clopidogrel) Package insert. Bridgewater, NJ: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; 2015
Lewis HD, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina: results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309:396–403. This landmark multicenter study conducted at the Veterans Affairs healthcare system demonstrated the efficacy of a 12-week regimen of aspirin compared to placebo in reducing the composite endpoint of death or myocardial infarction (MI) in patients with unstable angina. This study further established the efficacy of aspirin in secondary prevention, and reported a dramatic relative risk reduction of 51% in the incidence of death or MI.
Second International Study of Infarct Survival Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2(8607):349–60.
Theroux P, Ouimet H, McCans J, et al. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988;319(17):1105–11.
Antithrombotic Trialist Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Jneid H, Anderson JL, Wright RS, on behalf of the ACCF/AHA Task Force on Practice Guidelines, et al. 2012 American College of Cardiology Foundation/American Heart Association focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. Circulation. 2013;127:e663–828.
Levine GN, Bates ER, Blankenship JC, on behalf of the ACCF/AHA/SCAI Task Force, et al. 2011 American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the Society for Cardiovascular Angiography and Interventions Guideline for Percutaneous Coronary Intervention. Circulation. 2011;124:e574–651.
O’Gara PT, Kushner FG, Ascheim DD, on behalf of the ACCF/AHA Task Force on Practice Guidelines, et al. 2013 American College of Cardiology Foundation/American Heart Association guideline for the management of ST-elevation myocardial infarction. Circulation. 2013;127:e362–425.
CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363:930–42.
Sabatine MS, Cannon CP, Gibson CM, on behalf of the CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352(12):1179–89.
Mehta SR, Yusuf S, Peters RJ, on behalf of the PCI-CURE Investigators, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–33.
Steinhubl SR, Berger PB, Mann JT, on behalf of the CREDO Investigators, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–20.
Bhatt DL, Cryer BL, Contant CF, on behalf of the COGENT Investigators, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–17.
Abraham NS, Hlatky MA, Antman EM, et al. The American College of Cardiology Foundation (ACCF)/American College of Gastroenterology (ACG)/American Heart Association (AHA) 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol. 2010;105:2533–49.
Di Sciasco G, Patti G, Pasceri V, et al. Clopidogrel reloading in patients undergoing percutaneous coronary intervention on chronic clopidogrel therapy: results of the ARMYDA-4 RELOAD (Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty) randomized trial. Eur Heart J. 2010;31(11):1337–43.
Patti G, Pasceri V, Mangiacapra F, et al. Efficacy of clopidogrel reloading in patients with acute coronary syndrome undergoing percutaneous coronary intervention during chronic clopidogrel therapy (from the Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty [ARMYDA-8 RELOAD-ACS] trial. Am J Cardiol. 2013;112(2):162–8.
Montalescot G, Bolognese L, Dudek D, on behalf of the ACCOAST Investigators, et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med. 2013;369:999–1010.
Roe MT, Armstrong PW, Fox KA, on behalf of the TRILOGY ACS Investigators, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–309.
Bonaca MP, Bhatt DL, Cohen M, on behalf of the PEGASUS-TIMI 54 Steering Committee and Investigators, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800. This trial established the benefits of dual antiplatelet therapy, with aspirin and ticagrelor, in patients with stable coronary heart disease who had a myocardial infarction (MI) 1 to 3 years earlier. In that trial, dual antiplatelet therapy reduced the composite of cardiovascular death, MI or stroke at the expense of an increase in major bleeding risk after a median follow-up of 33 months.
Stone NJ, Robinson J, Lichtenstein AH, et al. 2013. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the ACC/AHA task force on practice guidelines. Circulation. 2013. Available online at: http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a.citation
Montalescot G, AW V’t h, Lapostolle F, on behalf of the ATLANTIC Investigators, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016–27.
Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–13.
Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33.
Fihn SD, Gardin JM, Abrams J, et al. The 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126:e354–471.
Ueno M, Ferreiro JL, Angiolillo DJ. Update on the clinical development of cangrelor. Expert Rev Cardiovasc Ther. 2010;8(8):1069–77.
Akers WS, Oh JJ, Oestreich JH, et al. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50:27–35.
Angiolillo DJ, Bhatt DL, Gurbel PA, et al. Advances in antiplatelet therapy: agents in clinical development. Am J Cardiol. 2009;103:40A–51.
Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI (CHAMPION PCI). N Engl J Med. 2009;361:2318–29.
Bhatt DL, Lincoff AM, Gibson CM, on behalf of the CHAMPION PLATFORM Investigators, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330–41.
Bhatt DL, Stone GW, Mahaffey KW, on behalf of the CHAMPION PHOENIX Investigators, et al. Effect of Platelet inhibition with cangrelor during PCI on ischemic events (CHAMPION PHOENIX). N Engl J Med. 2013;368:1303–13.
Steg PG, Bhatt DL, Hamm GW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382:1981–92.
Kereiakes DJ, Yeh RW, Massaro JM, et al. Antiplatelet therapy duration following bare metal or drug-eluting coronary stents. JAMA. 2015;313(11):1113–21.
Raber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer generation everolimus-eluting stent compared with early-generation drug-eluting stents. Circulation. 2012;125:1110–21.
Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–82.
Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129(3):304–12.
Collet JP, Silvain J, Barthelemy O, on behalf of the ARCTIC Investigators, et al. Dual antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomized trial. Lancet. 2014;384(9954):1577–85.
Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015–26.
Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to reduce late loss after Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505–13.
Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (Real Safety and Efficacy of 3-month dual antiplatelet therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60(15):1340–8.
Feres F, Costa RA, Abizaid A, et al. Three vs. twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510–22.
Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64(20):2086–97.
Schulz-Schupke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36(20):1252–63.
Gilard M, Barragan P, Noryani AA, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol. 2015;65(8):777–86.
Udell JA, Bonaca MP, Collet JP, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 31 August 2015. Available online at: http://eurheartj.oxfordjournals.org/content/early/2015/08/28/eurheartj.ehv443
Huang H, Li Y, Sun Meina. Shorter (≤6 months) vs. longer (≥12 months) dual antiplatelet therapy after second-generation drug-eluting stents implantation: a meta-analysis of randomized controlled trials. Eur Heart J. 29 February 2016. Available online at: http://eurheartjsupp.oxfordjournals.org/content/18/suppl_A/A54
Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66.
Giugliano RP, White JA, Bode C, on behalf of the EARLY ACS Investigators, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009;360(21):2176–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael Gillette, Kathleen Morneau, Vu Hoang, Salim Virani, and Hani Jneid declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Coronary Heart Disease
Rights and permissions
About this article
Cite this article
Gillette, M., Morneau, K., Hoang, V. et al. Antiplatelet Management for Coronary Heart Disease: Advances and Challenges. Curr Atheroscler Rep 18, 35 (2016). https://doi.org/10.1007/s11883-016-0581-6
Published:
DOI: https://doi.org/10.1007/s11883-016-0581-6