Abstract
Chronic rhinosinusitis (CRS) is a prevalent disease that is associated with significant costs and quality of life impairments. Currently, patients are classified into subgroups based on clinical characteristics, most often the presence or absence of nasal polyps. However, despite medical and surgical treatment, many of these patients continue to have symptoms. Recent efforts have focused on gaining a more complete understanding of the inflammatory mechanisms that drive pathogenesis in CRS, and it is becoming clear that the inflammatory processes in CRS are quite complex. As our understanding of these complex phenotypes improves, it may become possible to classify patients into endotypes based on unique inflammatory patterns within the sinus mucosa. This information may also lead to the identification of appropriate targeted therapies for different endotypes. This review will discuss our current understanding of endotypes in CRS along with the unique adaptive immune responses that may contribute to these different endotypes and, finally, some potential targeted therapeutics for the next generation of CRS treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a chronic disease of the upper respiratory system that affects about 10–12% of the population [1, 2]. Despite the high prevalence, quality of life impairment, and costs associated with CRS, the key inflammatory mechanisms that drive inflammation and persistence in this disease remain unclear. Currently, patients with CRS have limited treatment options; medical management of CRS frequently fails to sufficiently improve symptoms and many patients ultimately have endoscopic sinus surgery to remove inflamed sinus tissues and relieve symptoms [3]. Many researchers have been focused on elucidating these mechanisms with the goal of identifying new treatment options for CRS patients. From this work, it is clear that the inflammatory process in CRS is complex, and it involves multiple arms of the immune response. The goal of this review is to summarize the current knowledge regarding different classifications of CRS patients and how the adaptive immune response plays a role in disease pathogenesis in different subgroups of CRS patients. The innate immune system also plays very important roles in the pathogenesis of CRS, but that topic is beyond the scope of this review and has recently been comprehensively summarized elsewhere [4].
Classification of CRS Patients
Clinical Classifications
CRS is clinically classified into two phenotypes based on the presence or absence of nasal polyps on endoscopic examination: CRS with (CRSwNP) and without (CRSsNP) nasal polyps [1, 5]. CRSwNP can be further divided to include a subset of patients with aspirin-exacerbated respiratory disease (AERD), which is characterized by severe manifestations of CRSwNP and asthma, in addition to respiratory reactions to aspirin and other inhibitors, such as NSAID, of the COX-1 enzyme [6]. There are additionally rare but unique subsets of CRS that can be attributed to a specific genetic disorders such as cystic fibrosis, a pathogenic organism such the fungal species causing allergic fungal rhinosinusitis, or dental conditions that will not be discussed in this review [5, 7]. Studies suggest that the overall symptom burden of CRSsNP and CRSwNP have significant overlap, with patients in both groups demonstrating similar overall total symptom scores on quality of life instruments. However, it has been suggested that the symptoms that cause the biggest problems for CRS patients are distinct between CRSsNP and CRSwNP groups, with facial pain/pressure, headache, and cough being more bothersome in CRSsNP, whereas CRSwNP patients are significantly more bothered by nasal obstruction and hyposmia/anosmia [8,9,10]. Given that patients with CRS have relatively few treatment options, and a significant subset of patients do not respond to these treatments, it is important to determine how to tailor treatments to optimize outcomes and reliably identify patients for the treatments best suited to their underlying pathophysiology.
Endotypes of CRS
It is apparent that these current clinical definitions of CRS patient subsets lack complete insight into the pathophysiologic underpinnings of these diseases. Many groups are now investigating whether CRS patients would be better classified on the basis of symptom patterns or using patterns of inflammation measurable within the mucosa or serum to create more specific CRS patient endotypes [11,12,13]. The proponents of endotyping in CRS suggest it will better identify patients for targeted therapy. Importantly however, currently available and approved treatments for CRS, such as endoscopic sinus surgery or corticosteroids, are effective for many patients, regardless of their inflammatory phenotype. Nonetheless, not all patients respond to these traditional treatments, and longer-term follow-up suggests that CRSwNP phenotype is associated with increased recurrence [14]. Furthermore, exciting new data from clinical trials has demonstrated efficacy for therapies specifically targeting type 2 inflammation in CRSwNP patients providing critical insights into disease pathogenesis (discussed below) [14,15,16].
