Abstract
Purpose of Review
Self-reported penicillin allergies are frequently reported, though more than 95% of those are not truly allergic when challenged. These patients are more likely to receive alternative antibiotic regimens resulting in the use of broad-spectrum antibiotics that may be less effective, more toxic, and/or more expensive than preferred agents. Given the significant burden on patient outcomes and the healthcare system, the ability to reconcile an allergy and broaden future antibiotic options is essential.
Recent Findings
This is a narrative review describing risk stratification for penicillin skin testing, practical advice for implementation, and future directions. A summary of studies within the last 5 years is provided. The trend over the past several years has been to offer oral drug challenges to low-risk patients and skin testing to high-risk patients with a reported penicillin allergy.
Summary
This review provides support for risk stratification assessment of reported penicillin allergy to optimize antibiotic use and prevent emergence of antimicrobial resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse reactions to antibiotics are frequently encountered in clinical practice with allergy to the penicillin (PCN) family of antibiotics being the most commonly reported [1]. In fact, 10% of all patients self-reporting a drug allergy acknowledge an allergy to PCN [1]. However, 95% of those are not truly allergic when tested and therefore are likely incorrectly labeled [2•]. Patient-reported allergic reactions can range from mild to life-threatening; however, many self-reported reactions consist of historical childhood events with limited recollection of the details. Additionally, symptoms of the reported “allergy” are often non-allergic adverse events. Furthermore, some patients who truly have a PCN allergy have been found to have the hypersensitivity wane over time rendering the patient non-allergic [3,4,5].
Patients with reported PCN allergy are more likely to receive alternative antibiotic regimens resulting in the use of broad-spectrum antibiotics that may be less effective, more toxic, and/or more expensive than preferred agents [6,7,8,9,10]. A PCN allergy label has also been associated with longer hospital stays, higher drug costs, and more frequent healthcare complications [6, 9, 11,12,13].
Given the significant burden on patient outcomes and the healthcare system, the ability to reconcile an allergy and broaden future antibiotic options is essential. Formal evaluation of patients reporting a PCN allergy has been recommended by several large clinical societies, including the Centers for Disease Control and Prevention, the Infectious Diseases Society of America, and the American Academy of Allergy, Asthma, and Immunology [14,15,16]. Penicillin skin testing (PST) is a favorable option that can reliably and safely rule out IgE-mediated allergy with greater than 95% negative predictive value [17, 18••]. Table 1 includes a summary of PST studies published within the past 5 years [19,20,21,22,23,24,25, 26•, 27,28,29,30,31,32,33,34]. Within this manuscript, we describe risk stratification for PST, practical advice for implementing PST for allergy delabeling, and future directions.
Eligible for Oral Drug Challenge
The most definitive test for delabeling a PCN allergy is tolerance of the drug on direct oral drug challenge. The literature highlights that oral drug challenge (ODC) with PCN to a selected low-risk population is a safe and effective strategy to remove the PCN allergy label [22, 25, 35,36,37,38]. For example, a recent systematic review found approximately 95% of over 5000 participants with a reported history of PCN allergy tolerated ODC without any adverse reaction [39]. Hence, recent practice guidelines recommend that patients with suspected low-risk PCN allergy be primarily investigated using ODC without skin tests [18••, 40]. Although there is no consistent definition of “low-risk” in the literature, previous studies confirmed that ODC without skin test is appropriate in the following scenarios [8,9,10,11,12,13]: (1) reported history is not suggestive of allergy such as isolated gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea) or headache, (2) family history of penicillin allergy, (3) delayed reaction (> 1 h from exposure) with isolated benign maculopapular exanthem, and (4) unknown allergic reaction [18••, 40,41,42, 43••, 44].
Amoxicillin is recommended for the ODC as it includes a potentially antigenic side chain and accounts for the majority of PCN-class antibiotic usage. Therefore, tolerance of amoxicillin excludes selective amoxicillin allergy due to side-chain reactivity and reassures tolerance to the wider PCN family. However, there is significant heterogeneity in current protocols for ODC. The 2018 consensus statement of the American Academy of Allergy, Asthma & Immunology and the World Allergy Organization on β-lactam hypersensitivity testing acknowledged that there is no established “best protocol” for ODC [41]. We suggest the following principles to guide an ODC protocol:
-
1.
The dose should ideally be close to a therapeutic dose or a 10–25% aliquot when a graded oral challenge is done.
