Abstract
Purpose of Review
Occupational rhinitis is an underdiagnosed disease with significant morbidity and implications in the workplace. Multiple factors associated with this disease continue to pose a challenge to investigators. This review aims to summarize recent literature in occupational rhinitis, including classifications, pathogenesis, diagnosis, and treatment, as well as the impact of occupational rhinitis on individuals. Additionally, it identifies areas in need of further research and investigation.
Recent Findings
We highlight current research on the association between occupational rhinitis and occupational asthma and the role of immunotherapy in this disease. Discussion includes the impact of social trends on workers and the wider consequences of occupational rhinitis including decreased work productivity, absenteeism, and socioeconomic burden.
Summary
Occupational rhinitis remains a challenging disease entity due to the numerous potential causative factors, reduced recognition, morbidity in asthma, and therapeutic limitations. Additional research is needed to better identify disease predictors and develop effective management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nasal mucosa is the first point of entry to airborne particles in the environment. This includes common aeroallergens as well as pollutants and agents encountered in the workplace. Dusts, gases, vapors, and other chemicals are responsible for causing inflammation and irritation of the nasal mucosa [1]. Occupational respiratory diseases are common in the workplace and are associated with a broad variety of work environmental exposures. Occupational rhinitis (OR) is an upper airway inflammatory disease associated with the development of nasal symptoms including rhinorrhea, nasal congestion, sneezing, and itching directly linked to workplace exposures and not associated with factors that occur outside of the workplace [2]. Work-related rhinitis encompasses two subgroups of rhinitis: those directly caused by exposures or conditions of the workplace (OR) and rhinitis exacerbated by workplace exposures in an individual with pre-existing rhinitis prior to occupational exposures (work-exacerbated rhinitis) [3]. It is important to differentiate between these two entities as the early recognition of occupational rhinitis can lead to the prevention of occupational asthma [1]. While seen as a low-risk disease, rhinitis carries a high socioeconomic burden in affected individuals, the healthcare system, and the work environment. A systematic review of studies assessing the effect of allergic rhinitis suggested a significant impact on productivity and reduced performance while working (i.e., presenteeism) (35.9 mean percent impairment, 95% confidence interval (CI): 29.7-42.1) despite minimal impact on missed work time (i.e., absenteeism) (3.6 mean percent absenteeism, 95% CI: 2.4-4.8) [4]. Furthermore, the indirect costs that occurred due to loss of productivity represented 76% to 93% of the total allergic rhinitis costs [4]. However, the prevalence of occupational rhinitis is not well-defined due to several factors including the lack of a universal algorithm to diagnose occupational rhinitis [5, 6] and limited available longitudinal studies.
The purpose of this review is to summarize the available literature addressing recent classifications of occupational rhinitis, pathogenesis, diagnosis, and treatment, as well as the impact that occupational rhinitis has on individuals. It will also explore future areas of needed research and interventions to prevent and/or alleviate disease manifestations.
Rhinitis and Classifications
Rhinitis is diagnosed by the presence of either nasal congestion, rhinorrhea, sneezing, or itching [7]. While sometimes falsely considered as a non-consequential disease, rhinitis is a complex illness that involves a wide variety of underlying etiologies, triggers, and pathogenic mechanisms. Importantly, uncontrolled rhinitis adversely contributes to patients’ quality of life (QoL) as a considerable source of morbidity [8].
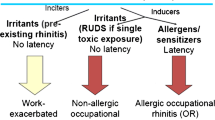
Work-related rhinitis (WRR) is a type of rhinitis that can be subcategorized as 1) occupational rhinitis or 2) work-exacerbated rhinitis (Fig. 1). While symptoms of work-related rhinitis, as its name implies, occur at the workplace, occupational rhinitis refers to rhinitis that is directly caused by occupational exposure in a previously unaffected individual [3]. As such, symptoms tend to improve when the workplace exposure is no longer present. For example, in occupational rhinitis there is typically symptom resolution either during weekends or time-off from the workplace for extended periods of time. In occupational rhinitis, the symptoms are attributed to the work environment and not to stimuli outside of it. Work-exacerbated rhinitis, on the other hand, refers to rhinitis that was present prior to the occupational exposure and is worsened by irritant triggers in the work environment. These irritants are usually nonallergic, such as smoke, strong odors, or dust. Occupational rhinitis can be further categorized into allergic and non-allergic etiologies based on the causative agent and the clinical history. Both types can contribute to decreased productivity in the workplace, creating significant stressors on the affected individual and their work environment.
