Abstract
Purpose of Review
The aim of this study was to identify risk factors of asthma among children < 6 years old (preschool age) for predicting asthma during the preschool age and early school age (≤ 10 years of age).
Method of the study
MEDLINE, Cochrane, EMBASE, and Google Scholar databases were searched until June 30, 2017. Prospective or retrospective cohort and case-control studies were included. Studies had to have evaluated risk factors or a predictive model for developing asthma in children ≤ 6 years of age or persistent asthma in early school age.
Recent Findings
A total of 17 studies were included in the analysis. Factors associated with developing asthma in children ≤ 10 years of age (both pre-school and early school age) included male gender (pooled OR = 1.70, P < 0.001), atopic dermatitis (pooled OR = 2.02, P < 0.001), a family history of asthma (pooled OR = 2.20, P < 0.001), and serum IgE levels ≥ 60 kU/l or having specific IgE (pooled OR = 2.36, P < 0.001). A history of exposure to smoke or wheezing was also associated with persistent asthma in early school age (pooled OR = 1.51, P = 0.030 and pooled OR = 2.59, P < 0.001, respectively). In general, asthma predictive models (e.g., API, PIAMA, PAPS) had relatively low sensitivity (range, 21% to 71.4%) but high specificity (range, 69% to 98%).
Summary
The study found that male gender, exposure to smoke, atopic dermatitis, family history of asthma, history of wheezing, and serum IgE level ≥ 60 kU/l or having specific IgE were significantly associated with developing asthma by either preschool or early school age. Asthma predictive models can be developed by those risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is the most common chronic airway disease of childhood and the leading cause of childhood morbidity from chronic disease [1]. It often begins in early childhood with up to 50% of people with asthma having had symptoms, such as wheeze, in the first 6 years of life, and about 40% of toddlers will experience continued wheezing later in childhood [2]. Although the typical respiratory symptoms of asthma are wheezing and cough, those symptoms are also common in children without asthma, particularly in those 0 to 2 years of age [1, 3]. Other causes of wheezing can be upper and lower respiratory infection [1, 3]. Determining whether recurrent clinical presentation of wheezing is indicative of childhood asthma is difficult [1, 3]. In fact, a study in China found that the missed diagnosis rate of asthma in urban children was as high as 30% [4].
The clinical characteristics of asthma for children < 6 years old generally begin before the age of 3 years [1, 5]. Currently, no specific diagnostic tools or surrogate markers for detecting asthma in infancy or very young children exist [6]. Evaluating pulmonary function can be difficult in young children, although children aged 4 to 5 years can often produce reproducible spirometry with guidance [3, 7]. The persistent asthma-associated pulmonary function impairment in children also begins at preschool age. Therefore, it is clinically significant to assess the risk of developing persistent asthma at preschool age and to estimate the requirement for initiating a long-term asthma control program [5]. The Global Initiative for Asthma Guidelines (GINA) recommend that the diagnosis of asthma in children ≤ 5 years of age be based on: (1) symptom patterns (recurrent wheeze, cough, breathlessness, and nocturnal or early morning symptoms or awakenings), (2) presence of risk factors for development of asthma, and (3) therapeutic response to asthma controller and/or reliever treatment [3]. A position paper on the diagnosis and management of asthma in preschoolers in Canada proposes that the diagnosis of asthma in preschoolers requires the objective documentation of signs (or convincingly reported symptoms) of airflow obstruction and reversibility of airflow obstruction (i.e., improvement of signs and symptoms with asthma medication) [7]. A reliable tool or prediction rule to specifically predict the risk of preschool children in developing diagnosed-asthma may also reinforce the diagnosis.
Several models/scoring systems have been developed to help the healthcare provider identify children who are at risk of developing asthma during childhood (age 6 years or older), such as the stringent and loose forms of the Asthma Predictive Index (API) [8], cumulative risk score [9], severity score for obstructive airway disease [10], PIAMA risk score [11], Persistent Asthma Predictive Score (PAPS) [12], and other risk prediction based on the presence of risk factors for asthma development [13]. These models have included risk factors in preschool age associated with the development of asthma during the early childhood.
The aim of this study was to further evaluate potential risk factors of asthma present in children < 6 years of age that are associated with asthma in preschool children (< 6 years of age) or persistent asthma in early school age children (between age 6 and age10). In addition, the different published asthma prediction tools or models used to predict asthma among preschoolers or persistent asthma in early school age children were systematically reviewed.