-
2.
To streamline implementation in clinical practice and avoid potential medication errors, choose a dose that is easy to order, prepare, and administer.
-
3.
To respect population preferences and underlying disease states, the dose should be available in more than one form (e.g., liquid, capsule).
Eligible for PCN Skin Testing
Penicillin allergy skin testing is ideally performed in higher risk patients when there is concern for a type I IgE-mediated reaction. This type of reaction is generally characterized by skin and/or mucosal tissue involvement with pruritis, urticaria, flushing, and/or angioedema; bronchospasm, wheezing, stridor, or other respiratory symptoms; gastrointestinal symptoms (e.g., abdominal pain, bloating, vomiting, or diarrhea) in conjunction with other signs of IgE-mediated symptoms; and/or hypotension, shock, and more than one organ involvement implicating anaphylaxis. The majority of IgE-mediated reactions are “immediate reactions” occurring within minutes to an hour of administration of the culprit drug.
Typical PST, when applied to higher risk patients with histories consistent with immediate reactions, consists of two steps. The first is an epicutaneous test consisting of a prick, puncture, or scratch. If the epicutaneous test remains negative 15 min after application, then intradermal skin testing is performed. A negative result indicates that there is no underlying IgE-mediated hypersensitivity. Most patients that undergo PST are negative. In prior studies, the percentage of patients that developed apparent IgE-mediated reactions to PCN following a negative skin test ranges from 1.2 to 2.9% [45, 46]. Administration of an ODC following a negative PST is the standard to rule out an IgE-mediated drug allergy more definitively. The combination of negative skin testing with an oral challenge has more than a 99% negative predictive value in excluding an IgE-mediated PCN allergy [47, 48].
Determining which patients benefit most from a skin test versus utilization of a direct ODC without a preceding skin test is important. Studies suggest that patients who report a prior history of anaphylaxis or urticaria have significantly higher rates of positive skin tests (17.4% and 12.4%, respectively) than patients with a history of an exanthem (4%) [46]. Additionally, studies have demonstrated that positive skin test results wane over time and the majority (nearly 70%) of patients who reported a reaction greater than 5–10 years prior will tolerate the skin test [3, 49,50,51]. In summary, skin testing should be considered when (1) the reaction occurred within the prior 5 years, (2) the patient has a history of anaphylaxis or clear IgE-mediated symptoms, or (3) the patient/provider is wary of ODC without skin prior skin testing.
PCN allergy delabeling can be accomplished even among more complex patient populations. Trubiano et al. described ODC among inpatient and outpatient adult cancer patients with a reported history of penicillin allergy deemed to be low-risk. All 23 cancer patients and 23 control patients (who had a prior history of cancer) were successfully challenged and delabeled [52]. Similarly, other studies have shown success with ODC in low-risk patients in the emergency department and inpatient setting [25, 53]. However, in patients with a reported PCN allergy with multiple drug allergy syndrome (defined by more than two allergies to unrelated drugs) and those with suspected IgE-mediated reactions, PST still remains commonplace. Patients with true PCN allergy are candidates for drug desensitization when PCN is of the preferred treatment [43••].
Not Eligible for Any Challenge
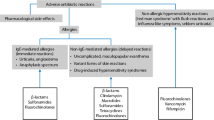
Delabeling PCN allergy via standardized skin and oral drug challenge protocols are useful for the majority of patients; however, there are certain instances in which testing with either modality is not recommended. Prior to determining which test to perform, an in-depth history with the patient should be utilized to identify rare reactions that include cutaneous and systemic reactions that may preclude these testing modalities (Fig. 1). Systemic reactions in which a patient should not undergo a drug challenge include drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DiHS), Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN), or serum sickness-like reactions.
PCN agents have been implicated in the development of DRESS/DiHS and therefore require careful consideration prior to initiating a drug challenge [54]. In general terms, the reaction can be characterized by the following criteria: (1) rash covering > 50% body surface area developing more than 2 weeks after initiating treatment, (2) prolonged clinical symptoms, (3) fever, (4) leukocyte abnormalities (leukocytosis and/or atypical lymphocytosis and/or eosinophilia), (5) elevation of liver enzymes, (6) lymphadenopathy, (7) lack of an alternative diagnosis, and (8) supportive histopathology. However, the reaction is defined differently by Japanese and European criteria [54, 55].
