Abstract
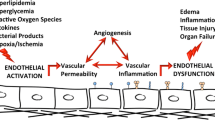
Endothelial inflammation is an important risk factor in the initiation and development of vascular disease. Therefore, signaling cascades and patho-physiological outcomes of endothelial inflammation are important questions in vascular biology. Recent studies suggest that sphingosine-1-phosphate receptor subtype 2 (S1PR2) signaling in endothelial cells (ECs) play a critical role in endothelial inflammation. For example, ECs present in atherosclerotic plaques exhibit senescence phenotype. Levels of S1PR2 are markedly increased in cultured senescent ECs and in lesion regions of atherosclerotic endothelium. Also, inflammatory cytokines and mechanical flow stress profoundly increase S1PR2 levels in ECs. Inhibition of endothelial S1PR2 signaling diminishes endothelial senescence-associated functional impairments and atherogenic stimuli-induced endothelial activation. In contrast, activation of endothelial S1PR2 stimulates the production of pro-inflammatory chemokines/cytokines and lipid mediators in ECs. In this article, we will review signaling and functions of sphingosine-1-phosphate (S1P) receptors in endothelial biology, with particular focus on endothelial S1PR2 signaling-mediated endothelial inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sphingosine-1-phosphate (S1P), a serum-borne sphingolipids (SPLs), is converted from plasma membrane sphingomyelin (SPM) by the sphingolipid catabolic pathway. Alternatively, S1P can be synthesized de novo by the sphingolipid biosynthetic pathway [1, 2]. Signaling and functions of S1P have been extensively elucidated in various physio-pathological events during the past 15 years. At least two milestone discoveries in late 1990s–early 2000s contribute to this blossoming period of S1P research: the identification of endothelial differentiation gene (EDG) family of G protein-coupled receptors as S1P receptors (S1PRs) [3–5] and the characterization of FTY720 (Fingolimod, a reagent known for immunosuppressive function) as an agonist of S1PRs [6]. These two discoveries have been leading to a tremendous progress in our understanding of S1P’s functions in vasculature, immune cell trafficking, inflammation, tumor progression, etc. Consequently, FTY720 was recently approved by FDA for the treatment of multiple sclerosis.
S1P regulates an array of biological activities in various cell types [7–10]. S1P can function either as an extracellular ligand or intracellular mediator [3, 11, 12]. When functioning as an extracellular ligand, S1P-regulated activities are mediated by the S1P family of G protein-coupled-receptors (S1PR1-S1PR5) [3, 5, 13, 14]. Distinct S1P receptor subtypes are expressed in different cell types, which regulate different signaling pathways and biological activities [15–18, 19•]. In endothelial biology, S1P was initially characterized as a beneficial and protective lipid mediator which regulates angiogenesis [15], chemotaxis [20], vasculature maintenance [21], and endothelial integrity function [22]. However, emerging evidence suggests that S1P also plays a pathological role in the endothelium, which depends on the expression of different S1P receptor subtypes on endothelial cell membrane in pathological conditions [16, 23•]. This review aims to focus on S1PRs’ mediated responses in endothelial cells (ECs), with particular emphasis on endothelial inflammation.
S1PR1 Signaling in Endothelial Biology
S1P/S1PR1 Signaling Regulates Angiogenic Responses
Following de-orphaning S1PRs [3, 24], researchers began to characterize signaling pathways and biological activities mediated by S1PRs. Before identifying the ligand, S1PR1 was suggested to be a Giα-coupled G protein-coupled receptor (GPCR), because the S1PR1 interacted with Giα by a yeast two-hybrid screening assay and by co-immunoprecipitation analysis [25]. Strikingly, S1PR1-over-expressing cells exhibited a distinct morphology. They formed network-like structures similar to the tubular structures in differentiated endothelial cells (ECs), through the increased formation of adherens junctions [3]. By using these S1PR1 stable transfectants, it was shown that the ligand for S1PR1 was present in serum and the ligand-S1PR1 interaction resulted in this aggregated morphology by up-regulation of adherens junction formation. Using this morphological screening read-out together with biochemical analyses, S1P, a serum-borne bioactive lipid, was shown to be the cognate ligand of S1PR1 [3]. The S1PR1 (old nomenclature EDG-1) was cloned from PMA-induced differentiating ECs [26]. Subsequently, it was showed that S1P promotes morphogenesis of ECs in vitro and angiogenesis in the Matrigel implantation animal model [15] via the activation of endothelial S1PR1. This S1PR1-mediated endothelial morphogenic response depends on the presence of its cognate ligand, S1P, and S1P/S1PR1 signaling activated the ERK-mediated cell survival pathway and small GTPase-mediated adherens junction (AJ) assembly in cultured ECs. Knockdown of endothelial S1PR1 abrogated the S1P-induced angiogenic responses. Collectively, these data suggested that S1P/S1PR1 signaling is a novel modulator of angiogenesis. In agreement, Dr. Proia’s group showed that deletion of S1pr1 resulted in embryonic lethality due to a defect in vasculature [21]. Therefore, S1P/S1PR1 signaling plays critical roles in neo-vessel formation and vasculature maintenance.
