Abstract
Purpose of Review
Ubiquitous environmental exposures, including ambient air pollutants, are linked to the development and severity of childhood asthma. Advances in our understanding of these links have increasingly led to clinical interventions to reduce asthma morbidity.
Recent Findings
We review recent work untangling the complex relationship between air pollutants, including particulate matter, nitrogen dioxide, and ozone and asthma, such as vulnerable windows of pediatric exposure and their interaction with other factors influencing asthma development and severity. These have led to interventions to reduce air pollutant levels in children’s homes and schools. We also highlight emerging environmental exposures increasingly associated with childhood asthma. Growing evidence supports the present threat of climate change to children with asthma.
Summary
Environmental factors play a large role in the pathogenesis and persistence of pediatric asthma; in turn, this poses an opportunity to intervene to change the course of disease early in life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Asthma is a common chronic childhood disease, affecting approximately one in fifteen children in the United States [1]. Characterized by airway inflammation, bronchospasm, and airflow obstruction, it presents with clinical symptoms such as wheeze, cough, and respiratory exacerbations, often in response to exposures (or “triggers”) such as aeroallergens and respiratory tract viruses. Beyond these two well-known categories of exposures, however, many factors in a child’s environment are linked to both asthma pathogenesis and disease activity. We review recent advances in the understanding of these associations, focusing primarily on ubiquitous airborne environmental exposures; we also discuss mechanistic insights that may suggest causal relationships and windows of vulnerability, including prenatal exposures. Increased attention has been placed on finer-scale exposure assessment; that is, more precise geographic and temporal resolution of potential environmental contaminants than what is measured regionally or locally. This includes monitoring in settings children spend the majority of their time, such as inside homes and schools. We review established airborne pollutants subject to regulation, including particulate matter (PM), ozone (O3), and nitrogen dioxide (NO2), referred to as “criteria” pollutants under the U.S. Clean Air Act. We also highlight other, emerging environmental exposures, including phthalates, polycyclic aromatic hydrocarbons, volatile organic compounds, radon, and organophosphates. Though tobacco smoke exposure is a well-known contributor to asthma development and morbidity, reviewing advances in that literature is beyond the scope of this article. Finally, there is increasing evidence that changes to environmental exposures related to anthropogenic climate change adversely affect children with asthma.
Established Associations between Criteria Pollutants and Asthma Pathogenesis
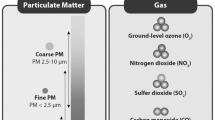
The association between criteria pollutants and asthma development in children has been convincingly demonstrated (Fig. 1). A meta-analysis in 2017 summarized much of the prior literature on components of traffic-related air pollution, including black carbon, coarse PM (PM10), fine PM (PM2.5), and NO2, in relation to incident asthma [2]. Regulatory efforts have led to reduced cases of asthma: reductions in PM2.5 and NO2 from 1993 to 2014 were associated with decreased asthma incidence in a prospective cohort of children in Southern California, with ozone and PM10 similarly trending toward an inverse association with asthma [3]. Nevertheless, ambient air pollution remains a significant contributor to asthma pathogenesis. A recent estimate of annual NO2 for the decade 2010–2019, linked to the Global Burden of Disease 2019 study, found that approximately 16% of asthma cases in urban areas worldwide could be attributed to NO2 [4].
Established and emerging environmental determinants of childhood asthma. “Criteria” pollutants under governmental regulation including particulate matter (PM), nitrogen dioxide (NO2), and ozone (O3) may arise from outdoor sources such as traffic and fossil fuel power plants or indoor sources such as cooking. Emerging ubiquitous environmental exposures in consumer plastics, combustion byproducts, food, household products, insecticides, and naturally occurring radioactive decay include phthalates, polycyclic aromatic hydrocarbons (PAH), volatile organic compounds (VOC), organophosphates, and radon
Prenatal exposures to criteria pollutants, when lung development is occurring, may constitute an important window of vulnerability. Prenatal PM2.5 and NO2 are consistently associated with asthma development across various birth cohort studies [5]. Recently, Hazlehurst and colleagues have suggested that PM2.5 exposure in the saccular phase of lung development, occurring between 24 and 36 weeks of gestation, particularly increases asthma risk [6••]. Though much of the literature has previously reported on cohort studies in the U.S., Canada, and Europe, there is increasing evidence replicating similar associations in low- and middle-income countries (LMIC), such as China and South Africa [7, 8]. The discovery that black carbon can cross the placental barrier leads to suggested mechanisms for fetal exposure to particulates [9]. NO2’s injurious impact on the developing lung prenatally has also been supported by an association with decreased levels of CC16, an anti-inflammatory protein protective against factors implicated in asthma pathogenesis [10, 11]. Nonetheless, remaining variability in recent study findings, such as a registry-based study in Sweden that did not find associations between prenatal ambient air pollutant exposure and asthma in offspring, suggests further work still needs to be done to elucidate the nature of this relationship [12].