Classically, CRSsNP has been characterized by increased levels of type 1 inflammatory mediators, including IFN-γ, while CRSwNP has been characterized by increased levels of type 2 inflammatory mediators, including IL-5 and eosinophils [1, 17]. Recently, two simultaneously published studies, one by our group and the other by an international consortium sampling from five countries across three continents, demonstrated that surgically managed CRSwNP in Western countries (e.g., Germany, Belgium, and the USA) are almost universally (∼80–90%) characterized by elevated levels of type 2 cytokines, such as IL-5, IL-13, and eosinophil granule proteins, including eosinophilic cationic protein (ECP) or Charcot Leyden Crystal protein [18, 19]. Unlike the Western populations studied, the Chinese populations studied had significantly less frequent eosinophilia and may have a distinct pathophysiology [19,20,21]. In contrast, CRSsNP appears endotypically more heterogeneous, with 30–50% of patients exhibiting elevated type 2 inflammation, making it the most common endotype identified. These results were quite surprising, given the long-held tenet that CRSsNP was primarily driven by type 1 inflammation. Moreover, within both types of CRS, there were smaller subsets of patients who had elevated IL-17 (∼15%) or IFN-γ (5–23%), particularly within the CRSsNP phenotype (Fig. 1). Together, these findings suggest that CRSwNP is pathobiologically more homogeneous than CRSsNP and that there is a sizable subset of patients with CRSsNP who are endotypically similar to patients with CRSwNP. These types of observations further support the concept of endotypic classification of CRS based on the inflammatory patterns found within nasal tissue. In fact, a recent study suggests that endotypes influence the prevalence of comorbidities [22]. However, the number of patients in each endotypic group in this study was small, and whether treatment outcomes or patient symptoms were distinct among the endotypes used remains unclear. Altogether, these studies suggest that endotyping has the potential to predict treatment outcomes and help select more effective therapies in the future, but significant validation of these endotypes will be needed before they can be clinically useful.
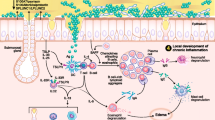
Adaptive immunity in CRS. CRSsNP (top) is characterized by a mixed inflammatory phenotype. This includes Th1, Th2, and Th17 cells. Cytokines produced by these cells, including IFN-g, IL-4, IL-13, and IL-17, may contribute to chronic inflammation through activation and/or skewing of innate effector cells such as dendritic cells and neutrophils, and these cytokines represent potential therapeutic targets. In addition, IgD-secreting plasma cells are elevated in the nasal mucosa of these patients, and this may be driven by IL-2. CRSwNP (bottom) is characterized by a type 2 inflammatory environment. This includes Th2 cells that produce IL-4, IL-5, and IL-13, all of which are potential therapeutic targets, and can contribute to the activation, recruitment, or skewing of innate effector cells such as dendritic cells, eosinophils, mast cells, and basophils. In addition, there is a large increase in the number of antibody-secreting cells in the nasal mucosa. These cells can produce autoreactive IgG and IgM that can bind to components of the basement membrane and activate compliment, as well as high levels of IgE, another potential therapeutic target. The cytokine BAFF is also elevated in CRSwNP tissues, may be important for local activation of B cells, and could also be a therapeutic target
Adaptive Immunity in CRS
T Cell Responses
Several groups have investigated the potential role for T cells in CRS (Fig. 1). In fact, T cells appear to be the predominant lymphocyte population in the nasal mucosa from CRS patients, particularly in CRSwNP nasal polyps [23,24,25]. Early studies that used immunohistochemistry found that CD3+ T cells were increased in both CRSsNP and CRSwNP tissues [26]. More recently, Derycke and colleagues used flow cytometry and intracellular cytokine staining to more fully characterize the T cells in sinus tissues of CRS patients [27]. They found that the frequency of CD3+ T cells was significantly increased in CRSwNP compared to CRSsNP or control nasal tissue, but they did not find an increased frequency of these cells in CRSsNP compared to control tissue [27]. Interestingly, they reported that Th1 cells, based on IFN-γ staining, were the most common T cell population in CRSwNP, CRSsNP, and control mucosa, but Th2 cells (IL-4 or IL-5 producing CD3+CD4+ cells) were only detected in CRSwNP tissues. They also detected other heterogeneous populations of T helper cells including Th1, Th22, Th17, and T follicular helper cells in both control and CRS tissues [27]. It is important to keep in mind that these T cell phenotypes were identified from surgically removed tissues, and it remains to be determined whether these T cell profiles can be identified based on the cytokines they produce, which could potentially be measured in nasal lavage fluids. Such studies will be needed before our knowledge of distinct T cell subsets in the nasal mucosa can be used to reliably group patients into a particular endotype for targeted treatment prior to surgery.