S1P Stimulates Endothelial Chemotaxis via the S1PR1/Akt Signaling Axis
S1P is a potent chemoattractant for various cell types [20, 27, 28]. S1P activates protein kinase Akt, which plays a critical role in S1P-mediated chemotaxis in ECs [20, 29]. S1PR1 is the predominant S1P receptor subtype expressed in cultured human umbilical vein endothelial cells (HUVECs) [15]. The consensus Akt phosphorylation sequence (R231xR233xxS/T236) is present in the third intracellular loop of S1PR1, and not in S1PR2 or S1PR3. It was shown that activated Akt phosphorylates Thr236 of S1PR1 [20]. Furthermore, a phosphorylation-defective S1PR1 mutant, in which Thr236 was mutated to Ala, inhibited the S1P-mediated Rac activation and chemotactic and morphogenetic responses in ECs. This result suggested that the Akt-mediated S1PR1 phosphorylation resulted in the transactivation of S1PR1, leading to Rac activation, cortical actin assembly, and chemotaxis in ECs.
S1P Promotes Cell Proliferation
The S1P-stimulated cell proliferation and survival has been shown to be independent of plasma membrane S1PRs. For example, it was shown that direct microinjection of S1P into cells resulted in a significant increase in DNA synthesis and inhibition of cell death [24].
S1P Signaling Regulates Endothelial Integrity Function
S1P/S1PR1-Mediated Signaling Enhances Endothelial Integrity
S1P was shown to stimulate cytoskeletal rearrangement [15, 20, 30, 31], activate integrin αvβ3 and β1-containing integrins [28, 32], and induce AJ formation [3, 15] via the activation of S1PR1 in ECs. Also, it was reported that zonula occludens-1 (ZO-1) played a dual role in the S1P/S1PR1 signaling-mediated endothelial chemotaxis and tight junction (TJ) formation [30]. Interestingly, two distinct ZO-1 complexes were characterized to regulate two different endothelial activities following S1P stimulation, namely ZO-1/cortactin complexes to regulate chemotactic response and ZO-1/α-catenin complexes to regulate endothelial barrier integrity. This observation suggested that the concerted operation of these two ZO-1 complexes may coordinate two important S1P-mediated functions (i.e., migration and barrier integrity) in vascular endothelial cells. These S1P-regulated endothelial activities were mainly mediated by the Rho family of small GTPases [3, 15, 20, 28, 30–32]. Because S1P/S1PR1 signaling regulated cytoskeletal architectures, integrin-extracellular matrix interactions, and intercellular interactions in ECs, it was reasonably to expect that S1P should modulate endothelial integrity function. Indeed, Dr. Garcia’s group showed for the first time that the activation of S1P/S1PR1 signaling profoundly enhanced barrier integrity in cultured ECs [22]. By utilizing the electrical cell-substrate impedance sensing (ECIS) technique, they showed that a robust increase of transendothelial electrical resistance (TEER) was observed immediately after S1P treatment in cultured ECs, suggesting that S1P was able to enhance endothelial barrier function. Also, we showed that the S1P-increased barrier integrity was sustained for more than 6–10 h after S1P treatment and thus was not a transient event. The S1P-enhanced TEER was markedly abrogated in S1PR1-knockdown ECs and was mediated by the endothelial Gi/PI3K/Akt/Rac signaling pathway [30]. The role of S1P/S1PR1 signaling in maintaining endothelial integrity function was further supported by the observation that FTY720P treatment, which induced S1PR1 internalization and degradation, stimulated vascular leakage in animals [33•].
