Abstract
Autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, have a strong association with an increased risk of atherosclerotic cardiovascular diseases (ASCVD), particularly ischemic heart disease (IHD). A majority of the autoimmune conditions occur predominantly in women, and as women continue to experience a higher cardiovascular mortality compared to men, this potential added risk factor must be recognized. Inflammation and immune mechanisms have been shown to be an underlying mechanism for the development of atherosclerosis, thus sharing a common mechanism with rheumatologic conditions. There is an under recognition, in both patient and physician, of the increased cardiovascular (CV) risk within the autoimmune population, with present CV risk profile algorithms performing poorly in these patients. Traditional risk factors play a role in the development of IHD in the autoimmune patient, but their overall significance is unclear and does not fully explain the elevated CV risk. The role of inflammation and risk factors in autoimmune conditions, and their link to the elevated CV risk will be explored within this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic autoimmune rheumatic diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SS), spondyloarthropathies, and vasculitides are inflammatory disorders which can all have cardiovascular (CV) manifestations. These manifestations may, in fact, be the initial presentation of the rheumatologic condition. Coronary atherosclerosis confers the most significant CV risk to the autoimmune patient, despite the fact that any cardiac structure may be affected. The majority of these autoimmune conditions affects women more often than men and therefore need to be included when discussing gender-specific issues with relationship to cardiovascular disease (CVD). Current algorithms for CV risk assessment in women include autoimmune disease in the “at risk” category [1]. However, autoimmune conditions presently are not included in any CV risk calculator. Rheumatoid arthritis and SLE have long been recognized as having excess atherosclerotic cardiovascular disease (ASCVD) risk [2, 3]. The other autoimmune inflammatory disorders have been less well studied given their lower prevalence, but early reports in antiphospholipid antibody syndrome (APS), SS, and psoriatic arthritis suggest similar increased CV risk [4–6]. As therapies for autoimmune diseases have improved over the last several decades, increasing patient longevity, the risk of premature atherosclerosis has become more clinically evident. It is now accepted that accelerated atherosclerosis is the leading cause of morbidity and mortality in the patient with a systemic autoimmune disease. Atherosclerosis itself has been identified as an inflammatory condition [7], and it is well established that systemic markers of inflammation independently predict ischemic heart disease [8]. Similarities exist between the inflammatory and immune-mediated mechanisms of both atherogenesis and autoimmune diseases, allowing the understanding of two diseases under one pathobiology.
Inflammation and Immunity in Atherosclerosis and Autoimmune Diseases
Atherosclerosis is an insidious disease of the arteries that begins early in life and progresses. Hypercholesterolemia was recognized to play a major role in the development of atherosclerosis in the twentieth century. Mechanical and chemical stresses were felt to be initiators of the migration of smooth muscle cells into arterial lesions. When it was identified that lipid-laden foam cells were monocyte-derived macrophages, immunity was recognized as playing a major part [9]. The theory that cellular immunity played a major role was proposed when T lymphocytes and their subsequent activation were detected within human atherosclerotic plaques [10, 11]. Subsequently, other advances, including the discovery of autoantibodies, T cell subtypes, and humoral and cellular immunity in atheroprotection, have led to greater understanding of the relationship between atherosclerosis and autoimmune diseases. Atherosclerosis is a chronic disease where both innate and adaptive immuno-inflammatory mechanisms are involved. Inflammation is pivotal at all stages of the development of atherosclerosis. Autopsy studies indicate that in a patient with RA there might be more inflammation within the coronary atherosclerotic plaque, with a greater proportion of B cells, than what is seen in the non-RA patient, indicating that the mechanism of ischemic heart disease may be different between the two groups [12].
Early in the course of atherosclerosis, plaque formation is favored in vascular beds characterized by higher expression of proinflammatory mediators. This is the process of inflammatory signaling. These mediators include leukocyte adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1), vascular endothelial growth factors, and cytokines [13]. Proinflammatory cytokines, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha) expressed by the endothelium promote further leukocyte infiltration, proliferation, and activation. Immune dysregulation enhances this process.
Innate immunity is independent of antigenic stimulation and thus represents a nonspecific inflammatory response. Macrophage foam cells mediate this response in atherosclerosis and are the first immune cells at the arterial wall, serving as an on-site lipid reservoir. These foam cells become the source for the proinflammatory mediators. Superoxide anion is produced within the plaque via the macrophages phagocytic actions. All of these functions promote further inflammation and further progression of the plaque [14].
An antigen-specific, or adaptive, immunity has also been recognized in atherosclerosis [14, 15]. Adaptive immunity refers to T cells, immunoregulatory cytokines, and antibodies involved in plaque progression. Oxidized low-density lipoprotein (LDL), heat shock proteins, beta2 glycoprotein 1b, and infectious agents are autoantigens delivered by antigen-presenting cells, within atherosclerotic lesions, to the T cells. Of these, oxidized LDL appears to be a primary antigen [16]. Detection of anti-oxidized LDL antibodies in atherosclerosis further supports the role of an immune reaction against the oxidized LDL that is present within the atherosclerotic plaque.