As alveolarization of the lung continues through early childhood, early life has also been posited as an important time window for exposure to criteria pollutants. For instance, NO2 exposure has been associated with onset of asthma before, but not after, age 5 in a longitudinal Canadian cohort [13]. Recent work has supported the persistence of these effects throughout childhood: PM2.5 and NO2 exposures associated with home address at birth were associated with asthma at age 20 in a Dutch birth cohort, while lung function declined in children with asthma up to age 15 in relation to PM2.5 exposures in the first year of life [14, 15]. Indoor sources of air pollutants are also increasingly recognized in asthma pathogenesis, both in developed countries and in LMIC [16, 17]. Though there is a substantial body of work on the relationship between indoor air pollutants and asthma morbidity, including interventions to improve indoor air quality discussed below, ascertainment of indoor criteria pollutant exposures in the prenatal and early life periods to assess their relevance to asthma pathogenesis is relatively sparse. The American Academy of Pediatrics Council on Environmental Health issued a policy statement in 2021 recognizing the adverse impact of air pollution on the child, supporting targeted interventions to reduce exposure in pregnant women and young children who may be particularly susceptible [18]. In turn, better understanding of indoor air pollutant exposures during vulnerable windows may lead to personalized interventions to reduce the risk of asthma development, complementing further policy and regulatory action to reduce ambient air pollutant levels.
Emerging Environmental Determinants of Asthma Development
A variety of environmental exposures, including environmental chemicals, has been studied in relation to asthma development. We highlight several common exposures here because of their established links to other health effects and/or mixed findings in the literature (Fig. 1).
Phthalates are a class of plasticizer compounds in widespread use, commonly found in food storage and personal use products, leading to skin, dietary, and inhaled exposures [19]. They have been linked to perinatal health effects such as preterm birth, possibly through endocrine disruption [20, 21]. In 2014, Whyatt and colleagues first described a link between prenatal phthalate exposures and child asthma in a New York City birth cohort [22]. A meta-analysis of fourteen studies in 2020 found consistent associations between mono-benzyl phthalate (MBzP) and metabolites of di-2-ethylhexyl phthalate (DEHP) and risk of childhood asthma [23]; since then, several further prospective cohorts have reported on associations, or lack thereof, between phthalates and asthma, in both prenatal and early life exposure windows [24,25,26,27].
Children may be exposed to polycyclic aromatic hydrocarbons (PAH), produced from incomplete combustion, through inhalation from traffic-related sources and ingestion of foods with PAH. PAH have been linked to health effects including cancer, changes in neurodevelopment, early wheeze, and allergen sensitization [28,29,30,31,32,33]. In the largest cohort analysis of prenatal PAH, females but not males were at risk of asthma development [34, 35]. These inconclusive findings require further clarification.
Volatile organic compounds (VOC), gaseous at room temperature, arise from a variety of sources including household cleaners, paint, furniture, and combustion [36]. While there is evidence for prenatal VOC exposure on early life respiratory outcomes such as wheeze and decreased lung function, no data exist on persistence and transformation of these into childhood asthma [37,38,39]. Leveraging existing cohorts with measures of VOC during putatively vulnerable periods of exposure such as prenatally and in early life [40, 41], in addition to prospectively constructed studies, could help define any role of VOC in asthma pathogenesis.
Of note, children are exposed to phthalates, PAH, and VOC as diverse mixtures of chemicals, rather than as individual compounds, posing challenges in exposure assessment and analytical design. These challenges may contribute to the variability in associations found between these chemicals and development of asthma. We hope that analyses that thoughtfully consider mixtures of these chemicals using contemporary methods such as weighted quantile sum or Bayesian kernel machine regression will help capture the totality of exposure-outcome relationships in these emerging pollutants [42].