Balance of S1PR1- and S1PR2-Mediated Signaling Controls Microvascular Permeability in Animals
Research data strongly indicated that S1P treatment enhanced endothelial integrity in cultured ECs via the activation of S1PR1 signaling. However, how vascular permeability is modulated in normal physiological setting remained to be characterized. Therefore, the vessel leakage model in Sprague Dawley (SD) rat was employed to examine the physiological effect of S1P in the regulation of vascular permeability [34]. FITC-tagged BSA was injected via the carotid artery, together with S1P or vehicle control. Ten minutes later, vessel leakage was induced by treating the surgically exposed cremaster muscle vessel with histamine. Interstitial fluorescence in the vascular bed of the cremaster muscle (an indicator of blood vessel leakage) was quantitated. Surprisingly, it was shown that S1P was unable to protect against histamine-induced venular leakage [34]. Both S1PR1 and S1PR2 were detected in the endothelium of the cremaster muscle vascular bed. S1PR2 was suggested to mediate the “inhibitory” signaling of S1P [35]. Therefore, antagonists and agonists specific for S1PRs were used to examine roles of S1PR1 and S1PR2 in modulating vascular permeability. Treatments of SEW2871 and FTY720, two agonists of S1PR1, significantly inhibited histamine-induced microvascular leakage. Also, treatment with VPC 23019 to antagonize S1PR1-regulated signaling greatly potentiated histamine-induced venular leakage. Moreover, after inhibition of S1PR2 signaling by JTE-013, a specific antagonist of S1PR2, S1P was able to protect microvascular permeability in SD rats. Furthermore, in cultured ECs, endothelial tight junctions and barrier function were regulated by S1PR1- and S1PR2-mediated signaling in a concerted manner that is similar to the observed regulation of vessel leakage in animals [34]. In agreement, it was shown that activation of S1PR2 in cultured ECs disrupted endothelial adherens junctions and increased paracellular permeability in cultured ECs, and inhibition of S1PR2 diminished the H2O2-induced permeability in the rat lung perfused model [36]. These data together suggested that the balance between S1PR1 and S1PR2 signaling regulates the homeostasis of microvascular permeability in the peripheral circulation.
S1PR2 in Endothelial Senescence-Associated Functional Impairments and Inflammation
Up-regulation of S1PR2 in Senescent, Aged, and Atherosclerotic ECs
ECs have a finite lifespan in vitro and eventually enter a growth arrest state called “senescence.” Evidence suggests that endothelial senescence is functionally implicated in vascular aging and associated cardiovascular dysfunctions including inflammation [16, 37–39, 40••]. Using the in vitro endothelial model, it was shown that S1PR2 receptors, which are expressed at low levels in young ECs, are significantly increased in senescent ECs [16]. The endothelial activities, including chemotaxis, wound-healing response, Rac activation, and morphogenesis, were markedly diminished in senescent ECs. Ectopically expressing S1PR2 in young ECs to a level which is similar to that in senescent ECs induced the senescence-associated endothelial impairments [16]. In contrast, the senescence-associated endothelial impairments were abrogated by knocking-down S1PR2 in senescent ECs. It was demonstrated that atherosclerotic endothelia exhibit senescence phenotype [38, 41]. The patho-physiological relevance of S1PR2 up-regulation in cultured senescent ECs was supported by the following observations: (1) Levels of S1PR2 were found to be markedly increased in lesion regions of atherosclerotic endothelium [16], and (2) S1PR2 levels were shown to be profoundly increased in the aorta from aged animals. These data suggest that S1PR2 signaling, particularly in the context of endothelium, plays a critical role in endothelial senescence-associated functional impairments and inflammation. Skoura et al. demonstrated that S1PR2 function in myeloid cells regulates vascular inflammation and atherosclerosis [42••]. However, whether endothelial S1PR2 signaling contributes to vascular pathologies remains to be investigated in animals.
Atherogenic Stimuli Increase S1PR2 Levels in ECs
-
(a)
Pro-inflammatory cytokine increases S1PR2 in ECs.