In rheumatoid arthritis, there is evidence that both immune dysregulation and systemic inflammation lead to the development of atherosclerosis, which may actually be identified before RA disease manifestations. Some evidence suggests that the excess CV risk precedes the diagnosis of RA [17]. Mortality from premature ASCVD is seen early in the course of seropositive RA [18], suggesting that preclinical inflammation is already exacting an influence on the coronary vasculature. The chronic inflammatory infiltrates seen in RA synovitis are similar to the cellular infiltrates seen within an atherosclerotic plaque. Mononuclear cell recruitment, proinflammatory cytokines, along with upregulation of adhesion molecules are present within inflamed RA-involved joints as well as within atherosclerotic lesions [19]. The erosions of cartilage and bone in RA are promoted by collagen-digesting enzymes, which also appear to play a role in atherosclerotic plaque rupture [4]. TNF-alpha, a cytokine prominent in RA, promotes a cascade of inflammatory mediators which include IL-1, IL-6, IL-8, prostaglandins, and matrix metalloproteinases which perpetuate and accelerate the inflammation seen in atherosclerosis [19]. This process of accelerated inflammation is fostered by an immune dysregulation seen in the autoimmune rheumatologic patient. Autoantibodies to oxidized LDL have been found in greater numbers in patients with autoimmune rheumatic diseases than in controls [20]. Confirming the link between atherosclerosis and RA is the presence of an abnormal CD4+ T cell in both processes [21]. Signaling Toll-like receptor (TLR), which is expressed by innate immune cells, plays a role in regulating antibody production in RA and SLE and is seen in atherosclerosis [22].
Similar to RA, commonalities exist between atherogenesis and SLE. Lupus may be considered a classic model of a systemic autoimmune disease. The pathogenesis of SLE includes the formation of immune complexes with complement activation. The autoantibodies, along with local inflammation as described above with RA, underlie the vascular injury and thrombosis seen in SLE [23]. Complex autoantibody production in SLE imparts the major role in the atherosclerosis production and progression [24].
Other rheumatologic conditions that are more common in women, such as SS and APS, have also been shown to exhibit accelerated atherosclerosis [4, 25]. Inflammation and autoimmunity underscore the pathogenesis of atherogenesis seen in these conditions, similarly to RA and SLE (but possibly in varying degrees). In APS, the presence of circulating antiphospholipid antibodies (aPL) appears to be the mediator of proinflammatory and prothrombotic effects on the vasculature [26]. Some of these antibodies seem to be able to cross react with oxidized LDL in SLE [27].
Epidemiology of Atherosclerotic Cardiovascular Disease in Autoimmune Rheumatologic Diseases
Understanding the similarities between atherosclerosis and autoimmune diseases establishes the basis for the recognition of the increased CV morbidity and mortality seen in this patient population [3–6]. SLE and RA have the largest body of available literature describing the increased burden of ASCVD, but smaller studies suggest an increased burden of ASCVD risk in APS and SS. Sjogren’s, another female predominant autoimmune rheumatologic disease, has several clinical manifestations similar to SLE, including circulating autoantibodies, and also shares some features with RA, such as rheumatoid factor (RF) and joint inflammation, however has not consistently been shown to have a higher prevalence of ASCVD.
SLE is a disease that affects 5 to 6 people per 100,000. It is nine times more common in women than men. The disease can have an onset at any point in life, but typically is seen most commonly between the ages of 16 to 55. Patients with lupus have a considerably increased morbidity and mortality related to ASCVD [28]. In SLE, there is a bimodal pattern of mortality, with an early peak (within the first three of years of diagnosis) related to active disease, sepsis, and nephritis, and a later peak (greater than 5 years from diagnosis) due to myocardial infarctions [3]. Patients with SLE have a fivefold to sixfold increased risk of CV events compared to the general population [29] and have been identified as having more atherosclerotic plaque within coronary arteries, as assessed by coronary calcium computed tomography [30]. Young women with SLE were more than two times more to experience myocardial infarctions (MI) compared with young women without lupus in a retrospective study from California. In this study, women older than 45 experienced no difference in the rate of hospitalization for acute MI whether they had SLE or not [28]. In the Pittsburgh SLE cohort, 67 % of women with lupus were less than 55 years of age at the time of their first cardiac event [29]. This translated to women between the ages of 35–44 having a relative risk of MI over 50 times that observed in age-matched controls. The relative risk in older women was also increased but only two- to fivefold higher. In patients who undergo coronary revascularization, there does not appear to be any significant difference between SLE and non-SLE patients in the prevalence of multivessel disease or in the reasons for revascularization (asymptomatic coronary artery disease (CAD), stable angina, unstable angina, and MI). The need for urgent and emergent procedures was also similar between groups. However, the patient with SLE who did undergo percutaneous coronary interventions (PCI) had more adverse events following these procedures, such as repeat PCI and myocardial infarction MI’s, compared with non-SLE patients after adjustments for covariates [31, 32]. A 2004 study out of California did not find a difference in in-hospital mortality after an acute MI between patients with SLE and those without, after adjusting for co-variables [33]. Methodology may account for some of these differences.