Stevens-Johnson syndrome and toxic epidermal necrolysis are severe cutaneous adverse reactions that affect both adults and children. Clinical features include history of drug exposure/febrile illness, painful rash, rash progressing to vesicles and bullae, and ultimately mucositis with painful erosions. These diseases, which occur across a spectrum of severity, are differentiated by the amount of body surface area involvement of clearly detached or Nikolsky sign positive skin. This life-threatening condition has been rarely known to occur in both adults and children who have received PCN agents. A particular concern is where a drug such as PCN is started following onset of SJS/TEN symptoms and then is subsequently and erroneously implicated as the causative agent. A complete history should be taken for alternative therapies and herbal supplements, which can also be the cause of SJS/TEN. When there is not a clear associated drug, SJS-/TEN-like illness may be associated with Mycoplasma and other respiratory illnesses known as recurrent reactive infectious mucocutaneous eruption (RIME), which is more common in children [56]. Any history of severe or suspected SJS/TEN in a patient reporting PCN allergy should avoid testing.
Serum sickness-like reactions (SSLR) have also been known to occur with the use of PCNs and are more common in children. These reactions are characterized by fever, cutaneous eruptions, edema, arthralgias, and lymphadenopathy typically occurring within days but certainly within 1 to 2 weeks after exposure to the inciting agent. SSLRs have a tendency to wane over time; future testing and re-challenge may be reasonable [57,58,59].
Future Directions
Healthcare providers frequently encounter patients reporting a PCN allergy, which interferes with the ability to prescribe the preferred antibiotic. Appropriate ODC or skin testing is important, especially in the era of increasing antimicrobial resistance. However, once a patient is delabeled, the prevention of re-labeling is a challenge. This is likely due to the myriad of medical records that may exist for a single patient (e.g., different healthcare systems, pharmacies, clinics) along with gaps in knowledge translation to the family regarding the new allergy status. A universal electronic medical record could help with not only delabeling (early identification, symptom checking, prompt to de-label) but could also assist with keeping the patient delabeled. Another area to explore in the future would be how PCN allergy delabeling impacts antibiotic prescriptions, durations of therapy, and potentially cost-implications to both families and healthcare systems. Lastly, the development of genetic testing which may predict drug allergy before it occurs could be another exciting approach to help us navigate a world with increasing antibiotic resistance.
Conclusion
Given efforts to optimize antibiotic use and prevent emergence of antimicrobial resistance, there is a current demand for formal risk stratification and assessment of penicillin allergy labels. A reported PCN allergy has serious implications for patient care and antimicrobial stewardship. Therefore, healthcare providers should take an active role in evaluating and/or referring patients for proper testing and delabeling. The trend over the past several years has been to offer oral drug challenges to low-risk patients with a reported PCN allergy. Many patients report low-risk symptoms of allergy; however, there is a select population of patients who report intermediate or high-risk symptoms in which PST is a rational option to evaluate a PCN allergy and improve clinical outcomes for patients. The practice of PST typically occurs in allergy clinics, but hospitals or outpatient offices may be sites of intervention as well. The practice of pharmacy-driven skin testing could also be another option to better facilitate the testing process and expand on allergy clinic options. Unique characteristics of each practice site will inform successful protocol development and implementation strategies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zhou L, Dhopeshwarkar N, Blumenthal KG, Goss F, Topaz M, Slight SP, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71:1305–13.
• Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72(9):1288–96 COMMENT: Meta-analysis describing the population-weighted mean for a negative skin test in inpatient cohorts was 95.1%.
Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5 Pt 1):918–24.
Chandra RK, Joglekar SA, Tomas E. Penicillin allergy: anti-penicillin IgE antibodies and immediate hypersensitivity skin reactions employing major and minor determinants of penicillin. Arch Dis Child. 1980;55(11):857–60.
Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171–80.
Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunology. 2014;133(3):790–6.
Al-Hasan MN, Acker EC, Kohn JE, Bookstaver PB, Justo JA. Impact of penicillin allergy on empirical carbapenem use in gram-negative bloodstream infections: an antimicrobial stewardship opportunity. Pharmacotherapy. 2018;38(1):42–50.
Lee CE, Zembower TR, Fotis MA, Postelnick MJ, Greenberger PA, Peterson LR, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000;160(18):2819–22.