S1PR2 was shown to be significantly up-regulated in the atherosclerotic endothelium. Because atherosclerosis is an inflammatory vascular disease, a recent study examined the role of S1PR2 signaling in endothelial inflammation [23•]. Treatment with tumor necrosis factor-α (TNFα, a pro-inflammatory cytokine) increased levels of S1PR2 in cultured human aortic ECs (HAECs). TNFα treatment also enhanced sphingosine kinase 1 expression and increased S1P production in HAECs. Pharmacological inhibition or knockdown of S1PR2 profoundly abrogated the TNFα-induced endothelial activation including the increased expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) in ECs. In contrast, pharmacological inhibition or knockdown of other S1P receptor subtypes (i.e., S1PR1 and S1PR3) had no effect on the TNFα-stimulated ICAM-1 and VCAM-1 expression. Moreover, ectopic expression of S1PR2 increased VCAM-1 and ICAM-1 expression in ECs following the stimulation of S1P [23•]. Utilizing the reporter luciferase assay, it was shown that the S1P/S1PR2 signaling stimulated NFκB activation in ECs. Pharmacological inhibition of S1PR2 signaling markedly inhibited the TNFα-stimulated NFκB activation. Moreover, the S1P/S1PR2-stimulated VCAM-1/ICAM-1 expression was completely abolished by the pharmacological inhibitor of NFκB. Collectively, these data suggest that proinflammatory cytokine treatment activates the autocrine S1P/S1PR2 signaling, which subsequently activates NFκB and leads to endothelial activation by up-regulating VCAM-1 and ICAM-1 expression.
-
(b)
Disturbed flow up-regulates S1PR2 in ECs.
Atherosclerotic lesions develop at bends and bifurcations in arteries where the fluid flow behavior can be characterized as “disturbed flow” (low shear stress oscillatory flow) [43, 44]. Dr. Sethu recently developed an enabling technology in the Endothelial Cell Culture Model (ECCM) which provides the most physiologically relevant platform for short- and long-term culture of ECs as it accurately reproduces clinically observed pulsatile pressure, flow, and stretch waveforms [45–47].
Role of sphingolipid signaling in flow stress-regulated endothelial patho-physiological responses is poorly understood. To examine whether S1P signaling regulates the disturbed flow stress-mediated endothelial dysfunction, HAECs were cultured within the ECCM and subject to either normal pulsatile or disturbed/retrograde flow for 12 h. HAECs cultured in static condition were used as a control. qPCR and flow cytometry analysis showed a significant increase of S1PR2 in ECs subjected to disturbed flow (unpublished observation). Levels of S1PR1 and S1PR3 were not altered in disturbed flow condition, and S1PR4 and S1PR5 were undetected. Also, qPCR analysis showed a significant increase of endothelial pro-inflammatory markers including ICAM-1 in cells exposed to disturbed flow in comparison to cells exposed to normal and static conditions. Using siRNA to knockdown S1PR2 significantly diminished the disturbed flow-stimulated ICAM-1 expression in HAECs. These data suggest that mechanical flow stress activates endothelial S1PR2 signaling, which plays a critical role in disturbed flow stress-triggered endothelial activation.
S1PR2-Mediated Signaling Pathways in Endothelial Inflammation
Endothelial S1PR2 Signaling Stimulates Expression of CCL-2, IL-6, and Cox-2
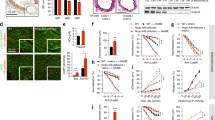
To investigate molecular details of endothelial S1PR2 signaling which may functionally regulate pro-inflammatory responses in ECs, young ECs (cumulative population doubling levels, CPDLs = 10) were transduced with 100 m.o.i. of adenoviral particles carrying either S1PR2 or control (β-galactosidase) vector. This transduction scheme resulted in levels of S1PR2 in young ECs which are equivalent to that in senescent ECs [16]. The expression of S1PR2 was validated by qPCR (data not shown). Following stimulating with or without S1P, levels of pro-inflammatory signaling molecules were measure by qPCR quantitation. We observed that mRNA and protein levels of pro-inflammatory chemokines/cytokines including chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemotactic protein 1 (MCP1) and interleukin-6 (IL-6) were significantly increased in ECs ectopically expressing S1PR2 (Fig. 1). Recruitment and infiltration of monocytes plays a critical role in the initiation and progression of pro-inflammatory responses, and CCL-2/MCP-1 is known to be a potent chemoattractant of monocytes. Therefore, endothelial S1PR2 activation not only increases the generation of monocyte chemoattractant (CCL-2) but also up-regulates the expression of adhesion molecules (e.g., ICAM-1 and VCAM-1) for monocyte attachment. Moreover, accumulating data suggest that IL-6, a target of NFκB, is an upstream inflammatory cytokine that plays a central role in amplifying the downstream inflammatory signaling (e.g., signal transducers and activators of transcription 3, STAT3) [48–50]. Furthermore, we found that endothelial S1PR2 signaling profoundly up-regulates mRNA and protein levels of cyclooxygenase-2 (Cox-2) (Fig. 1). The Cox-2-generated prostaglandins and the downstream eicosanoids and docosanoids (e.g., thromboxane, leukotrienes, resolvins, protectins, lipoxins etc.) play critical roles in inflammation. Collectively, these data highlight that endothelial S1PR2 signaling plays a key role in mediating endothelial inflammation.