Antiphospholipid antibody syndrome occurs either as a primary condition or in the setting of an underlying disease, usually SLE. The incidence of the APS is around 5 new cases per 100,000 persons per year. The syndrome is defined by the presence of one type of autoantibody (aPL) and the occurrence of either venous or arterial thromboses or pregnancy morbidity (or a combination). APS is characterized by a prothrombotic states where recurrent venous and arterial thromboembolisms occur. The development of atherosclerosis appears to be linked to the presence of the circulating aPL and may be the first manifestation of APS [34, 35]. A substudy of the Helsinki Heart Study evaluated the relationship of aPL (specifically anti-cardiolipin antibodies (aCL)) to myocardial infarctions. Although only men were studied, those with the highest aCL levels had a relative risk for myocardial infarction of 2.0, compared with the remainder of the population and independent of confounding factors, such as age, smoking, systolic blood pressure, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) [36]. However, given variabilities in study results, the role of aPL and/or APS as independent risk factors for atherosclerosis remains somewhat unclear [37]. The pro-coagulant activity of antiphospholipid antibodies may be the driver for ischemic events rather than atherosclerotic plaque formation [38].
Systemic sclerosis is a disease that affects the microcirculation, which is manifested as Raynaud’s phenomenon, pulmonary arterial hypertension, and scleroderma renal crisis. Endothelial dysfunction and inflammation may lead to macrovascular dysfunction and ultimately atherosclerosis. In a population-based cohort of patients with SS, the risk of incident MI was increased approximately twofold compared to matched controls [39]. In a recent Chinese study, SS was associated with an increased risk of acute myocardial infarction; however, less than 40 % had obstructive coronary artery disease, indicating that microvascular abnormalities may be the primary mechanism rather than epicardial coronary artery stenosis [5].
Rheumatoid arthritis has the most abundant epidemiological data given its greater prevalence. RA prevalence is estimated at approximately 1 % in Caucasians. The annual incidence is around 40 per 100,000 and affects women two to three times more often than men. The mean age at onset is around 56 years old. Over a period between 1955 and 1994, higher rates of death were noted in the RA patient, with women having more excessive mortality compared to men [40]. The increased morbidity and mortality in RA has been found to be due to ASCVD. In fact, woman with RA have higher risk of an acute MI than men with RA [41]. A meta-analysis, encompassing 24 observational studies of RA mortality between 1970 and 2005, found that CV mortality was significantly increased (overall standard mortality ratio of 1.50 with 95 % confidence interval (CI)). The standard mortality ratio for ischemic heart disease (IHD) was similarly increased at 1.59 (95 % CI). In a prospective cohort study involving over 110,000 women who participated in the Nurse’s Health Study, women with RA had a twofold higher risk of myocardial infarction [42]. Post-MI 30-day CV mortality has been shown to be higher in RA compared to non-RA patients (odds ratio (OR) for CV death in RA patients following a first acute CV event, which included MI and stoke, was 1.6 (95 % CI 1.2–2.2)). The increased mortality was accounted for almost entirely by excess deaths following MIs [43]. Despite the increased risk of IHD, patients with RA are less likely to experience angina and more likely to have no symptoms before sudden cardiac death. The risk of sudden death in RA is two times more likely than in a non-RA patient and an unrecognized MI is also twice as common [44]. An increased CAD risk may actually predate the diagnosis of RA; patients diagnosed with RA have a three times higher likelihood of a prior MI compared with non-RA individuals [44]. This suggests that premature or accelerated atherosclerosis may occur before or with RA symptom onset, but likely still before RA diagnosis. Others have not identified an increased risk of IHD before RA symptom onset [45]. There have been some reports of a decline in ASCVD mortality in the RA patient (specifically in acute MI mortality) [46]; however, this decline has not been as dramatic as what has been observed in the general population, thus the mortality gap appears to be widening [47]. There have been varying reports on the approaches to treatment of MIs between the RA patient and the non-RA patient. There have been reports suggesting a lower likelihood of an RA patient to receive acute reperfusion after an acute MI, along with lower rates of utilization of proven post-MI medications [48]. Others have found in patients presenting with MIs, those with RA may in fact receive more acute reperfusion and have an in-hospital survival advantage [49, 50]. Long-term mortality has been shown however to be poorer in the RA patient post-MI, despite similar MI treatments, indicating possibly the influence of the RA disease characteristics as the determinant for long-term survival after an MI [51•].