Methods
Search Strategy
The study was performed in accordance with the PRISMA guidelines. The following databases were searched on June 30, 2017: Medline, Cochrane, EMBASE, and Google Scholar using the following search terms: Asthma AND (predictive OR prediction OR predicting) AND risk factors AND (preschool OR infants). Prospective or retrospective cohort and case-control studies were included. Included studies investigated patients < 6 years of age (pre-school) and evaluated the relevant risk factors for predicting diagnosed asthma in pre-school children or the persistence of asthma in children aged between 6 to 10 years (early pre-school). Studies evaluating the asthma predictive model for preschool children to predict the risk of developing asthma at early school age (between 6 to 10 years of age) were also included. In addition, studies should evaluate outcomes of interest quantitatively. Letters, comments, editorials, case reports, proceedings, and personal communications were excluded. Studies that evaluated children treated with inhaled corticosteroids and studies that assessed asthma risk factors during pregnancy, infancy, or children > 10 years of age, adolescence, and young adults were also excluded.
Data Extraction
The following information/data were extracted from the included studies: the name of the first author, year of publication, study design, number of participants in each group, age, gender, event of asthma, major risk factors, and outcomes of multivariate analysis. Factors assessed for predicting the risk of developing asthma included gender, exposure to smoke, atopic dermatitis, family history of asthma, history of wheezing, and IgE levels. For the asthma predictive model, the sensitivity, specificity, and positive and negative predictive value of the model were extracted.
Statistical Analysis
Outcomes evaluated had to be reported in ≥ 3 studies. The odds ratio (OR) with 95% confidence intervals (CIs) was extracted for each individual study included in the meta-analysis. A χ2-based test of homogeneity was performed, and the inconsistency index (I2) and Q statistics were determined. Heterogeneity was determined using the I2 statistic and was defined as follows: 0 to 24% = no heterogeneity; 25 to 49% = moderate heterogeneity; 50 to 74% = large heterogeneity; 75 to 100% = extreme heterogeneity. Because the number of studies included in the meta-analysis was small, heterogeneity tests had low statistical power. When tests for heterogeneity are underpowered, random-effects models are used routinely [14]. The National Research Council report recommends the use of random-effects approaches for meta-analysis and the exploration of sources of variation in study results [15]. Pooled effects were calculated, and a two-sided P value < 0.05 was considered to indicate statistical significance. Sensitivity analysis was carried out using the leave-one-out approach to investigate the validity and robustness of the results. The leave-one-out approach involves performing a meta-analysis on a subset of the studies in which each study is left out in turn. Publication bias using a funnel plot was not performed as > 10 studies are necessary for this type of analysis [16]. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Search Results
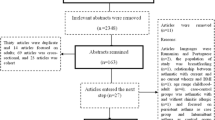
The flowchart of the search results is shown in Fig. 1. Of the original 326 articles initially identified, 296 were excluded based on the inclusion/exclusion criteria, and 30 studies were fully assessed for eligibility (Fig. 1). After full-text review, 13 additional articles were excluded because of the investigation of predictors for wheezing (n = 7), for atopy (n = 1), for school age adolescents and young adults (n = 3), and not reporting outcomes of interest (n = 2). A total of 17 articles were eligible for qualitative analysis [8, 11, 12, 17,18,19,20,21,22,23,24,25,26,27,28,29,30], and 11 articles were included in the meta-analysis [11, 12, 17, 20,21,22,23,24, 26, 27, 29].
Of the 17 included studies, 3 investigated the risk factors for predicting asthma events during the preschool age (age < 6 years), 8 investigated the risk factors for predicting persistent asthma at the early school age (age between 6-10 years old), and 10 investigated the asthma predictive models for preschool children to predict the risk of developing asthma at early school age (≤ 10 years of age) (Table 1). Four studies investigated both asthma predictors and the predictive model. The age of the participants ranged from newborn to 60 months (5 years of age). Male gender ranged from 49% to 74.4%, and the percentage of asthma events at the end of follow-up ranged from 5% to 75.2%.
Qualitative Analysis
The end points of the studies were diverse (Table 1). For the studies that investigated the predictors of asthma events at the preschool age, the end points were either asthma at 5 years of age [17, 20] or physician-diagnosed asthma in children aged 0 to 4 years [21]. For most of the studies that investigated predictors of persistent asthma in early school age, the end point was asthma at ages 6 to 8 years [11, 12, 22,23,24, 29]; only two studies had the end point of asthma in children up to the age of 10 years [26, 27].