Epidemiology
While chronic rhinitis (CR) is prevalent amongst the general population, the incidence of occupational rhinitis is not well-known. Avdeeva et al. report an incidence of chronic rhinitis of 40% amongst the general population in the Netherlands, consistent with a similar prevalence across Europe [9]. Over half of the working participants with non-allergic rhinitis in the Netherlands study had nasal complaints while at work. However, only 8% had improvement during weekends or vacation days away from work [9].
A review conducted by Siracusa et al. evaluating the prevalence of occupational rhinitis across nearly 60 different occupations, found that occupational rhinitis was three times more prevalent than occupational asthma (OA) [5]. Furthermore, a study by Maoua et al. evaluating this association in the textile industry of Tunisia, reported that rhinitis symptoms preceded asthma symptoms in 96.12% of the cases [10]. Ameille et al. conducted an analysis of 555 cases of newly reported occupational asthma attributed to identifiable agents and showed that occupational rhinitis was associated with occupational asthma in 58.4% of cases. Occupational rhinitis was also more prevalent after exposure to high molecular weight (HMW) agents compared to those of low molecular weight (LMW) (73.6% vs 51.4%, respectively, p<0.001) [11]. Moscato et al. also described an association between the severity of occupational rhinitis and occupational asthma, reporting that moderate-severe persistent occupational rhinitis was a predictor for persistent occupational asthma (OR: 19, 95% CI: 3.5-102.3) [12]. This emphasizes the close relationship between upper and lower airways as suggested by the united airway disease model, but additional studies are needed to further evaluate the association between these entities in the workplace environment [13]. Unfortunately, part of the difficulty associated with identifying the true incidence of occupational disease comes from the difficulty to confirm the diagnosis of occupational respiratory disease and the likely fear of workers to seek medical care due to the possibility of losing their job [14].
Occupational rhinitis has been described across multiple work environments and occupations due to exposure to both HMW and LMW agents (Table 1). With HMW agents, there is classically a sensitization period prior to the development of allergic occupational rhinitis. Food handlers, for example, can become sensitized by either direct transcutaneous exposure or via the inhalation of aerosolized particles while handling food. This has been recently described in a machine operator becoming sensitized to lentil and split pea while working in a packaging factory [15], as well as in two slaughterhouse workers becoming sensitized to pork and developing occupational rhinitis [16]. Workers at food processing plants can become sensitized not only to the food they are handling but also to the additives used in the work environment, such as enzymes. Phytase, for example, are fungal enzymes used to increase the rate of weight gain in animals that have been linked to the development of rhinitis, asthmatic symptoms, and contact urticaria in the workplace [17].
While food is a well-recognized sensitizer in the food-processing industry, the diverse use of chemical products in other work fields can make it difficult to identify a specific culprit. A recent study by da Paz et al. showed that the presence of symptoms suggestive of rhinitis and self-reports of rhinitis were more common amongst cleaning workers compared to office-workers regardless of the work environment of cleaning workers (e.g., hospital, university, housekeepers) [14]. Interestingly, there was no association between the length of time performing cleaning work and the nasal cellularity observed in nasal swabs [14].
In the automotive industry, isocyanates are well-recognized as strong LMW sensitizers that have been known to induce occupational rhinitis as well as occupational asthma. A series of 26 patients with occupational allergic rhinitis and asthma to isocyanates described manifestations developing 8.15 ± 5.13 years after initial exposure, with most patients experiencing nasal pruritus and rhinorrhea [18]. Persulphate salts are known to be common agents responsible for the development of occupational asthma and rhinitis in hairdressers, with those using bleaching powder being 20 times more likely to develop wheezing, breathlessness, and runny nose compared to individuals without an occupational exposure [19, 20]. A study comparing the prevalence of sneezing, rhinorrhea, and nasal congestion amongst hairdresser apprentices found a significant increase in the prevalence of these symptoms in recent graduates when compared to apprentices in their first year of training (p=0.001) [21]. Amongst the hairdresser products evaluated, bleaching products were the most common cause of rhinoconjunctival symptoms and cough [21]. It is important to note that new products, particularly in hair and cosmetic work, continue to be identified as causing agents of occupational rhinitis.