Traditional Cardiovascular Risk Factors in Autoimmune Diseases
Traditional risk factors for coronary artery disease include advancing age, the present of hypertension (HTN), hyperlipidemia, diabetes mellitus (DM), and male gender. These traditional risk factors have been used in CV risk scoring calculators such as the Framingham score and the Systemic Coronary Risk Evaluation (SCORE) which are most often used in Europe. In addition to these classic risk factors, the metabolic syndrome, which requires three or more of the following: abdominal obesity, increased fasting blood sugar (≥100 mg/dL), elevated triglycerides (TG) (≥150 mg/dL) or treatment of an elevated TG, blood pressure ≥130/85 mmHg or drug treatment for elevated blood pressure, and low serum HDL (<40 mg/dL in men and <50 mg/dL in women), incurs an increased risk of cardiovascular disease as well as an increased risk of developing type II DM [52]. Thus, the question regarding the increased ASCVD seen in the rheumatologic patient is whether these traditional risk factors are more prevalent than that seen in the general population or whether their occurrence is similar to the general population but act differently. Again, the literature regarding these questions is primarily in the RA patient and secondarily in the SLE patient. The focus thus will be how these traditional risk factors play a role in these two groups.
Tobacco use is a very important risk factor for IHD as well as being a risk factor for the development of RA, particularly seropositive RA (those with positive rheumatoid factor and more importantly positive anticitrullated protein antibody (ACPA)) [53, 54]. In the Nurses’ Health Study, past cigarette smoking was more likely in the women with RA that in those without RA (48 versus 38 %) [42]. A meta-analysis reported in 2011, which included 15 studies, also found the prevalence of smoking to be higher in the RA patient with an odds ratio of 1.56 (85 % CI; 1.35–1.80) with a p value of <0.00001 [55]. Thus, smoking risk may explain part of the increased CV risk in the RA patient. In fact, carotid intimal medial thickness (CIMT), a marker of early atherosclerosis, has been shown to be greater in current RA smokers compared to nonsmoking RA patients [56]. However, the importance of this risk factor may be less in the RA patient compared with the smoking risk in a non-RA patient. A retrospective study which reviewed whether traditional CV risk factors conferred the same risk among RA and non-RA patients found that smoking had less of a risk in the RA patient compared to control (the hazard ratio was 1.3 in the RA smoker compared to 2.2 in the non-RA smoker; 95 % CI) [57]. So, although smoking is not only a risk factor for the development of RA and does impart a risk for IHD in RA, its overall effect may be less significant than in the patient without RA. In SLE, there have been some conflicting reports on the prevalence of smoking, with the Toronto cohort showing similar smoking rates between SLE patients and the general population (relative risk 0.86, 95 % CI) [58], but the Systemic Lupus International Collaborative Clinics (SLICC) registry revealed increasing trends of smoking in the SLE population over a 3-year period [59]. Smoking in SLE has been related to vascular events in both the Toronto group [60] and the SLICC registry [61], but in the Johns Hopkins Lupus Cohort, smoking was not related to CAD [62], nor was it related to the presence of carotid plaque in the Pittsburgh Lupus cohort [63].
Cholesterol abnormalities have become a vital component in our CV risk algorithms such as Framingham. In the general population, using the cholesterol profile has become a routine part of the CV risk assessment. In RA, hyperlipidemia (elevated TC or LDL cholesterol) appears to occur less commonly than in the general population [64, 65•] but, despite this, results in higher rates of acute myocardial infarctions and ischemic stroke [66]. Women with RA have significantly lower TC and LDL cholesterol compared to the general population but interestingly with no significant differences in HDL based on a study of 2005 RA patients [65•]. The decrease in TC and LDL cholesterol actually appears to occur 3–5 years prior to the onset of the diagnosis of RA [67]. High-grade inflammation (either during acute episodes or with chronic high levels of inflammation) suppresses TC and LDL levels but results in an even greater suppression of HDL cholesterol, which produces the deleterious atherogenic index (TC/HDL cholesterol ratio) [68]. This seems to explain why the elevated TC and LDL occur less commonly in the RA patient. A meta-analysis suggested that in RA, the most common lipid abnormality was actually a depressed HDL cholesterol [55]. In fact, a paradoxical relationship now appears to exist between lipid abnormalities and ASCVD risk in the RA patient, whereby lower TC and LDL levels are associated with increased cardiovascular risk [69]. In this population-based incidence cohort of RA patients, the level of inflammation (particularly erythrocyte sedimentation rates (ESR)) correlated most closely with ASCVD risk. As ESR increased, lower TC/HDL ratios were associated with higher CV risk; thus, the association of lipids to ASCVD risk is affected by the level of inflammation present [69]. In SLE, elevated TC and LDL cholesterol do not seem to occur with any greater frequency compared to the general population, although higher TG and very low-density lipoproteins (VLDL) have been observed [58]. However, if there is a combination of SLE and hypercholesterolemia, an 18-fold increased risk of MI is observed compared to the general population [41].