Of the studies included in the meta-analysis, the predictors in which the odds ratio (OR) and 95% confidence intervals (CIs) were reported in ≥ 3 studies were male gender, exposure to smoke, atopic dermatitis, family history of asthma, history of wheezing and elevated IgE ≥60 kU/l, and specific IgE (Table 2). Predictors of asthma reported in two to three studies were not be included in meta-analysis, which were age at entry (baseline), prematurity (low birth weight) and preterm birth, positive asthma predictive index (API), atopy and allergy, blood eosinophils, respiratory infections, pulmonary disorders relating to prematurity, and wheezing (Supplemental Table 1). Odd ratios for the different predictors ranged from 1.11 to 9.3.
Type of asthma predictive score used in the different studies included loose API and stringent API [8, 19, 26], modified API (also referred to as the University of Cincinnati API [ucAPI]) [28, 30], asthma prediction tool (APT) [24, 25], clinical asthma prediction score (CAPS) [23], persistent asthma predictive score (PAPS) [12], and prevention and incidence of asthma and mite allergy (PIAMA) [18, 19] (Table 3).
The predictive probabilities of the different models/scoring systems (sensitivity, specificity, positive predictive value, negative predictive value) are summarized in Table 4. Loose API had a relatively higher sensitivity (range, 56.5% to 71.4%) but lower specificity (range, 33.3% to 81%) than stringent API (range, 27.5% to 42% for sensitivity; range, 79.2% to 96.3% for specificity) [8, 19, 26]. Modified API had relatively low sensitivity (range, 21% to 44%) but high specificity (range, 93% to 98%) [28, 30]. The PIAMA score and PAPS also had relatively low sensitivity (range, 54.5% to 63.8% for PIAMA; 42.6% for PAPS) but high specificity (range, 73.9% to 78.9% for PIAMA; 89.6% for PAPS) [12, 18, 19]. For APT, both sensitivity (72%) and specificity (71%) were moderately high [24], and external validation of APT found similar results (82% for sensitivity and 69% for specificity [25]).
Meta-Analysis
Three studies [11, 21, 27] reported ORs for gender and were included in the meta-analysis. No heterogeneity was observed among the three studies (Q statistic = 0.223, I2 = 0%). The pooled analysis revealed that male gender was significantly associated with asthma (pooled OR = 1.70, 95% CI: 1.54 to 1.88, P < 0.001, Fig. 2A).
Three studies [22, 27, 29] provided ORs for exposure to smoke and were included in the meta-analysis. No heterogeneity was observed among the studies (Q statistic = 1.843, I2 = 0%). The pooled analysis revealed that preschool children who were exposed to smoke were associated with risk of asthma at the early school age (age between 6-10 years old) (pooled OR = 1.51, 95% CI: 1.04 to 2.18, P = 0.030, Fig. 2B).
Three studies [12, 17, 29] reported ORs for atopic dermatitis and were included in the meta-analysis. No heterogeneity was observed among the three studies (Q statistic = 2.239, I2 = 10.66%). The pooled analysis showed that preschool children with atopic dermatitis were associated with a risk of asthma when they were ≤ 10 years of age (preschool and early school age) (pooled OR = 2.02, 95% CI: 1.58 to 2.57, P < 0.001, Fig. 2C).
Six studies [12, 17, 21, 23, 27, 29] provided ORs for family history of asthma and were included in the meta-analysis. Large heterogeneity was observed among the six studies (Q statistic = 17.160, I2 = 70.86%). The pooled analysis revealed that preschool children with a family history of asthma were associated with increased risk of asthma developing when < 6 years of age or 6 to 10 years of age (pooled OR = 2.20, 95% CI: 1.54 to 3.14, P < 0.001, Fig. 2D).
Three studies [11, 22, 29] reported ORs for history of wheezing and were included in the meta-analysis. No heterogeneity was observed among the three studies (Q statistic = 0.512, I2 = 0%). The pooled analysis showed that preschool children who have a history of wheezing were associated with a risk of developing asthma between 6 to 10 years of age (pooled OR = 2.59, 95% CI: 1.83 to 3.68, P < 0.001, Fig. 2E).
Four studies [12, 20, 23, 27] reported ORs for IgE levels. Moderate heterogeneity of serum IgE levels was observed among the four studies (Q statistic = 0.512, I2 = 0%). The pooled analysis showed that preschool children with serum IgE level ≥ 60 kU/l or having specific IgE were significantly associated with the risk of asthma developing when ≤ 10 years of age (pooled OR = 2.36, 95% CI: 1.56 to 3.58, P < 0.001, Fig. 2F).