Criterion Pollutant Exposures and Asthma Morbidity in Children
Similar to work on the likely causal role of criteria pollutants on asthma development, there is substantial evidence supporting a role for PM2.5, NO2, and O3 in asthma morbidity, including symptomatic exacerbations and decreased lung function [43, 44]. Recent work describing links between criteria pollutants and healthcare utilization among children with asthma in LMIC complement similar literature in developed regions around the world, highlighting the global impact of air pollution on asthma severity [45,46,47,48]. In 2020, a London coroner officially implicated traffic-related air pollution in the asthma death of 9-year-old Ella Kissi-Debrah. This ruling resulted in significant public and medical discourse of the harms of air pollution to particularly vulnerable groups—including children with asthma [49, 50]. Indeed, PM2.5 was a significant risk factor for pediatric asthma deaths in an analysis from 2001 to 2016 in North Carolina [51•]. Children with severe asthma at baseline are more significantly affected by exposures to criteria pollutants compared to those with mild asthma [52, 53]. Examination of interactions with other factors contributing to asthma severity can further help define children with asthma at particularly high risk. Among children who are obese, there is increased risk for asthma symptoms from NO2 exposure and increased deposition of PM2.5 in the lung, suggesting complex mechanistic links between asthma severity, obesity, and air pollution [54, 55]. Furthermore, the effects of air pollutants on asthma symptoms and lung function appear to persist despite baseline asthma control in children with verified maintenance use of inhaled corticosteroids [56], while mitigating mouse allergen exposure was more effective in improving asthma symptoms among children who were less exposed to indoor PM10 [57].
Mitigating personal exposure to criteria pollutants, particularly PM, has been an area of interest in recent years. In the last decade, landmark randomized controlled trials (RCT) of indoor air cleaners in children with asthma exposed to environmental tobacco smoke or woodstove smoke have shown decreases in symptoms and improvements in lung function [58,59,60]. Continued efforts to improve indoor air quality have shown varying levels of effectiveness in reducing asthma morbidity. Lung function improved with bedroom air purification and reduced PM2.5 in a RCT in Shanghai, China [61]. An exploratory trial reducing household NO2 appeared to lead to a reduction in asthma symptoms, though this effect did not reach statistical significance [62]. Though PM levels in schools, where children spend a substantial amount of time, are associated with asthma morbidity [63, 64], classroom HEPA air purifiers did not reduce symptoms in children with asthma [65••]. In a rural setting, where the makeup of airborne pollutants including the composition of particles may be different, HEPA air filters improved asthma control and reduced urinary leukotriene levels [66••]. Finally, a recent pilot RCT of ambient air pollution education for families improved asthma control [67]. We look forward to further work on fine-scale, indoor exposures to air pollution in the diverse places where children live and learn, such as the recently initiated Synair-G prospective cohort in European schools [68].
Novel Environmental Associations with Asthma Morbidity
Emerging evidence supports the influence of a range of ubiquitous environmental factors on asthma symptoms and lung function. As discussed above, these include phthalates, PAH, and VOC. Recent work on phthalates (particularly DEHP) and a related class of plasticizer compounds, bisphenols, strengthens the link between these chemicals and asthma morbidity [69,70,71]. PAH exposure has been associated with increased bronchodilator and systemic corticosteroid use in the following 30 days [72]; whether these effects are causal or represent effects of confounded co-exposures, such as combustion that also produces particulates, is unclear. In contrast, the association between VOC exposures and asthma symptoms is more established, with a recent meta-analysis estimating effect sizes among children with asthma approximately double that of criteria pollutants [73]. This builds on prior work suggesting children are more vulnerable than adults to the respiratory effects of VOC exposure [74, 75].
Other emerging environmental determinants of asthma morbidity include organophosphates, present in insecticides and therefore important for children with asthma living in non-urban areas, [76, 77] and radon, a noble gas that can accumulate inside poorly ventilated buildings, including homes and schools [78]. High summer ambient temperatures, independent of air pollutant, aeroallergen, or respiratory viral levels, also contribute to asthma exacerbations in children [79].