Hypertension is very common worldwide and imparts a significant but modifiable risk for the occurrence of myocardial infarctions [70]. As in the general population, HTN is common in patients with both RA and SLE. In RA, a recent meta-analysis showed that HTN has a similar prevalence compared to the general population (OR 1.09, 95 % CI) [55]. An older study with substantially larger number of patients suggested a higher prevalence of HTN in RA (relative risk 1.3) [71]; this study showed a similarly increased relative risk of HTN in the patient with psoriatic arthritis and ankylosing spondylitis [71]. A Greek study suggested that the presence of HTN incurred a significant risk for ASCVD (hazard ratio 3.76, 95 % CI 0.99–15.06). HTN however is complex in that is can by influenced by many other factors, such as inactivity, obesity, and certain medication; thus, its overall role in RA is more difficult to characterize independently. However, there are reports that HTN may be underdiagnosed in the RA patient, as well as being undertreated [72]. In SLE, HTN is significantly more common than the general population with a relative risk of 2.59 (95 % CI) [58]. Also, HTN has been linked to vascular events and mortality in SLE [61, 73].
Diabetes as well and insulin resistance occur with a higher prevalence in RA patients compared to controls [55, 74]. Insulin resistance is linked to inflammatory markers, such as TNFα, IL-6, ESR, and CRP, as well as disease activity in RA [75, 76]. Metabolic syndrome similarly is seen more commonly in RA [77, 78]. Abdominal obesity affects the glucose metabolism in RA [74] as it does in the general population. Likewise in SLE, the prevalence of diabetes is significantly increased [58], but interestingly has not been shown to confer an increased risk of CV events compared to the general population. Insulin resistance, which also in more frequent in SLE compared to the general population, is related to ESR [76], but also does not appear to predict ASCVD risk. Whether the increased prevalence of diabetes and metabolic syndrome is independently related to the autoimmune condition is unclear. These patients’ use of glucocorticoids along with abdominal obesity, sedentary lifestyle, and the underlying autoimmunity/inflammation all contribute adversely to glucose metabolism.
Obesity is known to decrease life expectance [79] and has been identified by the American Heart Association as an independent risk factor for ASCVD. Obesity has been associated with the development of RA [80] with those in the highest quartile of body mass index (BMI) having the highest risk relative to the lowest quartile (relative risk 1.4 (95 % CI = 1.0–2.0)) [81]. As in the general population, the presence of obesity in RA patients is associated with the presence of traditional risk factors [82]. However, there is an interesting association with BMI and mortality in RA. RA patients with a lower BMI have a significantly increased risk of CV death even after adjusted for co-variables [83]. This paradox may be related to uncontrolled systemic inflammation, with the highest ESR levels mitigating the protective effects of a high BMI [84]. In SLE, obesity has been shown to be a risk factor for atherosclerotic events [61]. An elevated BMI in SLE is also a major factor for the development of insulin resistance in patients with SLE [76].
So, we see that traditional risk factors are clearly important in the CVD risk assessment in the patient with rheumatologic diseases. However, not all factors have the same impact on overall CV risk in the autoimmune patient compared to the general population. In addition, these risk factors alone do not fully account for the accelerated CV risk observed in these patients [85••, 86]. Using the Framingham and Reynolds risk scores in patients with RA underestimates the CVD risk significantly, more so for the older patient and those who are seropositive [85••, 87]. The absolute ASCVD risk in RA is similar to the risk in a non-RA patient who is 5–10 years older [88]. The RA patient has a similar CV morbidity and mortality as the patient with diabetes [89], yet is not recognized as such with present risk algorithms. In patient with SLE and ASCVD, one fewer traditional risk factor is present compared to the general population with ASCVD [60, 90]. After adjusting for the Framingham score in the SLE patient, there remains a 7.5–17-fold increased risk of CV events [86]. So, there must be other factors to consider when determining the overall ASCVD risk in an autoimmune patient.
Nontraditional Cardiovascular Risk Factors in Autoimmune Diseases
As noted previously, the role of inflammation is vital to the understanding of atherosclerosis. The autoimmune patient thus has the increased burden of inflammation that imparts an added risk of ASCVD. Markers of inflammation such as fibrinogen and CRP levels have been found to be higher in woman with RA compared with controls in the Nurses’ Health Study [91]. Other potential factors that may play a role are the cytokines interleukin-17 [92] and TNF-alpha [93]. The markers of disease activity in RA, such as CRP and ESR, have been linked to CVD risk [44, 94]. Also, the disease severity, measured by rheumatoid factor (RF), ACPA, destructive joints with physical limitations, rheumatoid lung disease, and presence of rheumatoid nodules along with the use of corticosteroids, has been associated with CVD morbidity and mortality [95–98]. In SLE, along with inflammatory markers, evidence of organ involvement as well as disease-related damage scores has been associated with ASCVD [60, 99]. Going beyond the traditional cholesterol profile, a proinflammatory HDL, which does not confer protection for LDL cholesterol oxidation, has been seen in higher levels in patients with SLE and RA [100].