S1PR2 signaling increases levels of Cox-2, IL-6, and MCP-1 in ECs. a Levels of Cox-1, Cox-2, IL-6, and MCP-1 were measured by qPCR in ECs transduced with adenoviral control or S1PR2 vector, following S1P stimulation. Note that S1PR2 signaling up-regulates Cox-2, IL-6, and MCP-1. In contrast, S1PR2 signaling has no effect on Cox-1 expression. b Western blotting analysis shows that Cox-2, IL-6, and MCP-1 proteins are increased in ECs transduced with adenoviral-S1PR2 followed by S1P stimulation
Endothelial S1PR2 Signaling Stimulates Sphingolipid Biosynthesis
Atherosclerosis is a pro-inflammatory disease of vessel wall [51]. ECs present in atherosclerotic plaques are senescence [38, 41]. Also, senescent EC-mediated pro-inflammatory responses have been suggested to contribute to atherogenesis [37, 39]. However, atherosclerotic/senescent ECs-triggered endothelial pro-inflammatory responses, particularly in the context of pro-inflammatory lipid mediators, remain to be characterized. Several studies, as discussed above, suggest the critical role of endothelial S1PR2 signaling in endothelial inflammation and atherogenesis. Therefore, characterization of endothelial S1PR2-produced inflammatory lipid mediators is expected to greatly advance our knowledge in understanding endothelial inflammation.
Lipid mediators play important roles in inflammatory responses. However, what lipid mediators generated by inflamed ECs and what patho-physiological functions of these lipid mediators are poorly understood. Therefore, we conducted the LC-MS/MS lipidomic methods to characterize bioactive lipids generated by S1PR2 signaling in ECs. Our initial effort was focused on sphingolipids, since sphingolipids have been shown to regulate various physiological responses including inflammation. We have established very sensitive targeted multiple reaction monitoring (MRM) LC-MS/MS methods to quantify more than 50 species of sphingolipids, including different lengths and levels of saturation of acy fatty acid side chains [23•, 52•, 53]. This sensitive method can detect 50–200 pg of lipids on the column, depending on the analyte.
Young HAECs were transduced with adenoviral particles carrying either S1PR2 or control β-galactosidase vector. Culture media were collected 3 h in the presence or absence of S1P stimulation and measured for sphingolipids by LC-MS/MS methods (Fig. 2a). We observed that sphingosine (SPH), dihydrosphingosine (DiH-SPH), C16-ceramide (C16-Cer), C16-ceramide-1-phosphate (C16-C1P), dihydroceramide (DiH-Cer), and most sphingomyelin (SPM) species were significantly increased in S1PR2 expressing cells following S1P treatment. Levels of ceramide species secreted by S1PR2-expressing ECs are low (0.1–3 ng/105 cells). However, they were significantly increased (ranging from two- to six-folds) in S1PR2-expressing ECs after S1P stimulation (Fig. 2a). No significant difference in sphingolipid levels was observed in control adeno-β-galactosidase-transduced ECs treated with or without S1P (data not shown). Moreover, we characterized sphingolipid species in plasma of C56BL/6 mice fed with normal chow or an atherogenic diet (Harlan, TD88051) [54]. As shown in Fig. 2b, consistent with the elevated sphingolipidome in S1PR2-activated ECs in vitro, the same class of sphingolipids was significantly increased in plasma of atherogenic diet-fed mice. Although sources of these plasma sphingolipids in atherogenic diet-fed animals remain to be determined, these data suggest that these elevated sphingolipids may represent a novel signature of pro-inflammatory lipid mediators associated with inflamed ECs. Because S1PR2 are up-regulated in senescent, atherosclerotic, and inflamed endothelia [16, 23•], these S1PR2-increased sphingolipids may have critical roles in vascular inflammation and atherogenesis.