Jeffres MN, Narayanan PP, Shuster JE, Schramm GE. Consequences of avoiding beta-lactams in patients with beta-lactam allergies. J Allergy Clin Immunol. 2016;137(4):1148–53.
Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ. 2018;361:k2400.
Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66(3):329–36.
Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685–7.
Powell N, Honeyford K, Sandoe J. Impact of penicillin allergy records on antibiotic costs and patient length of hospital say: a single centre observational retrospective cohort. J Hosp Infect. 2020;S0195-6701(20):30282–6 (online ahead of print).
Centers for Disease Control and Prevention. Is it really a penicillin allergy? Evaluation and diagnosis of penicillin allergy for healthcare professionals. 2018. Available at: https://www.cdc.gov/antibiotic-use/community/pdfs/penicillin-factsheet.pdf. Accessed August 6, 2020.
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of American. Clin Infect Dis. 2016;62(10):e51–77.
American Academy of Asthma, Allergy, and Immunology. Position statement: penicillin allergy. Available at: https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Penicillian-Allergy-Testing-Febraury-17.pdf. Accessed August 6, 2020.
Bland CM, Bookstaver PB, Griffith NC, Heil EL, Jones BM, Ann Justo J, et al. A practical guide for pharmacists to successfully implement penicillin allergy skin testing. Am J Health Syst Pharm. 2019;76:136–47.
•• Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188–99 COMMENT: Excellent review describing evaluation of penicillin allergy before prescribing antibiotics as an important tool for stewardship.
Heil EL, Bork JT, Schmalzle SA, et al. Implementation of an infectious disease fellow-managed penicillin allergy skin testing service. Open Forum Infect Dis. 2016;3(3):ofw155.
King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67–71.
Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A proactive approach to penicillin allergy testing in hospitalized patients. J Allergy Clin Immunol Pract. 2017;5(3):686–93.
Confino-Cohen R, Rosman Y, Meir-Shafrir K, Stauber T, Lachover-Roth I, Hershko A, et al. Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract. 2017;5(3):669–75.
Marwood J, Aguirrebarrena G, Kerr S, Welch SA, Rimmer J. Delabeling self-reported penicillin allergy within the emergency department through the use of skin testing and oral drug provocation testing. Emerg Med Australas. 2017;29(5):505–15.
Sundquist BK, Bowen BJ, Otabor U, Celestin J, Sorum PC. Proactive penicillin allergy testing in primary care patients labeled as allergic: outcomes and barriers. Postgrad Med. 2017;129(8):915–20.
Vyles D, Adams J, Chui A, et al. Allergy testing in children with low-risk penicillin allergy symptoms. Pediatrics. 2017;140(2):e20170471.
• Ramsey A, Staicu ML. Use of penicillin allergy screening algorithm and penicillin skin testing for transitioning hospitalized patients to first-line antibiotic therapy. J Allergy Clin Immunol Pract. 2018;6(4):1349–55 COMMENT: Describes a process for using penicillin skin testing to transition patients to first line therapies.
Jones BM, Avramovski N, Concepcion AM, Crosby J, Bland CM. Clinical and economic outcomes of penicillin skin testing as an antimicrobial stewardship initiative in a community health system. Open Forum Infect Dis. 2019;6(4):ofz109.
Mustafa SS, Conn K, Ramsey A. Comparing direct challenge to penicillin skin testing for the outpatient evaluation of penicillin allergy: a randomized controlled trial. J Allergy Clin Immunol Pract. 2019;7(7):2163–70.
Solensky R, Jacobs J, Lester M. Lieberman, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7(6):1876–85.
Taremi M, Artau A, Foolad F, Berlin S, White C, Jiang Y, et al. Safety, efficacy, and clinical impact of penicillin skin testing in immunocompromised cancer patients. J Allergy Clin Immunol Pract. 2019;7(7):2185–91.
Harmon S, Richardson T, Simons H, Monforte S, Fanning S, Harrington K. The clinical and financial impact of a pharmacist-driven penicillin skin testing program on antimicrobial stewardship practices. Hosp Pharm. 2020;55(1):58–63.
Plager JH, Mancini CM, Fu X, Melnitchouk S, Shenoy ES, Banerji A, et al. Preoperative penicillin allergy testing in patients undergoing cardiac surgery. Ann Allergy Asthma Immunol. 2020;124(6):583–8.
Jones BM, Bland CM. Penicillin skin testing as an antimicrobial stewardship initiative. Am J Health Syst Pharm. 2017;74(4):232–7.