A number of medications used in autoimmune rheumatologic diseases may also play a role in the risk profile. Nonsteroidal anti-inflammatory drugs (NSAIDS) have been associated with ASCVD and their use must be tempered with the overall CV risk in an individual patient [101]. Corticosteroids, as well as NSAIDS, are associated with development of HTN. Steroids in addition can cause weight gain, insulin resistance, and adverse lipid profiles which can contribute to the increased ASVD risk. Use of steroid monotherapy has been linked to a 50 % increased risk of a CV event [102]. Use of methotrexate (MTX) and other disease-modifying drugs (DMARDS) in RA have been associated with decreased CV risk [103–105]. In fact, the use of MTX is being studied in the Cardiovascular Inflammation Reduction Trial (CIRT), funded by the National Heart, Lung, and Blood Institute (NHLBI) to assess whether MTX reduces vascular events in a high-risk population. Hydroxychloroquine, used in SLE, is associated with improved survival and a decrease in vascular events [106]. Anti-TNF agents have also been associated with improved CV outcomes [103]. The proposed mechanism for these potential benefits is related to the decrease in systemic inflammation. However, the true role of these agents in decreased CV risk remains unclear. These agents are prescribed based on levels of disease activity and severity as well as based on individual patient profiles, and are frequently used in combination; thus, their effects on ASVD is confounded by various factors.
Conclusion
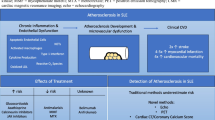
The autoimmune rheumatologic patient has an increased risk of ASCVD. There is a complex interplay between traditional and nontraditional risk factors in these patients (see Fig. 1). Inflammation appears to be the backbone on which the CV risk accelerates beyond the traditional risk factors. Present methods to evaluate CV risk in this patient population are underrepresenting the true risk. A collaborate approach to this high-risk patient population is essential. The traditional risk factors must be fully identified and treated aggressively. However, autoimmune disease severity and activity must be addressed with appropriate agents to modify the systemic inflammation. Whether initial evaluation of the autoimmune patient should include testing for early atherosclerosis, with surrogate markers such as CIMT, endothelial function assessment, and coronary artery calcium determination, is unclear, but warrants further study. The goal is to educate both the patient and provider regarding the elevated risk and incorporate multiple approaches to decrease that risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–62.
Cobb S, Anderson F, Bauer W. Length of life and cause of death in rheumatoid arthritis. N Engl J Med. 1953;249:553–6.
Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–5.
Shoenfeld Y, Gerli R, Doria A, Matsuura E, Cerinic MM, Ronda N, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112:3337–47.
Chu S-Y, Chen Y-J, Liu C-J, Tseng W-C, Lin M-W, Hwang C-Y, et al. Increased risk of acute myocardial infarction in systemic sclerosis: a nationwide population-based study. Am J Med. 2013;126:982–8.
Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27:174–82.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511.
Schaffner T, Taylor K, Bartucci EJ, Fischer-Dzoga K, Beeson JH, Glagov S, et al. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980;100:57–80.
Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–75.
Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714–7.
Aubry M-C, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007;34:937–42.
Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81.
Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–91.
Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–26.
Jara LJ, Medina G, Vera-Lastra O, Amigo M-C. Accelerated atherosclerosis, immune response and autoimmune rheumatic diseases. Autoimmun Rev. 2006;5:195–201.
Bartoloni E, Alunno A, Bistoni O, Gerli R. How early is the atherosclerotic risk in rheumatoid arthritis? Autoimmun Rev. 2010;9:701–7.
Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DPM. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–9.
Full LE, Ruisanchez C, Monaco C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2009;11:217.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Gerli R, Schillaci G, Giordano A, Bocci EB, Bistoni O, Vaudo G, et al. Cd4+Cd28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–8.
Bartoloni E, Shoenfeld Y, Gerli R. Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res. 2011;63:178–83.
Bruce IN. Atherogenesis and autoimmune disease: the model of lupus. Lupus. 2005;14:687–90.
Matsuura E, Lopez LR. Autoimmune-mediated atherothrombosis. Lupus. 2008;17:878–87.
Zinger H, Sherer Y, Shoenfeld Y. Atherosclerosis in autoimmune rheumatic diseases—mechanisms and clinical findings. Clin Rev Allergy Immunol. 2009;37:20–8.