S1PR2 signaling increases generation of sphingolipids which stimulate endothelial activation. a Adeno-S1PR2-transduced ECs were treated with vehicle or S1P for 3 h. Media were measured for sphingolipid levels by LC-MS/MS. Data, mean ± SD (n = 3), show 31 sphingolipids are significantly increased (p < 0.05). b C57BL/6 mice (n = 6) were fed with normal chow or an atherogenic diet for 4 weeks. Plasma levels of sphingolipids were analyzed by LC-MS/MS. Only sphingolipids having more than 1.5-fold increase and statistically significant (p < 0.05, t test) are shown. c Sphingolipids stimulate ICAM-1 and VCAM-1 expression in ECs. HAECs were treated with sphingolipids. Levels of ICAM-1 and VCAM-1 were quantitated by qPCR analysis. Data are mean ± SD (n = 3)
DiH-SPH, SPH, C16-C1P, DiH-Cer, C1P, Cer, and SPM are metabolites of the de novo sphingolipid biosynthetic pathway [55, 56], which is initiated by serine palmitoyltransferase (SPT) that synthesizes 3-ketosphinganine from l-serine and palmitoyl CoA [57, 58]. Subsequently, 3-ketosphiganine is converted to DiH-SPH, DiH-Cer, C1P, etc., via reactions catalyzed by a series of enzymes [55]. Although molecular details remain to be determined, our data suggest that endothelial S1PR2 signaling stimulates sphingolipid biosynthesis, which might be mediated by the activation of de novo sphingolipid biosynthetic pathway in inflamed ECs.
Next, we investigated roles of these sphingolipids in endothelial activation and inflammation. As shown in Fig. 2c, DiH-S1P, SPM, and Cer increased levels of endothelial ICAM-1, and DiH-S1P, DiH-SPH, SPM, and Cer induced expression of endothelial VCAM-1 in HAECs. Collectively, these results suggest that endothelial S1PR2 signaling stimulates sphingolipid biosynthesis which may be mediated by the activation of sphingolipid biosynthetic pathway. Subsequently, these S1PR2 signaling-generated sphingolipids trigger endothelial activation and inflammation via an autocrine reaction loop.
Conclusion
Endothelial inflammation plays a key role in mediating vascular disease such as atherosclerosis. Atherosclerosis, a chronic inflammatory process, is the primary cause of cardiovascular disease and cerebrovascular accident, two of the most common causes of illness and death worldwide [59]. Clinically, symptomatic atherosclerosis is typically associated with men in their 40s and women in their 50s to 60s. Inhibitors of HMG-CoA reductase (e.g., statins) have been used to control hypercholesterolemia and atherosclerosis. However, benefit of statins is questionable in patients with elevated cholesterol levels but without previous cardiovascular diseases [60, 61]. Also, side effects of statins include muscle pain, increased risk of diabetes, and abnormalities in liver enzyme tests [62]. Additionally, statins have rare but severe adverse effects, particularly muscle damage [63]. Therefore, efforts continue to identify novel therapeutic agents.
Levels of S1PR2 are markedly up-regulated in senescent ECs, as well as in lesion regions of atherosclerotic endothelium. Inflammatory cytokines and mechanical flow stress increased S1PR2 expression in ECs. Inhibition of S1PR2 signaling profoundly diminished endothelial activation and inflammation, whereas activation of endothelial S1PR2 signaling exhibits the opposite effects on ECs. Moreover, activation of endothelial S1PR2 signaling profoundly produced pro-inflammatory cytokines/chemokines and lipid mediators. Collectively, these studies strongly suggest that endothelial S1PR2 signaling plays a key role in endothelial inflammation, which may have functional implication in atherosclerosis development. Thus, targeting endothelial S1PR2 may provide an intervention point for the treatment of vascular inflammation and the associated cardiovascular disorders such as atherosclerosis in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2000;476(1–2):55–7.
Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870–81. doi:10.1002/bies.20081.
Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein coupled receptor EDG-1. Science. 1998;279(5356):1552–5.
An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417(3):279–82.
Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330(2):605–9.
Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–9. doi:10.1126/science.1070238.