Children with Asthma are Vulnerable to the Effects of Climate Change
Rising ambient temperatures are but one consequence of anthropogenic climate change that is likely to adversely affect children with asthma. Wildfire smoke, a complex environmental hazard that is an important source of ambient fine and ultrafine PM, is becoming more intense and more widespread, including outside traditionally wildfire-prone areas [80,81,82]. For instance, in 2023, smoke from wildfires of unprecedented size in Canada was associated with increased asthma-related healthcare utilization in New York City [83]. The harmful effects of wildfire smoke exposure on pediatric asthma seem to extend beyond “background” exposures to traffic-related air pollution [84]. In addition to efforts to reduce wildfire activity, there is an urgent need to develop personal interventions to mitigate exposures among vulnerable populations such as children with asthma [85]. We also highlight here recent work attempting to mitigate exposure to another source of particulates linked to climate change—desert dust storms—in which an intervention with home air purifiers and decreased home ventilation successfully reduced indoor infiltration of dust storm PM [86]. Furthermore, continued fossil fuel extraction, with consequent production of air pollution due to activities such as gas flaring, is associated with pediatric asthma morbidity [87]. A warming climate may lead to increases in environmental exposures known to be determinants of asthma and pose new challenges for children with asthma (Fig. 2).
Conclusions
Though the associations between PM, NO2, and O3 and childhood asthma are well-established, we continue to learn about the complexity of these relationships. Recent investigations have identified vulnerable windows of exposure and subgroups at particularly high risk. At the same time, more needs to be done to understand the causal pathways in order to continue developing interventions to mitigate exposure-related asthma morbidity. Increasing evidence is emerging to support ubiquitous chemicals in children’s indoor environments such as phthalates, PAH, and VOC as determinants of asthma. These environmental hazards, which are likely to evolve and increase in a changing climate, require a multipronged approach of regulatory action, translational research, and individual mitigation to protect children with asthma.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Most Recent National Asthma Data. Atlanta, GA: Centers for Disease Control and Prevention; 2023 [cited 2024 February 16]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31.
Garcia E, Berhane KT, Islam T, McConnell R, Urman R, Chen Z, et al. Association of changes in air quality with incident asthma in children in California, 1993–2014. JAMA. 2019;321(19):1906–15.
Anenberg SC, Mohegh A, Goldberg DL, Kerr GH, Brauer M, Burkart K, et al. Long-term trends in urban NO(2) concentrations and associated paediatric asthma incidence: estimates from global datasets. Lancet Planet Health. 2022;6(1):e49–58.
Bettiol A, Gelain E, Milanesio E, Asta F, Rusconi F. The first 1000 days of life: traffic-related air pollution and development of wheezing and asthma in childhood. A systematic review of birth cohort studies. Environ Health. 2021;20(1):46.
•• Hazlehurst MF, Carroll KN, Loftus CT, Szpiro AA, Moore PE, Kaufman JD, et al. Maternal exposure to PM(2.5) during pregnancy and asthma risk in early childhood: consideration of phases of fetal lung development. Environ Epidemiol. (Philadelphia, Pa). 2021;5(2). Largest (n = 1,469) prospective cohort study of prenatal PM exposure and asthma to date, examining different gestational ages corresponding to phases of lung development.
Xu M, Shao M, Chen Y, Liu C. Early life exposure to particulate matter and childhood asthma in Beijing, China: a case-control study. Int J Environ Health Res. 2024;34(1):526–34.
Hüls A, Vanker A, Gray D, Koen N, MacIsaac JL, Lin DTS, et al. Genetic susceptibility to asthma increases the vulnerability to indoor air pollution. Eur Respir J. 2020;55(3).
Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10(1):3866.
Beamer PI, Furlong M, Lothrop N, Guerra S, Billheimer D, Stern DA, et al. CC16 levels into adult life are associated with nitrogen dioxide exposure at birth. Am J Respir Crit Care Med. 2019;200(5):600–7.
Zhai J, Insel M, Addison KJ, Stern DA, Pederson W, Dy A, et al. Club cell secretory protein deficiency leads to altered lung function. Am J Respir Crit Care Med. 2019;199(3):302–12.
Olsson D, Forsberg B, Bråbäck L, Geels C, Brandt J, Christensen JH, et al. Early childhood exposure to ambient air pollution is associated with increased risk of paediatric asthma: an administrative cohort study from Stockholm. Sweden Environment international. 2021;155: 106667.
Lau N, Smith MJ, Sarkar A, Gao Z. Effects of low exposure to traffic related air pollution on childhood asthma onset by age 10 years. Environ Res. 2020;191: 110174.