Shoenfeld Y, Harats D, George J. Atherosclerosis and the antiphospholipid syndrome: a link unravelled? Lupus. 1998;7 Suppl 2:S140–3.
Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341:923–5.
Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:338–46.
Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol. 1997;145:408–15.
Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15.
Maksimowicz-McKinnon K, Selzer F, Manzi S, Kip KE, Mulukutla SR, Marroquin OC, et al. Poor 1-year outcomes after percutaneous coronary interventions in systemic lupus erythematosus: report from the National Heart, Lung, and Blood Institute dynamic registry. Circ Cardiovasc Interv. 2008;1:201–8.
SHAH MA, SHAH AM, KRISHNAN E. Poor outcomes after acute myocardial infarction in systemic lupus erythematosus. J Rheumatol. 2009;36:570–5.
Ward MM. Outcomes of hospitalizations for myocardial infarctions and cerebrovascular accidents in patients with systemic lupus erythematosus. Arthritis Rheum. 2004;50:3170–6.
Jara LJ, Medina G, Vera-Lastra O, Shoenfeld Y. Atherosclerosis and antiphospholipid syndrome. Clin Rev Allergy Immunol. 2003;25:79–88.
Jara LJ, Medina G, Vera-Lastra O. Systemic antiphospholipid syndrome and atherosclerosis. Clin Rev Allergy Immunol. 2007;32:172–7.
Vaarala O, Manttari M, Manninen V, Tenkanen L, Puurunen M, Aho K, et al. Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men. Circulation. 1995;91:23–7.
Artenjak A, Lakota K, Frank M, Cucnik S, Rozman B, Bozic B, et al. Antiphospholipid antibodies as non-traditional risk factors in atherosclerosis based cardiovascular diseases without overt autoimmunity. A critical updated review. Autoimmun Rev. 2012;11:873–82.
Gualtierotti R, Biggioggero M, Meroni P. Cutting-edge issues in coronary disease and the primary antiphospholipid syndrome. Clin Rev Allergy Immunol. 2013;44:51–6.
Man A, Zhu Y, Zhang Y, Dubreuil M, Rho YH, Peloquin C, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis. 2013;72:1188–93.
Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–8.
Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198–200.
Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7.
Van Doornum S, Brand C, King B, Sundararajan V. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2061–8.
Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–11.
Holmqvist ME, Wedren S, Jacobsson LT, Klareskog L, Nyberg F, Rantapaa-Dahlqvist S, et al. No increased occurrence of ischemic heart disease prior to the onset of rheumatoid arthritis: results from two Swedish population-based rheumatoid arthritis cohorts. Arthritis Rheum. 2009;60:2861–9.
Krishnan E, Lingala VB, Singh G. Declines in mortality from acute myocardial infarction in successive incidence and birth cohorts of patients with rheumatoid arthritis. Circulation. 2004;110:1774–9.
Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7.
Van Doornum S, Brand C, Sundararajan V, Ajani AE, Wicks IP. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
Francis ML, Varghese JJ, Mathew JM, Koneru S, Scaife SL, Zahnd WE. Outcomes in patients with rheumatoid arthritis and myocardial infarction. Am J Med. 2010;123:922–8.
Varghese JJ, Koneru S, Scaife SL, Zahnd WE, Francis ML. Mortality after coronary artery revascularization of patients with rheumatoid arthritis. J Thorac Cardiovasc Surg. 2010;140:91–6.
McCoy SS, Crowson CS, Maradit-Kremers H, Therneau TM, Roger VL, Matteson EL, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605–10. This study specifically identifies that patient with RA have worse outcomes after a myocardial infarction, despite similar treatment approach. RA patients are treated with guideline based therapy for their acute presentation like a non-RA patient, so their increased risk is likely due to their autoimmune disease itself.
Eckel RH, Alberti K, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2010;375:181–183.
Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20:1830–5.
Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol. 2009;21:279–83.
Boyer J-F, Gourraud P-A, Cantagrel A, Davignon J-L, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine. 2011;78:179–83.
Gerli R, Sherer Y, Vaudo G, Schillaci G, Gilburd B, Giordano A, et al. Early atherosclerosis in rheumatoid arthritis: effects of smoking on thickness of the carotid artery intima media. Ann N Y Acad Sci. 2005;1051:281–90.
Gonzalez A, Kremers HM, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–9.
Bruce IN, Urowitz MB, Gladman DD, Ibañez D, Steiner G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48:3159–67.
Urowitz MB, Gladman D, Ibañez D, Fortin P, Sanchez-Guerrero J, Bae S, et al. Accumulation of coronary artery disease risk factors over three years: data from an international inception cohort. Arthritis Care Res. 2008;59:176–80.
Urowitz MB, Ibanez D, Gladman DD. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J Rheumatol. 2007;34:70–5.