Spiegel S. Sphingosine 1-phosphate: a prototype of a new class of second messengers. J Leukoc Biol. 1999;65(3):341–4.
Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253(1):230–8.
Igarashi Y, Yatomi Y. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim Pol. 1998;45(2):299–309.
Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, et al. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol. 1999;58(2):201–7.
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–7. doi:10.1126/science.1176709.
Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–8. doi:10.1038/nature09128.
An SZ, Goetz EJ, Lee HY. Signaling mechanisms and molecular characteristics of G protein-coupled receptors for lysophosphatidic acid and sphingosine 1-phosphate. J Cell Biochem. 1998;147–57.
Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International union of pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54(2):265–9.
Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99(3):301–12.
Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, Mathis SP, et al. Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem. 2008;283(44):30363–75. doi:10.1074/jbc.M804392200.
Eskan MA, Rose BG, Benakanakere MR, Lee MJ, Kinane DF. Sphingosine 1-phosphate 1 and TLR4 mediate IFN-beta expression in human gingival epithelial cells. J Immunol. 2008;180(3):1818–25.
Hsu A, Zhang W, Lee JF, An J, Ekambaram P, Liu J, et al. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int J Oncol. 2012;40(5):1619–26. doi:10.3892/ijo.2012.1379.
Zhang W, Zhao J, Lee JF, Gartung A, Jawadi H, Lambiv WL, et al. ETS-1-mediated transcriptional up-regulation of CD44 is required for sphingosine-1-phosphate receptor subtype 3-stimulated chemotaxis. J Biol Chem. 2013;288(45):32126–37. doi:10.1074/jbc.M113.495218. This study characterized a novel ETS-1/CD44 pathway in S1P-stimulated chemotaxis.
Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8(3):693–704.
Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106(8):951–61. doi:10.1172/jci10905.
Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi:10.1172/jci12450.
Zhang W, An J, Jawadi H, Siow DL, Lee JF, Zhao J, et al. Sphingosine-1-phosphate receptor-2 mediated NFkappaB activation contributes to tumor necrosis factor-alpha induced VCAM-1 and ICAM-1 expression in endothelial cells. Prostaglandins Other Lipid Mediat. 2013;106:62–71. doi:10.1016/j.prostaglandins.2013.06.001. S1PR2 plays a key role in endothelial inflammation.
Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142(1):229–40.
Lee MJ, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem. 1996;271(19):11272–9.
Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265(16):9308–13.
Liu F, Verin AD, Wang P, Day R, Wersto RP, Chrest FJ, et al. Differential regulation of sphingosine-1-phosphate- and VEGF-induced endothelial cell chemotaxis. Involvement of G(ialpha2)-linked Rho kinase activity. Am J Respir Cell Mol Biol. 2001;24(6):711–9.
Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem. 2001;276(15):11830–7. doi:10.1074/jbc.M009422200.
Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276(22):19672–7. doi:10.1074/jbc.M009993200.
Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281(39):29190–200. doi:10.1074/jbc.M604310200.
Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem Cell Biol. 2006;126(3):297–304. doi:10.1007/s00418-006-0143-z.
Wang L, Lee JF, Lin CY, Lee MJ. Rho GTPases mediated integrin alpha v beta 3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem Cell Biol. 2008;129(5):579–88. doi:10.1007/s00418-008-0389-8.
Oo ML, Chang SH, Thangada S, Wu MT, Rezaul K, Blaho V, et al. Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121(6):2290–300. doi:10.1172/jci45403. This study used an animal model to show the role of S1PR1 in maintaining vascular permeability.
Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, et al. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296(1):H33–42. doi:10.1152/ajpheart.00097.2008.
Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23(5):1534–45.
Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27(6):1312–8. doi:10.1161/atvbaha.107.143735.
Sikora E, Bielak-Zmijewska A, Mosieniak G. Cellular senescence in ageing. Curr Vasc Pharmacol: Age-Related Disease and Longevity; 2013.
Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–4.
Minamino T, Miyauchi H, Yoshida T, Tateno K, Kunieda T, Komuro I. Vascular cell senescence and vascular aging. J Mol Cell Cardiol. 2004;36(2):175–83. doi:10.1016/j.yjmcc.2003.11.010.
Bai B, Liang Y, Xu C, Lee MY, Xu A, Wu D, et al. Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation. 2012;126(6):729–40. doi:10.1161/circulationaha.112.118778. This study identified SIRT1 being a target of CDK5, and characterized the critical role of CDK5/SIRT1 signaling axis in endothelial senescence and atherosclerosis.