Gehring U, Wijga AH, Koppelman GH, Vonk JM, Smit HA, Brunekreef B. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J. 2020;56(1).
Zhao Q, Kress S, Markevych I, Berdel D, von Berg A, Gappa M, et al. Air pollution during infancy and lung function development into adolescence: the GINIplus/LISA birth cohorts study. Environ Int. 2021;146: 106195.
Bédard MA, Reyna ME, Moraes TJ, Simons E, Turvey SE, Mandhane P, et al. Association between gas stove use and childhood asthma in the Canadian CHILD Cohort Study. Can J Public Health = Revue canadienne de sante publique. 2023;114(4):705–8.
Lu W, Wang LA, Mann J, Jenny A, Romero C, Kuster A, et al. Biomass smoke exposure and atopy among young children in the Western Highlands of Guatemala: a prospective cohort study. Int J Environ Res Public Health. 2022;19(21).
Brumberg HL, Karr CJ. Ambient air pollution: health hazards to children. Pediatrics. 2021;147(6).
Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics. 2019;7(2).
Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7.
American College of O, Gynecologists’ Committee on Obstetric P. Reducing Prenatal Exposure to Toxic Environmental Agents: ACOG Committee Opinion, Number 832. Obstet Gynecol. 2021;138(1):e40-e54.
Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: the Columbia Center for Children’s Environmental Health Cohort. Environ Health Perspect. 2014;122(10):1141–6.
Wu W, Wu C, Ji C, Diao F, Peng J, Luo D, et al. Association between phthalate exposure and asthma risk: a meta-analysis of observational studies. Int J Hyg Environ Health. 2020;228: 113539.
Jøhnk C, Høst A, Husby S, Schoeters G, Timmermann CAG, Kyhl HB, et al. Maternal phthalate exposure and asthma, rhinitis and eczema in 552 children aged 5 years; a prospective cohort study. Environ Health. 2020;19(1):32.
Adgent MA, Carroll KN, Hazlehurst MF, Loftus CT, Szpiro AA, Karr CJ, et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ Int. 2020;143: 105970.
Karramass T, Sol C, Kannan K, Trasande L, Jaddoe V, Duijts L. Bisphenol and phthalate exposure during pregnancy and the development of childhood lung function and asthma. The Generation R Study. Environ Pollut. (Barking, Essex : 1987). 2023;332:121853.
Navaranjan G, Diamond ML, Harris SA, Jantunen LM, Bernstein S, Scott JA, et al. Early life exposure to phthalates and the development of childhood asthma among Canadian children. Environ Res. 2021;197: 110981.
Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, et al. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS ONE. 2014;9(11): e111670.
Wallace ER, Ni Y, Loftus CT, Sullivan A, Masterson E, Szpiro AA, et al. Prenatal urinary metabolites of polycyclic aromatic hydrocarbons and toddler cognition, language, and behavior. Environ Int. 2022;159: 107039.
Sun B, Wallace ER, Ni Y, Loftus CT, Szpiro A, Day D, et al. Prenatal exposure to polycyclic aromatic hydrocarbons and cognition in early childhood. Environ Int. 2023:108009.
Miller RL, Garfinkel R, Lendor C, Hoepner L, Li Z, Romanoff L, et al. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatr Allergy Immunol. 2010;21(2 Pt 1):260–7.
Jung KH, Lovinsky-Desir S, Perzanowski M, Liu X, Maher C, Gil E, et al. Repeatedly high polycyclic aromatic hydrocarbon exposure and cockroach sensitization among inner-city children. Environ Res. 2015;140:649–56.
Substances AfT, Disease Registry. Toxicological profile for polycyclic aromatic hydrocarbons. Atlanta, Ga.]: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. 1995.
Loftus CT, Szpiro AA, Workman T, Wallace ER, Hazlehurst MF, Day DB, et al. Maternal exposure to urinary polycyclic aromatic hydrocarbons (PAH) in pregnancy and childhood asthma in a pooled multi-cohort study. Environ Int. 2022;170: 107494.
Sherris AR, Loftus CT, Szpiro AA, Dearborn L, Hazlehurst MF, Carroll KN, et al. Prenatal polycyclic aromatic hydrocarbon exposure and asthma at age 8–9 years in a multi-site longitudinal study. Res Sq. 2023.