Urowitz MB, Gladman D, Ibañez D, Bae SC, Sanchez-Guerrero J, Gordon C, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res. 2010;62:881–7.
Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–9.
Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60.
Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: an explorative analysis from the Incremental Decrease in Endpoints through Aggressive Lipid Lowering (IDEAL) trial. Rheumatology. 2011;50:324–9.
Liao KP, Cai T, Gainer VS, Cagan A, Murphy SN, Liu C, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res. 2013;65:2046–50. Review of the role lipid abnormalities have in the RA patient in regards to prevalence and overall impact in CV events.
Semb AG, Kvien TK, Aastveit AH, Jungner I, Pedersen TR, Walldius G, et al. Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the Apolipoprotein-Related Mortality Risk (AMORIS) study. Ann Rheum Dis. 2010;69:1996–2001.
Myasoedova E, Crowson CS, Kremers HM, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310–4.
Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007;28:69–75.
Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–7.
Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. The Lancet. 2004;364:937–952.
Han C, Robinson Jr DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–72.
Panoulas VF, Douglas KMJ, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology. 2007;46:1477–82.
Rahman P, Aguero S, Gladman D, Hallett D, Urowitz M. Vascular events in hypertensive patients with systemic lupus erythematosus. Lupus. 2000;9:672–5.
Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54:2765–75.
Dessein PH, Stanwix AE, Joffe BI. Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decreased insulin sensitivity and high-density lipoprotein cholesterol as well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res. 2002;4:R5.
Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58:2105–12.
Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196:756–63.
Crowson CS, Myasoedova E, Davis 3rd JM, Matteson EL, Roger VL, Therneau TM, et al. Increased prevalence of metabolic syndrome associated with rheumatoid arthritis in patients without clinical cardiovascular disease. Journal Rheumatol. 2011;38:29–35.
Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93.
Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case–control study in Norfolk, England. Arthritis Rheum. 1997;40:1955–61.
Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5:525–32.
Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Douglas KM, Nevill AM, Jamurtas AZ, et al. Associations of obesity with modifiable risk factors for the development of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:242–5.
Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450–7.
Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9.
Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420–4. Underscores how traditional CV risk scoring tools are not as beneficial in the patient with RA and that we must look beyond those factors to fully understand an RA patient’s overall risk of heart disease.
Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, Berger RD, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–7.
Crowson CS, Therneau TM, Davis JM, Roger VL, Matteson EL, Gabriel SE. Brief report: accelerated aging influences cardiovascular disease risk in rheumatoid arthritis. Arthritis Rheum. 2013;65:2562–6.
Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58:2268–74.
Peters MJL, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Care Res. 2009;61:1571–9.
Bruce IN. ‘Not only…But also’: factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology. 2005;44:1492–502.
Solomon DH, Curhan GC, Rimm EB, Cannuscio CC, Karlson EW. Cardiovascular risk factors in women with and without rheumatoid arthritis. Arthritis Rheum. 2004;50:3444–9.
Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Rantapää DS. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–91.
Szekanecz Z, Kerekes G, DÉR H, SÁNdor Z, SzabÓ Z, VÉGvÁRi A, et al. Accelerated atherosclerosis in rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:349–58.
Goodson NJ, Symmons DP, Scott DG, Bunn D, Lunt M, Silman AJ. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–9.
Farragher TM, Lunt M, Bunn DK, Silman AJ, Symmons DP. Early functional disability predicts both all-cause and cardiovascular mortality in people with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2007;66:486–92.
Liang KP, Maradit-Kremers H, Crowson CS, Snyder MR, Therneau TM, Roger VL, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009;36:2462–9.
Lopez-Longo FJ, Oliver-Minarro D, de la Torre I, Gonzalez-Diaz de Rabago E, Sanchez-Ramon S, Rodriguez-Mahou M, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:419–24.
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32.
Haque S, Gordon C, Isenberg D, Rahman A, Lanyon P, BELL A, et al. Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the Lupus and Atherosclerosis Evaluation of Risk (LASER) study. J Rheumatol. 2010;37:322–9.
McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–9.
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.
Solomon DH, Avorn J, Katz JN, Weinblatt ME, Setoguchi S, Levin R, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–8.
Naranjo A, Sokka T, Descalzo M, Calvo-Alen J, Horslev-Petersen K, Luukkainen R, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10:R30.
Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49:295–307.
van Halm V, Nurmohamed M, Twisk J, Dijkmans B, Voskuyl A. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8:R151.
Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA l). Ann Rheum Dis. 2007;66:1168–72.
Compliance with Ethics Guidelines
Conflict of Interest
Rekha Mankad declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Mankad, R. Atherosclerotic Vascular Disease in the Autoimmune Rheumatologic Patient. Curr Atheroscler Rep 17, 21 (2015). https://doi.org/10.1007/s11883-015-0497-6
Published:
DOI: https://doi.org/10.1007/s11883-015-0497-6