VASILE E, TOMITA Y, BROWN LF, KOCHER O, DVORAK HF. Differential expression of thymosin β-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15(2):458–66. doi:10.1096/fj.00-0051com.
Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(1):81–5. doi:10.1161/atvbaha.110.213496. This study demonstrated that S1PR2 signaling in myeloid cells plays a key role in atherosclerosis.
Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L529–33.
Li Y-SJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38(10):1949–71.
Estrada R, Giridharan GA, Nguyen M-D, Roussel TJ, Shakeri M, Parichehreh V. Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Anal Chem. 2011. doi:10.1021/ac2002998. null-null.
Estrada R, Giridharan G, Prabhu SD, Sethu P. Endothelial cell culture model of carotid artery atherosclerosis. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:186–9.
Estrada R, Giridharan GA, Nguyen M-D, Prabhu SD, Sethu P. Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics. 2011;5(3):032006–11.
Cantwell CA, Sterneck E, Johnson PF. Interleukin-6-specific activation of the C/EBPdelta gene in hepatocytes is mediated by Stat3 and Sp1. Mol Cell Biol. 1998;18(4):2108–17.
Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22(3):147–51. doi:10.1097/crd.0000000000000021.
Ogura H, Arima Y, Kamimura D, Murakami M. The gateway theory: how regional neural activation creates a gateway for immune cells via an inflammation amplifier. Biomed J. 2013;36(6):269–73. doi:10.4103/2319-4170.113187.
Dessi M, Noce A, Bertucci P, di Villahermosa MS, Zenobi R, Castagnola V, et al. Atherosclerosis, dyslipidemia, and inflammation: the significant role of polyunsaturated fatty acids. ISRN Inflamm. 2013;2013:191823. doi:10.1155/2013/191823.
Muradashvili N, Khundmiri SJ, Tyagi R, Gartung A, Dean WL, Lee MJ, et al. Sphingolipids affect fibrinogen-induced caveolar transcytosis and cerebrovascular permeability. Am J Physiol Cell Physiol. 2014;307(2):C169–79. doi:10.1152/ajpcell.00305.2013. This study demonstrated a novel role of sphingolipids in fibrinogen-mediated caveolar transcytosis, leading to increase cerebrovascular permeability.
Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, et al. Adipocyte lipolysis stimulated interleukin-6 production requires sphingosine kinase 1 activity. J Biol Chem. 2014. doi:10.1074/jbc.M114.601096. Sep 24. pii: jbc.M114.601096. [Epub ahead of print].
Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57(1):65–73.
Wattenberg BW. Role of sphingosine kinase localization in sphingolipid signaling. World J Biol Chem. 2010;1(12):362–8. doi:10.4331/wjbc.v1.i12.362.
Van Brocklyn JR, Williams JB. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: oxidative stress and the seesaw of cell survival and death. Comp Biochem Physiol B Biochem Mol Biol. 2012;163(1):26–36. doi:10.1016/j.cbpb.2012.05.006.
Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632(1–3):16–30.
Linn SC, Kim HS, Keane EM, Andras LM, Wang E, Merrill Jr AH. Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption. Biochem Soc Trans. 2001;29(Pt 6):831–5.
Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451(7181):904–13. doi:10.1038/nature06796.
Lipid modification—cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease—quick reference guide. National Institute for Health and Clinical Excellence May 2008, reissued March 2010.
Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;1, CD004816. doi:10.1002/14651858.CD004816.pub4. Online.
Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcome. 2013;6(4):390–9. doi:10.1161/circoutcomes.111.000071.
Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10(3):373–87. doi:10.1517/14740338.2011.540568.
Compliance with Ethics Guidelines
Conflict of Interest
Jiawei Zhao, Dante Garcia, Allison Gartung, and Menq-Jer Lee declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Vascular Biology
Rights and permissions
About this article
Cite this article
Zhao, J., Garcia, D., Gartung, A. et al. Sphingosine-1-Phosphate Receptor Subtype 2 Signaling in Endothelial Senescence-Associated Functional Impairments and Inflammation. Curr Atheroscler Rep 17, 26 (2015). https://doi.org/10.1007/s11883-015-0504-y
Published:
DOI: https://doi.org/10.1007/s11883-015-0504-y