New trends embracing organic and vegan products have increased the use of vegetable-based dyes. Indigo dye is one vegetable-based dye that has been linked to both occupational asthma and rhinitis. It is commonly used in powder form, thereby increasing its exposure to the respiratory tract. Haltia et al. report two cases of hairdressers who developed rhinorrhea, cough, and dyspnea 3 to 6 months after using vegetable dyes with confirmed sensitization and positive inhalation challenges to indigo dye [22•]. Another common vegetable dye used by hairdressers is henna. Henna can be seen in different colors, and both yellow (Cassia obovata) and red henna (Lawsonia inermis) have been linked to occupational rhinitis [23].
Occupational rhinitis and occupational asthma are also important comorbidities affecting bakers at a higher rate than the general population, with recent studies describing an incidence of 31% and 5%, respectively [24]. While this has been attributed to flour exposure in general, a recent study evaluating the types of flour used in traditional bakeries in Verona, Italy, showed that bakers handling wheat flour with additives, as well as those also handling multigrain, had a significantly increased risk of nasal symptoms (OR: 1.8, 95% CI:1.01-3.22; OR: 2.19, 95% CI: 1.03-4.65, respectively) compared to those handling wheat flour alone (OR 1.49, 95% CI 0.8-2.76). Symptoms suggestive of work-related asthma were also more common in bakers handling wheat flour, additives, and multigrain (OR: 3.77, 95% CI: 1.5-10.54) compared to those exposed to wheat flour alone (OR 1.41, 95% CI 0.55-3.63) [25••].
In an evolving market demanding organic, gluten-free, and vegan products, bakers have turned to alternative agents. Psyllium is a common baking substitute for gluten and eggs, and it has been reported to cause occupational rhinitis [26•]. Moreover, buckwheat flour, another gluten-free alternative, has also been linked to occupational rhinitis and asthma in the food industry, especially in cooks and bakers [27•].
The examples presented here illustrate the complexity of occupational diseases in a constantly evolving occupational environment. As the occupational industry adjusts to meet consumer needs, it is important for healthcare providers to modify approaches to evaluating individuals with rhinitis attributed to the workplace. Healthcare providers should consider expanding the clinical history and testing for other compounds when medically appropriate. For example, baker’s rhinitis and asthma can be linked to a variety of components and substitutes, making testing for other ingredients besides wheat equally important. Additionally, when the index of suspicion remains high despite negative testing, the provider should consider the possibility of currently unrecognized agents leading to occupational disease.
Pathogenesis
Occupational rhinitis can be subclassified based on its underlying mechanism of disease (Fig. 1). Allergic occupational rhinitis (also known as sensitizer-induced occupational rhinitis) is mediated by a T-helper 2 (Th2) driven immune response. Here, specific antibodies are formed, usually IgE, that lead to the sensitization to a particular antigen. Following sensitization there is a latent period during which the sensitized individual is repetitively exposed to the causative agent, ultimately driving the hypersensitivity response to it. In contrast, non-allergic occupational rhinitis (also known as irritant-induced rhinitis) is not IgE mediated and results from direct injury caused by irritation to the epithelium, including epithelial cells and neurons [28]. Pro-inflammatory mediators and neuromodulators (including substance P and neurokinins) are released after chemoreceptors in nerve fibers underneath the epithelium, such as transient response potential receptors (TRPs), are activated. This ultimately leads to parasympathetic and sympathetic modulation in the form of vasodilation, recruitment of inflammatory cells, and glandular secretion [1].
In some instances, such as with exposure to a very high concentration of an irritant agent, non-allergic occupational disease can occur, including asthma and rhinitis. This is known as reactive airway dysfunction syndrome (RADS) and reactive upper airway dysfunction syndrome (RUDS), respectively [29]. In both diseases, desquamation of the respiratory epithelium can be seen via electron microscopy [30, 31]. This can occur following exposure to a variety of strongly acidic or alkaline chemicals such as chlorine, ammonia, cleaning agents, and fire smoke [32]. Most recently, RADS has been recognized as one of three phenotypes of irritant-induced asthma (IIA) [33]. In occupational rhinitis, less is known about high concentration irritant-induced rhinitis pathogenesis.