Englert E, Weeks A. Pharmacist-driven penicillin skin testing service for adults prescribed non-preferred antibiotics in a community hospital. Am J Health Syst Pharm. 2019;76(24):2060–9.
Vezir E, Dibek Misirlioglu E, Civelek E, Capanoglu M, Guvenir H, Ginis T, et al. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol. 2016;27(1):50–4.
Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170(6):1–8.
Marrs T, Fox AT, Lack G, du Toit G. The diagnosis and management of antibiotic allergy in children: systematic review to inform a contemporary approach. Arch Dis Child. 2015;100(6):583–8.
Ibáñez MD. Rodríguez del Río P, Lasa EM, et al. Prospective assessment of diagnostic tests for pediatric penicillin allergy: from clinical history to challenge tests. Ann Allergy Asthma Immunol. 2018;121(2):235–44.
DesBiens M, Scalia P, Ravikumar S, Glick A, Newton H, Erinne O, et al. A closer look at penicillin allergy history: systematic review and meta-analysis of tolerance to drug challenge. Am J Med. 2020;133(4):452–62.
Stone CA Jr, Trubiano J, Coleman DT, Rukasin CRF, Phillips EJ. The challenge of delabeling penicillin allergy. Allergy. 2019;75(2):273–88.
Torres MJ, Adkinson NF, Caubet JC, et al. Controversies in drug allergy: beta-lactam hypersensitivity testing. J Allergy Clin Immunol Pract. 2019;7(1):40–5.
Reichel A, Röding K, Stoevesandt J, Trautmann A. De-labelling antibiotic allergy through five key questions. Clin Exp Allergy. 2020;50(4):532–5.
•• Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381(24):2338–51 COMMENT: Excellent review article describing penicillin allergy diagnosis, clinical implications, drug challenge, stewardship opportunities, and more.
Trubiano JA, Vogrin S, Chua KYL, Bourke J, Yun J, Douglas A, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745–52.
Sogn DD, Evans R, Shepherd GM, et al. Results of the National Institute of Allergy and Infectious Diseases collaborative clinical trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992;152(5):1025–32.
Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270(20):2456–63.
Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183–98.
Joint Task Force on Practice Parameter, American Academy of Allergy Asthma and Immunology and American College of Allergy Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–73.
Patriarca G, Schiavino D, Nucera E, Milani A. Positive allergological tests may turn negative with no further exposure to the specific allergen: a long-term, prospective, follow-up study in patients allergic to penicillin. J Investig Allergol Clin Immunol. 1996;6(3):162–5.
Voelker D, et al. Abstract P007. Presented at: American College of Allergy, Asthma and Immunology Annual Scientific Meeting; Nov. 15-19, 2018; Seattle.
Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5:813–5.
Trubiano JA, Smibert O, Douglas A, et al. The safety and efficacy of an oral penicillin challenge program in cancer patients: a multicenter pilot study. Open Forum Infect Dis. 2018;5(12):ofy306.
Ramsey A, Mustafa SS, Holly AM, Staicu ML. Direct challenges to penicillin-based antibiotics in the inpatient setting. J Allergy Clin Immunol Pract. 2020;8(7):2294–301.
Jurado-Palomo J, Cabañas R, Prior N, Bobolea ID, Fiandor-Román AM, López-Serrano MC, et al. Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: two case reports. J Investig Allergol Clin Immunol. 2010;20(5):433–6.
Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55(1):1–8.
Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child. 2013;98(12):998–1003.
Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol. 2001;86(3):330–4.
Clark BM, Kotti GH, Shah AD, Conger NG. Severe serum sickness reaction to oral and intramuscular penicillin. Pharmacotherapy. 2006;26(5):705–8.
Delli Colli L, Gabrielli S, Abrams EM, et al. Differentiating between β-lactam-induced serum sickness-like reactions and viral exanthem in children using a graded oral challenge. J Allergy Clin Immunol Pract. 2020. https://doi.org/10.1016/j.jaip.2020.08.047 Epub ahead of print.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Anaphylaxis and Drug Allergy
Rights and permissions
About this article
Cite this article
Zembles, T., Mitchell, M., Alqurashi, W. et al. Skin Testing for Penicillin Allergy: a Review of the Literature. Curr Allergy Asthma Rep 21, 21 (2021). https://doi.org/10.1007/s11882-021-00997-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11882-021-00997-x