Sensitivity Analysis
Sensitivity analyses were performed using the leave-one-out approach in which the meta-analysis was performed with each study removed in turn (Supplemental Table 2). Except exposure to smoke, the direction and magnitude of combined estimates did not vary markedly with the removal of the studies, indicating that the meta-analyses had good validity and robustness, and the data were not overly influenced by any one study. Sensitivity analysis with regard to exposure to smoke suggests that the studies of Arshad et al. (2005) [27] and Kotaniemi-Syrjänen et al. (2002) [29] may have overly impacted the findings.
Discussion
Identifying risk factors associated with the development of asthma in preschool children and persistent asthma in early school age children would facilitate the diagnosis and management of children with asthma. The meta-analysis found that being male, having atopic dermatitis, having a family history of asthma, and serum IgE levels ≥60 kU/l or having specific IgE were associated with asthma in preschool children (< 6 years of age) and persistent asthma in early school age children (≥ 6 and ≤ 10 years of age). A history of exposure to smoke or of wheezing was associated with developing persistent asthma in children aged 6 to 10 years.
The systematic review found that the different published models used to predict asthma varied with regard to sensitivity and specificity. Of all the models assessed, APT was the only scoring system that consistently had both good sensitivity and specificity (range, 72% to 82% for sensitivity; range, 69% to 71% for specificity). The other five models had either high sensitivity but low specificity or vice versa. Although the modified API model and stringent API model had high specificity (range, 79.2% to 98%), they had very low sensitivity (21% to 44%) suggesting these models would fail to identify many children who may develop asthma. Similarly, the PIAMA score and PAPs model had good specificity (range, 73.9% to 89.6%) but low sensitivity (range, 42.6% to 63.8%). These findings suggest that the APT model may be a more robust model for identifying children at risk for developing asthma, but additional studies are required to test the different models in clinical practice.
Our systematic review and meta-analysis excluded several other models/scoring systems used to predict asthma as they either are predictive for persistent wheezing [9, 20] or identified asthma-related risk factors in school children [10]. We also did not include assessment of risk factors that have been reported only once in the literature to be predictive of asthma. Those predictors are summarized in Supplemental Table 1.
A systematic review by Strina et al. (2014) evaluated risk factors associated with non-atopic asthma/wheeze in children and adolescents [31]. The systematic review included 43 studies, and they assessed 30 different risk factors. Only three factors showed consistent association with non-atopic asthma/wheeze. Similar to our findings, Strina et al. found family history of asthma was a risk factor for asthma. They also found dampness/mold in the household and lower respiratory infections in childhood were risk factors. Overweight/obesity and psychological/social factors also showed a consistent association with non-atopic asthma/wheeze; however, the number of studies investigating these factors was limited.
Rodriguez-Martinez et al. (2017) performed a systematic review of studies that identified factors that predict persistence of asthma symptoms among young patients with recurrent wheeze [32]. The review included 35 studies. They found that of the identified predictors the following were associated with asthma: family history of asthma or atopy; personal history of atopic disease; allergic sensitization early in life; and clinical patterns, frequency, or severity of wheezing/symptoms. Other risk factors included fraction of exhaled nitric oxide, eosinophils, age of onset ≥12 months, and male gender. Again, the typical respiratory symptoms of asthma are wheezing and cough, but those symptoms are also common in children without asthma, particularly in those 0 to 2 years of age [1, 3]. Therefore, the Rodriguez-Martinez’s study was meaningful in clinical practice. Our study also found that a history of wheezing was significantly associated with developing persistent asthma in children aged 6 to 10 years.
Two studies have evaluated risk factors associated with asthma in Chinese children [22, 33]. Zhao et al. (2014) performed a survey study in children (from newborn to 14 years of age) that evaluated the risk factors for asthma in Zhengzhou, China [33]. Using multivariate analysis, they found that allergic rhinitis, history of eczema, history of atopic dermatitis, food allergies, cesarean delivery, frequent use of antibiotic use within 1 year of age, use of wall decorating material, and use of a heater in the winter were risk factors for asthma. Yu et al. (2017) evaluated asthma risk factors in preschool wheezers among Chinese children before the age of 6 years [22]. They used multivariate analysis to identify independent risk factors for preschool wheeze. Yu et al. found female gender, positive skin prick test, and late-onset preschool wheeze increased the chance of asthmatic hospitalization after the age of 6 years. Allergic rhinitis and family history of asthma were associated with greater likelihood of asthma controller prescription after the age of six. It appears that more well-designed clinical study is needed to identify risk factors of asthma among preschool children for predicting asthma during the preschool and early school age (≤10 years of age).