The molecular weight of agents is also important as agents of different molecular weight often induce varying responses. HMW agents are those larger than 10kDA, and due to their size, they can participate in IgE mediated responses and consequentially a Th2 cell-driven response [3]. Vegetal and animal glycoproteins, as well as microorganisms, are common HMW sensitizers. Conversely, LMW agents such as persulfate salts and isocyanates, are less than 10kDA and induce rhinitis symptoms through mechanisms unrelated to IgE. LMW substances are usually synthetic chemicals and, while their pathophysiology remains unclear, they are thought to function as haptens by conjugation with other proteins, forming hapten-protein complexes [34].
Diagnosis
The diagnosis of occupational rhinitis can be challenging and relies on obtaining a thorough clinical history to differentiate it from other types of rhinitis. Due to the difficulties in diagnosing occupational rhinitis and the similarity in nasal symptoms with other forms of chronic rhinitis, occupational rhinitis should be considered in any individual being evaluated for new or persistent symptoms.
Individuals with a history of atopy and prior allergic disorders are at an increased risk for development of allergic occupational rhinitis. Therefore, understanding baseline risk factors prior to work initiation is essential to provide proper counseling. As a healthcare provider, obtaining a thorough occupational history is crucial for identifying possible triggers and causative agents. Important questions to consider when assessing a patient’s occupational history include their job description, workplace environment, and temporality of symptoms with respect to the workplace. Patients with work-related rhinitis typically experience improvement during time away from work, with recurrence of symptoms upon their return. It is important to emphasize that physicians should keep a high index of suspicion beyond typical occupational exposures when evaluating occupational disease, as atypical presentations of occupational rhinitis are possible. Laboratory workers working with mice are a commonly affected labor group. However, they can also develop occupational rhinitis from other uncommon causes. Nakonechna et al. described the case of a laboratory worker who became sensitized to casein after working with culture media powder, developing occupational asthma, rhinitis, and even a severe milk allergy [35••]. In another example, diesel generators acting as a source of electricity have also been reported to cause occupational rhinitis and asthma in the office setting [36]. Additionally, it is essential to review all medications currently used by the patient to evaluate the presence of drug-induced rhinitis, as it can be triggered by a variety of medications, including non-steroidal anti-inflammatory drugs (NSAIDs), calcium channel blockers, psychotropics, and intranasal decongestants [37]. Identifying medication-induced rhinitis is crucial as discontinuing the offending mediation can lead to resolution of symptoms.
Knowledge of the patient’s smoking history is also valuable. Some studies have suggested that current smoking is related to a greater impairment in rhinitis specific QoL [4]. However, its association with rhinitis remains controversial, with studies reporting mixed results regarding the association between smoking and rhinitis [38,39,40]. Most recently, smoking has even been linked to decreasing the risk of allergic rhinitis (Inverse variance weighted (IVW) OR: 0.29, 95% CI: 0.18-0.47)) while increasing the risk of vasomotor rhinitis (IVW OR: 1.3, 95% CI: 1.04-1.62) [41].
As with any other disease process, a thorough physical exam can help identify other conditions with overlapping symptoms, such as the identification of nasal polyps, nasal tumors, or septal wall abnormalities. Physical examination is also useful in assessing the involvement of other organs or organ systems. Occupational rhinitis has been reported to increase the risk of occupational asthma, with symptoms of occupational rhinitis usually presenting prior to lung involvement. Occupational rhinitis was associated with occupational asthma in 58.4% of cases, with rhinitis symptoms occurring more frequently before asthma symptoms in individuals exposed to HMW compared to LMW agents (52.3% vs 38.8%, p<0.01) [11].
Evaluating the skin for the presence of any rashes should also be performed as contact urticaria can also be seen in association with occupational diseases. For example, Lucas and colleagues report the case of a worker assigned to the salmon-filleting line who would develop daily symptoms of rhinitis with dry cough within minutes to an hour after starting his workday [42]. This worker would also develop urticaria after 30 minutes of coming in touch with water from salmon preservative tanks [42]. Romita et al. also highlights this concept in a case report describing a candy factory worker whose job consisted in kneading candies with gum Arabic. He originally developed recurrent nasal congestion and after 4 years, he noticed urticaria affecting his arms despite using personal protection equipment. His nasal congestion would be present year-round, but his rash was seasonal as he would “pull up his sleeves” during the summer [43].