In addition to inflammatory T cell subsets, T regulatory cells (Tregs) have also been studied in CRS. Early studies focused on analyzing the gene expression of FoxP3, the master transcriptional regulator of Tregs. These studies found that expression of FoxP3 was decreased, both at the mRNA and protein level, in CRSwNP. They also found decreased expression of the regulatory cytokines IL-10 and TGF-β, suggesting a deficiency or a dysfunction of Tregs in CRSwNP tissues [20, 28]. These findings were further supported in a separate study that found decreased percentages of FoxP3+CD8+ Tregs in both eosinophilic and non-eosinophilic nasal polyps [29]. In contrast, another study reported that the overall frequencies of CD4+ Tregs (CD3+CD4+CD25+FoxP3+) were significantly higher in CRS tissues compared with controls, while CD8+ Tregs (CD3+CD8+CD25+FoxP3+) were significantly reduced in CRSwNP by means of flow cytometric analysis [30]. Some of the discrepancies in these findings may be due to the fact that in humans FoxP3 is expressed by both Tregs and activated effector T cells. As such, additional markers were needed to accurately identify whether Tregs were responsible for these observations in human tissues. In a study by Miljkovic and colleagues, more specific markers of human Tregs were used to identify these cells in CRS tissues by flow cytometry. In contrast to the prior studies, this study found that Tregs (CD45+CD4+CD25+CD127low) were significantly elevated in CRSwNP nasal tissue compared to CRSsNP [31]. This suggests that perhaps the Tregs found in CRSwNP tissues are not effective at controlling inflammation in this environment. Importantly, another recent study has suggested that glucocorticoid treatment, which is commonly used to treat CRSwNP, may directly increase the numbers of Tregs (CD4+CD25+CD127low) in nasal polyp tissue, and thus, concurrent medical treatment may have significantly affected the frequency of Tregs detected [32]. Taken together, even though numerous studies indicate that Tregs play an important role in inflammatory diseases, further studies are needed to delineate the function of Tregs in CRS pathogenesis, as well as the effect of medical treatment on their numbers and function, given the conflicting results in the literature [4, 33].
B Cell Responses
As with T cells, there is mounting evidence that B cells may play and important role in the pathogenesis of CRS, especially in CRSwNP (Fig. 1). B cell activating factor of the TNF family (BAFF) plays a critical role in B cell survival and differentiation to plasma cells, and it has been shown to be highly elevated in nasal polyp tissues [34, 35]. In line with this, early studies demonstrated elevated expression of the B cell marker CD19 and the plasma cell marker CD138 in CRSwNP tissues [26]. In addition, using flow cytometry, we have reported that plasma cells, which are terminally differentiated antibody-secreting cells, are also highly elevated in nasal polyps [36]. More recently, it has been reported that B cell activation events that occur within nasal polyps are distinct from the classical activation mechanisms used in germinal centers, and this difference may be important for the overall increased levels of activated B cell subsets found in nasal polyps [37]. Another recent report has demonstrated that B cells that express CD180, which is an unconventional member of the toll-like receptor family that plays an important role in B cell activation and proliferation, are also elevated in nasal polyps compared to control sinus tissue [38]. Altogether, these data suggest that activated B cells are highly elevated in CRSwNP, and they may play an important role in the pathophysiology of disease. However, more studies are needed to determine the mechanisms by which B cells contribute to disease pathogenesis in CRSwNP.
Given that activated B cell subsets are highly elevated in CRSwNP, it is not surprising that several groups have reported elevated antibody levels of every isotype in nasal polyps as well [37, 39, 40]. In addition, it has been demonstrated that B cells in nasal polyps are activated locally to undergo class switch recombination, similar to what has been seen in allergic rhinitis [37, 39]. This suggests that the local inflammatory environment may play a key role in the development of potentially pathogenic antibody responses. While the specificity of these antibodies remains largely unknown, there is evidence that some of the IgE in nasal polyps is specific to proteins produced by Staphylococcus aureus and that some of the local IgG and IgA in nasal polyps are autoreactive [40,41,42,43]. Both of these types of antibodies could play very important roles in the inflammatory response that occurs in nasal polyps. In addition, recent work has identified elevated levels of activated components of the classical, or antibody-mediated, complement pathway [44]. This provides further evidence to support the notion that antibody-mediated events may play a role in the inflammatory processes in CRSwNP.
While increased levels of IgE, IgA, IgM, and IgG antibodies are found in CRSwNP, increased levels of soluble IgD (sIgD), along with abundant IgD+ plasmablasts, are found in nasal tissue in a subpopulation of CRS patients, more commonly among patients with CRSsNP [45]. Mucosal sIgD was associated with high local IL-2 levels and the presence of pathogenic bacteria in the sinonasal microenvironment. These findings suggest that IgD might contribute to enhancing protective mucosal immunity or, by contrast, represent a pathologic inflammatory response possibly driven by superantigenic effects. These investigations significantly advance our understanding of IgD and IgD-producing B cells in the setting of sinonasal inflammatory disease.