Indoor Air Quality Scientific Findings Resource Bank. Volatile Organic Compounds. Berkeley, CA: Lawrence Berkeley National Laboratory; 2022 [cited 2024 February 16]. Available from: https://iaqscience.lbl.gov/volatile-organic-compounds-topics.
Franck U, Weller A, Röder SW, Herberth G, Junge KM, Kohajda T, et al. Prenatal VOC exposure and redecoration are related to wheezing in early infancy. Environ Int. 2014;73:393–401.
Chaya S, Vanker A, Brittain K, MacGinty R, Jacobs C, Hantos Z, et al. The impact of antenatal and postnatal indoor air pollution or tobacco smoke exposure on lung function at 3 years in an African birth cohort. Respirology. 2023.
Gutiérrez-Delgado RI, Barraza-Villarreal A, Escamilla-Núñez MC, Hernández-Cadena L, Cortez-Lugo M, Sly P, et al. Prenatal exposure to VOCs and NOx and lung function in preschoolers. Pediatr Pulmonol. 2020;55(8):2142–9.
Farrow A, Taylor H, Northstone K, Golding J. Symptoms of mothers and infants related to total volatile organic compounds in household products. Arch Environ Health. 2003;58(10):633–41.
Boyle EB, Viet SM, Wright DJ, Merrill LS, Alwis KU, Blount BC, et al. Assessment of exposure to VOCs among pregnant women in the National Children’s Study. Int J Environ Res Public Health. 2016;13(4):376.
Day DB, Sathyanarayana S, LeWinn KZ, Karr CJ, Mason WA, Szpiro AA. A permutation test-based approach to strengthening inference on the effects of environmental mixtures: comparison between single-index analytic methods. Environ Health Perspect. 2022;130(8):87010.
Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: systematic review and multilevel meta-analysis. PLoS ONE. 2017;12(3): e0174050.
Li X, Chen Q, Zheng X, Li Y, Han M, Liu T, et al. Effects of ambient ozone concentrations with different averaging times on asthma exacerbations: a meta-analysis. Sci Total Environ. 2019;691:549–61.
Zhang Y, Xu X, Zhang G, Li Q, Luo Z. The association between PM2.5 concentration and the severity of acute asthmatic exacerbation in hospitalized children: a retrospective study in Chongqing, China. Pediatr Pulmonol. 2023;58(10):2733–45.
Yadav R, Nagori A, Mukherjee A, Singh V, Lodha R, Kabra SK, et al. Effects of ambient air pollution on emergency room visits of children for acute respiratory symptoms in Delhi, India. Environ Sci Pollut Res Int. 2021;28(33):45853–66.
Hardell J, Silver EJ, Kavouras I, Lee DS, Gross E. Childhood asthma in the Bronx, NY; the impact of pollutants on length of hospital stay. J Asthma. 2023;60(12):2160–9.
Kim HS, Kim K, Rhee EH, Kim WK, Song DJ, Park JS, et al. Atmospheric environment and persistence of pediatric asthma: a population-based cohort study. Asian Pac J Allergy Immunol. 2024.
Dyer C. Air pollution from road traffic contributed to girl’s death from asthma, coroner concludes. BMJ. 2020;371: m4902.
Varghese D, Clemens T, McMurray A, Pinnock H, Grigg J, Cunningham S. Near-fatal and fatal asthma and air pollution: are we missing an opportunity to ask key questions? Arch Dis Child. 2023.
• Mirabelli MC, Flanders WD, Vaidyanathan A, Beavers DP, Gower WA. Ambient air quality and fatal asthma exacerbations among children in North Carolina. Epidemiology. 2023;34(6):888–91. Registry-based case-crossover study of all childhood asthma deaths in North Carolina 2001-2016, linked to daily PM2.5 exposure. Compared to the lowest tertile of PM2.5 exposure, the highest tertile was associated with 2.2 times the odds of mortality.
Kelchtermans J, Mentch F, Hakonarson H. Ambient air pollution sensitivity and severity of pediatric asthma. J Expo Sci Environ Epidemiol. 2023.
Rabinovitch N, Strand M, Gelfand EW. Particulate levels are associated with early asthma worsening in children with persistent disease. Am J Respir Crit Care Med. 2006;173(10):1098–105.
Afshar-Mohajer N, Wu TD, Shade R, Brigham E, Woo H, Wood M, et al. Obesity, tidal volume, and pulmonary deposition of fine particulate matter in children with asthma. Eur Respir J. 2022;59(3).