As part of the evaluation, it can be advantageous to differentiate allergic from non-allergic occupational rhinitis. Laboratory evaluation may show evidence of eosinophilia in allergic occupational rhinitis compared to its absence in irritant-induced rhinitis [44]. Skin prick testing (SPT) and serum specific IgE (sIgE) are two useful immunologic tests to confirm the sensitization to a suspected allergen, especially those of HMW. However, negative SPT and sIgE alone do not rule out the presence of allergic rhinitis. In local allergic rhinitis (LAR) for example, specific nasal IgE antibodies are produced while serum IgE is absent and SPT remains negative. T-cell mediated hypersensitivity reactions have also been reported. Touati et al. described a case of occupational rhinitis suspected to be secondary to a T-cell mediated hypersensitivity to sodium metabisulphite (SMBS) in a coffee factory worker responsible for making coffee pods [45•]. This worker had negative initial SPT that became positive hours later. Confirmatory patch testing was positive for SMBS at 24 hours, suggesting a possible role of non-immediate patch testing coupled with SPT in the assessment of T cell hypersensitivity reactions driving allergic occupational rhinitis [45•]. This case report highlights the importance of a thorough clinical history as local allergic rhinitis should still be considered in workers despite negative sIgE and SPT. In these clinical scenarios, further evaluation via nasal provocation testing (NPT) can be useful. In NPTs, a suspected allergen is exposed to the nasal mucosa in a controlled setting to elicit symptoms and objective measurements in a sensitized individual, making it an important part of translational and clinical research studies [46]. Whereas nasal provocation testing is considered the “gold standard” for diagnosing allergic occupational rhinitis, this diagnostic method is difficult to perform in-office, is time-consuming, and can be limited by the lack of standardized agents, usually requiring adapted protocols [47••].
Ultimately, the diagnosis of occupational rhinitis requires an integrated approach including a high index of suspicion and a thorough clinical history supported by physical examination and immunological testing. NPT can be considered when further tests are required to confirm the diagnosis.
Prevention and Management
Addressing occupational rhinitis requires a multifaceted approach involving prevention, reducing exposure to causative agents, and medical management. Prevention is an effective way of reducing the incidence of occupational rhinitis and decreasing its socioeconomic burden. Primary prevention relies on avoiding exposure to hazardous agents in the workplace and the proper use of protective equipment. Secondary prevention focuses on identifying early symptoms to halt disease progression. This may also allow for interventions to prevent the possible development of occupational asthma and a decrease in patients’ QoL. Tertiary prevention implies the use of targeted medical management to reduce the severity of disease. The management of occupational rhinitis, regardless of whether it is caused by allergens or irritants, relies on minimizing exposure to the causative agent. While avoiding the culprit agent can prevent progression of disease, complete avoidance may prove difficult as it can result in socioeconomic consequences for patients. In this context, reducing the exposure to the causative agent rather than completely avoiding it can be a reasonable option to balance this predicament [48].
Medical therapy in occupational rhinitis should be individualized for each patient. Specific studies evaluating the effectiveness of medical management in occupational rhinitis are lacking. Intranasal corticosteroids and intranasal antihistamines can be considered in both allergic- and non-allergic occupational rhinitis. In allergic occupational rhinitis, the use of oral antihistamines is also reasonable. However, it is important to emphasize that minimizing exposure to the causative agent should be prioritized.
While there is no definite role for immunotherapy in occupational rhinitis due to the lack of standardized extracts, several studies have evaluated its use as a possible therapeutic modality. Subcutaneous immunotherapy (SCIT) has been used to reduce morbidity associated to maize-based occupational rhinitis [49], while sublingual immunotherapy (SLIT) has been used in the management of baker’s rhinitis and asthma [50••]. In a small study of 6 participants, Wagoner et al. reported preliminary results in abstract form showing a mean symptom improvement of 52% (p=0.02) in 83% of patients with maize based occupational rhinitis after treatment with Phleum pretense SCIT [49]. Dubini et al. evaluated the use of SLIT with a wheat flour extract in 5 workers suffering from allergic rhinitis and/or asthma to wheat flour. After 3 years of therapy, patients reported symptom improvement and were able to step down asthma therapy [50••]. Moreover, there was a reduction in total IgE (mean reduction of 49.4 ± 18.54 KUA/L, p=0.0312) albeit there was no difference for specific IgE to wheat, barley, rye, and corn. They also reported decreased eosinophil cation protein (ECP) (mean reduction 23.32 ± 6.65 µg/L, p=0.0312) and decreased exhaled nitric oxide levels (FeNO) (average reduction 12.2 ± 2.44 parts-per-billion, p=0.0312) [50••].