Across the different studies and systematic reviews summarized above as well as the current study, a family history of asthma was found to be a risk factor for asthma. This may indicate a genetic component underlying asthma. Prior studies have found that many of the symptoms of asthma result from an interaction of environmental and genetic factors that lead to an immune response caused by infiltration of inflammatory cells into the airways [34, 35]. Multiple genetic factors are thought to influence asthma [35]. Genetic factors are thought to underlie about 40% to 60% of variance in the allergen-specific IgE level, 30% to 60% in the positive skin test to dust mite etiology, and 30% of log eosinophil count [35]. The genetic pathways involved in asthma are not well understood but are crucial for designing a precision medicine in the future.
The study has several limitations that should be considered. For each risk factor evaluated by meta-analysis, the number of studies included was small. Moreover, we assessed specific factors already indicated to be involved in asthma. It is possible that ethnic background and/or cultural/lifestyle differences may influence what factors are predictive of developing asthma. Additional analyses are required to test other factors that may also be predictive of asthma in preschool children and persistent asthma in young school age children.
Conclusions
In summary, male gender, exposure to smoke, atopic dermatitis, family history of asthma, history of wheezing, and serum IgE level ≥60 kU/l or having specific IgE were significantly associated with the chance of developing asthma by either preschool or early school age. The risk factors identified by our meta-analysis can be used to develop a predictive model for asthma at preschool age and early childhood with high sensitivity and specificity.
References
Bacharier LB, Guilbert TW. Diagnosis and management of early asthma in preschool-aged children. J Allergy Clin Immunol. 2012;130:287–96. quiz 97-8
Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8.
2017 GINA Report, Global Strategy for Asthma Management and Prevention [cited 3. October 1]
National Cooperative Group on Childhood Asthma; Institute of Environmental Health and Related Product Safety, Chinese Center for Disease Control and Prevention; Chinese Center for Disease Control and Prevention. [Third nationwide survey of childhood asthma in urban areas of China]. Zhonghua Er Ke Za Zhi. 2013; 51, 729-35. (in Chinese)
Subspecialty Group of Respiratory Diseases, Society of Pediatrics, Chinese Medical Association.; Editorial Board, Chinese Journal of Pediatrics. [Guideline for the diagnosis and optimal management of asthma in children(2016)]. Zhonghua Er Ke Za Zhi. 2016;54(3):167-81. (in Chinese)
Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5–34.
Ducharme FM, Dell SD, Radhakrishnan D, Grad RM, Watson WT, Yang CL, et al. Diagnosis and management of asthma in preschoolers: A Canadian Thoracic Society and Canadian Paediatric Society position paper. Paediatr Child Health. 2015;20:353–71.
Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6.
Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent disease among children who wheeze during early life. Eur Respir J. 2003;22:767–71.
Devulapalli CS, Carlsen KC, Haland G, Munthe-Kaas MC, Pettersen M, Mowinckel P, et al. Severity of obstructive airways disease by age 2 years predicts asthma at 10 years of age. Thorax. 2008;63:8–13.
Caudri D, Wijga A, Schipper ACM, Hoekstra M, Postma DS, Koppelman GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol. 2009;124:903-10.e1–7.
Vial Dupuy A, Amat F, Pereira B, Labbe A, Just J. A simple tool to identify infants at high risk of mild to severe childhood asthma: the persistent asthma predictive score. J Asthma. 2011;48:1015–21.
Savenije OE, Kerkhof M, Koppelman GH, Postma DS. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–31.
Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150:206–15.
National Research Council. Combing Information: Statistical Issues and Opportunities for Research. Washington, DC: National Academy Press; 1992.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002.
Wen HJ, Chiang TL, Lin SJ, Guo YL. Predicting risk for childhood asthma by pre-pregnancy, perinatal, and postnatal factors. Pediatr Allergy Immunol. 2015;26:272–9.
Hafkamp-de Groen E, Lingsma HF, Caudri D, Levie D, Wijga A, Koppelman GH, Duijts L, Jaddoe VW, Smit HA, Kerkhof M, Moll HA, Hofman A, Steyerberg EW, de Jongste JC, Raat H. Predicting asthma in preschool children with asthma-like symptoms: validating and updating the PIAMA risk score. J Allergy Clin Immunol 2013; 132: 1303-1310.
Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Discriminative properties of two predictive indices for asthma diagnosis in a sample of preschoolers with recurrent wheezing. Pediatr Pulmonol. 2011;46:1175–81.
Holt PG, Rowe J, Kusel M, Parsons F, Hollams EM, Bosco A, McKenna K, Subrata L, de Klerk N, Serralha M, Holt BJ, Zhang G, Loh R, Ahlstedt S, Sly PD. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol 2010; 125: 653-9, 9.e1-653-9, 9.e7.
Schaubel D, Johansen H, Dutta M, Desmeules M, Becker A, Mao Y. Neonatal characteristics as risk factors for preschool asthma. J Asthma. 1996;33:255–64.
Yu P-T, Chan J, Poon F, Lee R, Leung S-Y, Ng J, et al. The predictive factors in preschool wheezers for subsequent asthma hospitalization after the age of 6 years. Pediatric Respirology and Critical Care Medicine. 2017;1:11–6.
van der Mark LB, van Wonderen KE, Mohrs J, van Aalderen WM, ter Riet G, Bindels PJ. Predicting asthma in preschool children at high risk presenting in primary care: development of a clinical asthma prediction score. Prim Care Respir J 2014; 23: 52-59.
Pescatore AM, Dogaru CM, Duembgen L, Silverman M, Gaillard EA, Spycher BD, et al. A simple asthma prediction tool for preschool children with wheeze or cough. J Allergy Clin Immunol. 2014;133:111-8.e1–13.
Grabenhenrich LB, Reich A, Fischer F, Zepp F, Forster J, Schuster A, et al. The novel 10-item asthma prediction tool: external validation in the German MAS birth cohort. PLoS One. 2014;9:e115852.
Leonardi NA, Spycher BD, Strippoli MP, Frey U, Silverman M, Kuehni CE. Validation of the Asthma Predictive Index and comparison with simpler clinical prediction rules. J Allergy Clin Immunol. 2011;127:1466-72.e6.
Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127:502–8.
Amin P, Levin L, Epstein T, Ryan P, LeMasters G, Khurana Hershey G, et al. Optimum predictors of childhood asthma: persistent wheeze or the Asthma Predictive Index? J Allergy Clin Immunol Pract. 2014;2:709–15.
Kotaniemi-Syrjanen A, Reijonen TM, Korhonen K, Korppi M. Wheezing requiring hospitalization in early childhood: predictive factors for asthma in a six-year follow-up. Pediatr Allergy Immunol. 2002;13:418–25.
Chang TS, Lemanske RF Jr, Guilbert TW, Gern JE, Coen MH, Evans MD, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1:152–6.
Strina A, Barreto ML, Cooper PJ, Rodrigues LC. Risk factors for non-atopic asthma/wheeze in children and adolescents: a systematic review. Emerg Themes Epidemiol. 2014;11:5.
Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Factors predicting persistence of early wheezing through childhood and adolescence: a systematic review of the literature. J Asthma Allergy. 2017;10:83–98.
Zhao K, Song GH, Gu HQ, Liu S, Zhang Y, Guo YR. Epidemiological survey and risk factor analysis of asthma in children in urban districts of Zhengzhou, China. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:1220–5. (in Chinese)
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25.
Meng J-F, Rosenwasser LJ. Unraveling the Genetic Basis of Asthma and Allergic Diseases. Allergy, Asthma & Immunology Research. 2010;2:215–27.
Acknowledgments
The authors would like to thank Dr. Elizabeth Goodwin of MedCom Asia for providing the necessary writing assistance and editorial support during development of the manuscript funded by AstraZeneca China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Yixiao Bao and Zhimin Chen Co-first authors.
This article is part of the Topical Collection on Pediatric Allergy and Immunology
Electronic Supplementary Material
ESM 1
(DOCX 31.7 kb)
Rights and permissions
About this article
Cite this article
Bao, Y., Chen, Z., Liu, E. et al. Risk Factors in Preschool Children for Predicting Asthma During the Preschool Age and the Early School Age: a Systematic Review and Meta-Analysis. Curr Allergy Asthma Rep 17, 85 (2017). https://doi.org/10.1007/s11882-017-0753-7
Published:
DOI: https://doi.org/10.1007/s11882-017-0753-7