Permaul P, Gaffin JM, Petty CR, Baxi SN, Lai PS, Sheehan WJ, et al. Obesity may enhance the adverse effects of NO(2) exposure in urban schools on asthma symptoms in children. J Allergy Clin Immunol. 2020;146(4):813-20.e2.
Rosser FJ, Han YY, Forno E, Guilbert TW, Bacharier LB, Phipatanakul W, et al. Long-term PM(2.5) exposure and lung function change in children with asthma receiving inhaled corticosteroids. Am J Respir Crit Care Med. 2023.
Sadreameli SC, Ahmed A, Curtin-Brosnan J, Perzanowski MS, Phipatanakul W, Balcer-Whaley S, et al. Indoor environmental factors may modify the response to mouse allergen reduction among mouse-sensitized and exposed children with persistent asthma. J Allergy Clin Immunol Pract. 2021;9(12):4402–9 e2.
Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165(8):741–8.
Lanphear BP, Hornung RW, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011;127(1):93–101.
Noonan CW, Semmens EO, Smith P, Harrar SW, Montrose L, Weiler E, et al. Randomized trial of interventions to improve childhood asthma in homes with wood-burning stoves. Environ Health Perspect. 2017;125(9): 097010.
Cui X, Li Z, Teng Y, Barkjohn KK, Norris CL, Fang L, et al. Association between bedroom particulate matter filtration and changes in airway pathophysiology in children with asthma. JAMA Pediatr. 2020;174(6):533–42.
Gent JF, Holford TR, Bracken MB, Plano JM, McKay LA, Sorrentino KM, et al. Childhood asthma and household exposures to nitrogen dioxide and fine particles: a triple-crossover randomized intervention trial. J Asthma. 2023;60(4):744–53.
Gaffin JM, Hauptman M, Petty CR, Haktanir-Abul M, Gunnlaugsson S, Lai PS, et al. Differential effect of school-based pollution exposure in children with asthma born prematurely. Chest. 2020;158(4):1361–3.
Mentz G, Robins TG, Batterman S, Naidoo RN. Effect modifiers of lung function and daily air pollutant variability in a panel of schoolchildren. Thorax. 2019;74(11):1055–62.
•• Phipatanakul W, Koutrakis P, Coull BA, Petty CR, Gaffin JM, Sheehan WJ, et al. Effect of school integrated pest management or classroom air filter purifiers on asthma symptoms in students with active asthma: a randomized clinical trial. JAMA. 2021;326(9):839–50. A factorial randomized controlled trial of HEPA air purifiers and integrated pest management in schools with children with asthma in the Northeast US. The primary outcome was asthma symptom-days over 2 weeks. No difference between intervention and sham groups was found.
•• Drieling RL, Sampson PD, Krenz JE, Tchong French MI, Jansen KL, Massey AE, et al. Randomized trial of a portable HEPA air cleaner intervention to reduce asthma morbidity among Latino children in an agricultural community. Environ Health. 2022;21(1):1. A randomized controlled trial of HEPA air purifiers in homes of children with asthma in an agricultural community in Washington. Four primary outcomes were selected: asthma control test scores, symptom days, clinical utilization, and urinary leukotriene E4 concentration. Findings suggest improvements in the intervention group, though some did not reach statistical significance.
Rosser FJ, Rothenberger SD, Han YY, Forno E, Celedón JC. Air quality index and childhood asthma: a pilot randomized clinical trial intervention. Am J Prev Med. 2023;64(6):893–7.
Papadopoulos NG, Akdis CA, Akdis M, Damialis A, Esposito G, Fergadiotou I, et al. Addressing adverse synergies between chemical and biological pollutants at schools-the ‘SynAir-G’ hypothesis. Allergy. 2024;79(2):294–301.
Quiros-Alcala L, Hansel NN, McCormack M, Calafat AM, Ye X, Peng RD, et al. Exposure to bisphenols and asthma morbidity among low-income urban children with asthma. J Allergy Clin Immunol. 2021;147(2):577–86 e7.
Babadi RS, Riederer AM, Sampson PD, Sathyanarayana S, Kavanagh TJ, Krenz JE, et al. Longitudinal measures of phthalate exposure and asthma exacerbation in a rural agricultural cohort of Latino children in Yakima Valley, Washington. Int J Hyg Environ Health. 2022;243: 113954.