While the studies mentioned above are small, they highlight the potential use of immunotherapy in occupational disease. Standardized reagents and larger studies evaluating the role and safety of immunotherapy in occupational rhinitis are required prior to recommending the use of this treatment modality in individuals.
Impact Beyond Symptoms
Few studies have evaluated the effects of occupational rhinitis in the healthcare system and the quality of life (QoL) of the affected individual, partly due to the difficulty in diagnosing occupational rhinitis. Work-related rhinitis appears to have a significant impact on general QoL and work productivity. Vandenplas et al. found that allergic rhinitis had an overall work productivity impairment of 39.4% (95% CI: 34.8-44%) – a work productivity impairment similar to that of other chronic diseases including depression, chronic obstructive pulmonary disease, and irritable bowel syndrome [4]. In particular, allergic occupational rhinitis was associated with decreased job productivity and overall daily activity limitations in a cross-sectional study performed by Maoua et al. [51]. Namely, overall work and activity impairment were positively correlated with age (p=0.045 and p=0.037, respectively) [51].
In a similar fashion, those affected by work-related rhinitis have a higher occurrence of disability which can cause workers to decrease their time at work, change their job responsibilities, or leave their job altogether [52••] Collectively, this creates additional socioeconomic stressors, highlighting the impact of occupational disease in the community. Unless provided with a diagnosis and medical care, individuals are unable to remain in the workforce due to debilitating symptoms, ultimately experiencing financial hardship and job insecurity. Unfortunately, recent case reports continue to show a significant delay from the initiation of symptoms to the time of diagnosis while highlighting delays from employers in the processing of compensation claims [15, 42].
While the understanding of the pathogenesis of occupational rhinitis and its causative agents has improved, there are still opportunities to make an early diagnosis and decrease the morbidity associated with this disease, ultimately improving the QoL of affected individuals.
Conclusions
Occupational rhinitis is a commonly underdiagnosed and under-reported disease that should be considered in any patient presenting with chronic rhinitis. A thorough evaluation and a detailed occupational history is vital in identifying occupational rhinitis. While the understanding and recognition of occupational rhinitis has increased, further studies are needed to better describe the true incidence and pathogenesis of this condition. Early recognition and avoidance of the causative agent is important to achieve a better prognosis, improved QoL, and decreased socioeconomic implications for affected individuals. With the continuous emergence of new products in the food and industrial sectors, it is likely that we will continue to see newer agents being described as causes of occupational rhinitis. Future research and better understanding of this disease can lead to the development of improved preventative and therapeutic options to protect patients and minimize the impact of occupational rhinitis in society.
REFERENCES
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hox V, et al. Occupational upper airway disease: how work affects the nose. Allergy. 2014;69(3):282–91.
Moscato G, Siracusa A. Rhinitis guidelines and implications for occupational rhinitis. Curr Opin Allergy Clin Immunol. 2009;9(2):110–5.
Dykewicz MS, et al. Rhinitis 2020: A practice parameter update. J Allergy Clin Immunol. 2020;146(4):721–67.
Vandenplas O, et al. Impact of Rhinitis on Work Productivity: A Systematic Review. J Allergy Clin Immunol Pract. 2018;6(4):1274-1286 e9.
Siracusa A, Desrosiers M, Marabini A. Epidemiology of occupational rhinitis: prevalence, aetiology and determinants. Clin Exp Allergy. 2000;30(11):1519–34.
Vandenplas O, Hox V, Bernstein D. Occupational Rhinitis. J Allergy Clin Immunol Pract. 2020;8(10):3311–21.
Hellings PW, et al. Non-allergic rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2017;72(11):1657–65.
Segboer CL, et al. Quality of life is significantly impaired in nonallergic rhinitis patients. Allergy. 2018;73(5):1094–100.
Avdeeva KS, et al. The prevalence of non-allergic rhinitis phenotypes in the general population: A cross-sectional study. Allergy. 2022;77(7):2163–74.
Maoua M, Rouis H. Occupational Rhinitis and Asthma in the Textile Sector of the Central Region of Tunisia. Int J Respiratory Pulmon Med. 2018;5.
Ameille J, et al. Occupational asthma and occupational rhinitis: the united airways disease model revisited. Occup Environ Med. 2013;70(7):471–5.
Moscato G, et al. Occupational rhinitis affects occupational asthma severity. J Occup Health. 2016;58(3):310–3.
Balogun RA, Siracusa A, Shusterman D. Occupational rhinitis and occupational asthma: Association or progression? Am J Ind Med. 2018;61(4):293–307.
da Paz ER, et al. Airway inflammatory profile among cleaning workers from different workplaces. BMC Pulm Med. 2022;22(1):170.
Sonday Z, Jeebhay M. Occupational rhinitis and asthma due to lentil and split pea allergy in a food-handler 2022. Curr Opin Allergy Clin Immunol. 2022;35:35–43.
Jungewelter S, Airaksinen L, Pesonen M. Occupational rhinitis, asthma, and contact urticaria from IgE-mediated allergy to pork. Am J Ind Med. 2019;62(1):80–4.
Kuske M, et al. Occupational allergy to phytase: case series of eight production workers exposed to animal feed additives. J Dtsch Dermatol Ges. 2020;18(8):859–65.
Chemingui S, et al. RF-417 Occupational Rhinitis and Asthma Caused by Isocyanates. BMJ Publishing Group Ltd. 2021.
Hollund BE, et al. Prevalence of airway symptoms among hairdressers in Bergen. Norway Occup Environ Med. 2001;58(12):780–5.
Macan J, et al. Respiratory toxicity of persulphate salts and their adverse effects on airways in hairdressers: a systematic review. Int Arch Occup Environ Health. 2022;95(8):1679–702.
Foss-Skiftesvik MH, et al. High occurrence of rhinitis symptoms in hairdressing apprentices. Int Forum Allergy Rhinol. 2017;7(1):43–9.
• Haltia T, et al. Occupational asthma, rhinitis, and contact urticaria from indigo (Indigofera tinctoria) hair dye. J Allergy Clin Immunol Pract. 2021;9(9):3500-3502. Describes sensatization to organic products amongst hairdressers.
Villalobos V, et al. Occupational Asthma and Rhinitis due to Yellow and Red Henna in a Hairdresser. J Investig Allergol Clin Immunol. 2020;30(2):133–4.
Pyana Kitenge J, et al. Occupational rhinitis and asthma in bakers: a cross-sectional study in the former Katanga province of DR Congo. International Archives of Occupational and Environmental Health. 2022;95(1):293–301.
•• Olivieri M, et al. Exposure to additives or multigrain flour is associated with high risk of work-related allergic symptoms among bakers. Occup Environ Med. 2021;78(2):112-116. Describes the complexity of baker's rhinitis and asthma and the effect of different flour components in symptoms.
• Jungewelter S, Suomela S, Airaksinen L. Occupational IgE-mediated psyllium allergy in contemporary gluten-free and vegan baking: A case of allergic rhinitis. Am J Ind Med. 2021;64(5):431-434. Highlights the effect of social trends in the recognition of new agents leading to occupational rhinitis.
• Jungewelter S, Airaksinen L, Pesonen M. Occupational buckwheat allergy as a cause of allergic rhinitis, asthma, contact urticaria and anaphylaxis-An emerging problem in food-handling occupations? Am J Ind Med. 2020;63(11):1047-1053. Presents the case of workers becoming sensitized to buckwheat when used as a gluten free-alternative in the workplace.
Bachert C. Persistent rhinitis - allergic or nonallergic? Allergy. 2004;59 Suppl 76:11-5; discussion 15.
Kotz S, et al. Occupational rhinitis Allergol Select. 2021;5:51–6.
Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest. 1985;88(3):376-84.
Meggs WJ, et al. Nasal pathology and ultrastructure in patients with chronic airway inflammation (RADS and RUDS) following an irritant exposure. J Toxicol Clin Toxicol. 1996;34(4):383–96.
Lemiere C, et al. Irritant-Induced Asthma. J Allergy Clin Immunol Pract. 2022;10(11):2799–806.
Vandenplas O, et al. EAACI position paper: irritant-induced asthma. Allergy. 2014;69(9):1141–53.
Ogi K, et al. Trimellitic anhydride facilitates transepithelial permeability disrupting tight junctions in sinonasal epithelial cells. Toxicol Lett. 2021;353:27–33.