Fandiño-Del-Rio M, Matsui EC, Peng RD, Meeker JD, Quirós-Alcalá L. Phthalate biomarkers and associations with respiratory symptoms and healthcare utilization among low-income urban children with asthma. Environ Res. 2022;212(Pt B): 113239.
Uong SP, Hussain H, Thanik E, Lovinsky-Desir S, Stingone JA. Urinary metabolites of polycyclic aromatic hydrocarbons and short-acting beta agonist or systemic corticosteroid asthma medication use within NHANES. Environ Res. 2023;220: 115150.
Alford KL, Kumar N. Pulmonary health effects of indoor volatile organic compounds-a meta-analysis. Int J Environ Res Public Health. 2021;18(4).
Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59(9):746–51.
Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health. 2007;80(8):711–9.
Benka-Coker WO, Loftus C, Karr C, Magzamen S. Characterizing the joint effects of pesticide exposure and criteria ambient air pollutants on pediatric asthma morbidity in an agricultural community. Environ Epidemiol (Philadelphia, Pa). 2019;3(3).
Benka-Coker W, Hoskovec L, Severson R, Balmes J, Wilson A, Magzamen S. The joint effect of ambient air pollution and agricultural pesticide exposures on lung function among children with asthma. Environ Res. 2020;190: 109903.
Mukharesh L, Greco KF, Banzon T, Koutrakis P, Li L, Hauptman M, et al. Environmental radon and childhood asthma. Pediatr Pulmonol. 2022;57(12):3165–8.
Schinasi LH, Kenyon CC, Hubbard RA, Zhao Y, Maltenfort M, Melly SJ, et al. Associations between high ambient temperatures and asthma exacerbation among children in Philadelphia, PA: a time series analysis. Occup Environ Med. 2022;79(5):326–32.
Abatzoglou JT, Williams AP. Impact of anthropogenic climate change on wildfire across western US forests. Proc Natl Acad Sci USA. 2016;113(42):11770–5.
Borchers Arriagada N, Horsley JA, Palmer AJ, Morgan GG, Tham R, Johnston FH. Association between fire smoke fine particulate matter and asthma-related outcomes: Systematic review and meta-analysis. Environ Res. 2019;179(Pt A): 108777.
Rice MB, Henderson SB, Lambert AA, Cromar KR, Hall JA, Cascio WE, et al. Respiratory impacts of wildland fire smoke: future challenges and policy opportunities. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2021;18(6):921–30.
Thurston G, Yu W, Luglio D. An evaluation of the asthma impact of the June 2023 New York City Wildfire Air Pollution Episode. Am J Respir Crit Care Med. 2023;208(8):898–900.
Moore LE, Oliveira A, Zhang R, Behjat L, Hicks A. Impacts of wildfire smoke and air pollution on a pediatric population with asthma: a population-based study. Int J Environ Res Public Health. 2023;20(3).
Adibi A, Barn P, Shellington EM, Harvard S, Johnson KM, Carlsten C. HEPA air filters for preventing wildfire-related asthma complications, a cost-effectiveness study. Am J Respir Crit Care Med. 2023.
Achilleos S, Michanikou A, Kouis P, Papatheodorou SI, Panayiotou AG, Kinni P, et al. Improved indoor air quality during desert dust storms: the impact of the MEDEA exposure-reduction strategies. Sci Total Environ. 2023;863: 160973.
Willis M, Hystad P, Denham A, Hill E. Natural gas development, flaring practices and paediatric asthma hospitalizations in Texas. Int J Epidemiol. 2021;49(6):1883–96.
Acknowledgements
Figures were created with BioRender.com.
Funding
Dr. Sun is a Fellow in the Pediatric Scientist Development Program, supported by NIH/NICHD grant K12HD000850 and the Cystic Fibrosis Foundation. Dr. Gaffin is supported by NIH/NIEHS grants R01ES030100, P30ES000002 and the American Lung Association.
Author information
Authors and Affiliations
Contributions
Dr. Sun drafted the manuscript and prepared the figures. Dr. Gaffin conceived of the work and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Sun declares no conflicts of interest. Dr. Gaffin reports personal fees from medical-legal consulting, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, B.Z., Gaffin, J.M. Recent Insights into the Environmental Determinants of Childhood Asthma. Curr Allergy Asthma Rep 24, 253–260 (2024). https://doi.org/10.1007/s11882-024-01140-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-024-01140-2