•• Nakonechna A, Matthews D, Sargur R. Occupational asthma, rhinitis, contact dermatitis, and severe milk allergy caused by primary occupational exposure to casein. Ann Allergy Asthma Immunol. 2019;123(2):224-225. Presents a rare case of occupational asthma, rhinitis, urticaria, and severe milk allergy in a laboratory worker sensitized to casein after working with culture media powder containing casein.
Sibanda E, Makaza N. Health effects of diesel engine exhaust emissions exposure (DEEE) can mimic allergic asthma and rhinitis. Allergy Asthma Clin Immunol. 2019;15:31.
Varghese M, Glaum MC, Lockey RF. Drug-induced rhinitis. Clin Exp Allergy. 2010;40(3):381–4.
Lee WH, et al. Effects of cigarette smoking on rhinologic diseases: Korean National Health and Nutrition Examination Survey 2008–2011. Int Forum Allergy Rhinol. 2015;5(10):937–43.
Hisinger-Molkanen H, et al. Smoking, environmental tobacco smoke and occupational irritants increase the risk of chronic rhinitis. World Allergy Organ J. 2018;11(1):6.
Konno S, et al. The prevalence of rhinitis and its association with smoking and obesity in a nationwide survey of Japanese adults. Allergy. 2012;67(5):653–60.
Wang S, et al. Smoking behavior might affect allergic rhinitis and vasomotor rhinitis differently: A mendelian randomization appraisal. World Allergy Organ J. 2022;15(2): 100630.
Lucas D, et al. Occupational asthma, rhinitis and contact urticaria in a salmon-processing worker. Int Marit Health. 2022;73(3):112–4.
Romita P, et al. Occupational allergic rhinitis and contact urticaria caused by Gum Arabic in a candy factory worker. Contact Dermatitis. 2018;78(6):427–8.
Sokol KC, Hamilos DL. Occupational Rhinitis. In: Bernstein JA, editor. Rhinitis and Related Upper Respiratory Conditions: A Clinical Guide. Cham: Springer International Publishing; 2018. p. 59–66.
• Touati N, et al. An Unusual Case of Occupational Rhinitis. J Investig Allergol Clin Immunol. 2020;30(3):207-208. Describes the use of patch testing in conjunction with SPT in the assessment of OR with suspected underlying T-cell mediated hypersensitivity reactions.
Cantone E, Detoraki A, De Corso E. Local Allergic Rhinitis: A Different Rhinitis Endotype? Literature Overview. Appl Sci. 2022;12. https://doi.org/10.3390/app122111141.
•• Ronsmans S, et al. Diagnostic approach to occupational rhinitis: the role of nasal provocation tests. Curr Opin Allergy Clin Immunol. 2020;20(2):122-130. Describes the role of NPTs in ocuppational rhinitis and considerations to keep in mind when using HMW and LMW agents that are not standardized.
Hellgren J, Karlsson G, Toren K. The dilemma of occupational rhinitis: management options. Am J Respir Med. 2003;2(4):333–41.
Wagoner WW, et al. Treatment of Maize-Based Occupational Rhinitis By Phleum Pretense Subcutaneous Immunotherapy. J Allergy Clin Immunol. 2017;139(2, Supplement):AB152.
•• Dubini M, et al. Occupational asthma and rhinitis due to wheat flour: sublingual specific immunotherapy treatment. Med Lav. 2020;111(3):203-209. Highlights the use of SLIT in the management of baker's rhinitis and asthma.
Maoua M, et al. Quality of Life and Work Productivity Impairment of Patients with Allergic Occupational Rhinitis. Tanaffos. 2019;18(1):58–65.
•• Vandenplas O, et al. The Impact of Work-Related Rhinitis on Quality of Life and Work Productivity: A General Workforce-Based Survey. J Allergy Clin Immunol Pract. 2020;8(5):1583-1591 e5. Presents evidence showing that WRR has a significant impact on QoL and work productivity.
Funding
JAP receives funding from National Institute for Occupational Safety and Health (R01:OH012045), Department of Defense (PR200793) and Central States Center of Agricultural Safety and Health (CS-CASH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
JAP has received research regents (no monies) from AstraZeneca and is clinical site recruiter for asthma and sinus disease studies for GlaxoSmithKline, AstraZeneca, and Regeneron Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamora-Sifuentes, J., Poole, J.A. Occupational Rhinitis: An Update. Curr Allergy Asthma Rep 23, 579–587 (2023). https://doi.org/10.1007/s11882-023-01103-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-023-01103-z