Targeting Treatment to Inflammatory Patterns in CRS
As presented above, it is evident that adaptive immune responses are upregulated in the mucosa of CRS patients, including local elevations of B and T cell subsets, and the antibodies and/or cytokines produced by these cells [33, 46]. In recent years, the repertoire of approved treatments for specifically targeting aspects of the adaptive immune system have dramatically increased. However, our persistent lack of understanding of the key inflammatory mechanisms driving CRS has limited the availability of these novel treatments for use in CRS. Thus, few of these targeted medications have been specifically studied in CRS, and none are currently approved for use in CRS. When considering targeting specific aspects of the adaptive immune response, it is also important to consider that the primary impairment suffered by patients with CRS is on their quality of life. Thus, while epidemiologic studies do associate CRS with incident lower airway disease and cerebrovascular events, the efficacy of novel targeted treatments for CRS has to be weighed against potential risks and costs of treatment.
To date, all published randomized controlled trials (RCTs) of targeted therapies in CRS have examined the efficacy of monoclonal antibodies targeting aspects of type 2 inflammation in CRSwNP patients (Table 1). In the first omalizumab (humanized anti-IgE) study, 14 patients (12/14 CRSwNP) were randomized to receive placebo or subcutaneous omalizumab every 4 weeks for 6 months [48]. Patients in this study had non-significant decreases in radiographic disease severity from 76.1 to 60% pre- to post-treatment. Similarly, none of the secondary outcomes, including nasal endoscopy scores, SNOT-20 scores, olfactory testing, nasal peak inspiratory flow, or eosinophil levels in nasal lavage, were significantly different in the treatment group. In contrast, a separate study randomized 24 allergic and non-allergic patients with CRSwNP to receive 4–8 doses of subcutaneous omalizumab or placebo [47]. Patients in the treatment arm were noted to have significantly decreased endoscopic nasal polyp scores and Lund-Mackay CT scores, regardless of their atopic status. Patient reported measures including nasal and asthma QoL measures, and symptom questionnaires also demonstrated significant differences. The fact that this treatment was effective in both atopic and non-atopic patients suggests that omalizumab may have therapeutic benefits that are independent from decreasing IgE. The contrasting findings presented in these two studies may be due to differences in patient selection criteria, length of treatment, and/or timing of the measurements after the treatment.
Similarly, mixed results have been published for monoclonal antibodies targeting IL-5. A phase 1 study of 24 CRSwNP patients compared placebo to a single 1- or 3-mg/kg intravenous dose of reslizumab (humanized anti-IL-5) [49]. The study authors noted that the drug was well tolerated in patients but that there were no significant differences in nasal polyp size across treatment groups, although subgroup analysis demonstrated potentially increased efficacy among patients with high tissue levels of IL-5 [49]. A separate study examined 20 CRSwNP patients who were randomized to receive mepolizumab (anti-IL-5) or placebo [50]. This study found that 60% of the treated group, compared to 10% of the placebo group, had reductions in polyp size, postnasal drip, and improved olfaction. In addition, patients in the treated group had decreased blood eosinophil levels, ECP, and blood IL-5R expression at 8 weeks. Again, these studies suggest that there may be a benefit for treatment with anti-IL-5, at least in a subset of CRSwNP patients.
The most recent randomized trial examined the efficacy of dupilumab, a fully humanized antibody targeting the common alpha subunit of the IL-4 and IL-13 receptor. In this multicenter study, 60 CRSwNP patients were randomized to receive weekly dupilumab or placebo for 16 weeks [15]. Seventy percent of treated patients, compared to 20% in the placebo group, had polyp size reduction at 16 weeks. Significant improvements were also reported in Lund-Mackay CT scores, patient reported measures, and olfactory scores. Together these studies establish that type 2 inflammation, especially those mediated by IL-4 and IL-13, are likely to be pathogenic in patients with CRSwNP. Active ongoing and future clinical trials will help to better delineate the length of treatment and the patient subgroups most likely to benefit from these novel therapies.
As discussed above, the proliferation of B cells and antibody production may have pathogenic consequences in CRSwNP. There is mounting evidence demonstrating the development of autoreactive B cell responses in nasal polyps and an increased activation of the classical complement pathway in nasal polyps [42,43,44, 51]. These findings suggest that the growing armamentarium of anti-B cell therapies used in treating autoimmune diseases may also be of therapeutic value in CRS, especially in patients with nasal polyps (Table 2). The best-characterized anti-B cell agent in clinical use has been rituximab (anti-CD20), which is indicated for use in rheumatoid arthritis and granulomatous polyangiitis [52]. However, rituximab use is also associated with increased rates of sinopulmonary infections, low, but concerning, rates of serious infections, and rarely progressive multifocal leukoencephalopathy. Unfortunately, this risk profile is likely unsuitable for treating a quality of life illness like CRS, and the potential for increased sinopulmonary infections is unlikely to be acceptable to CRS patients who already suffer an increased frequency of airway infections [53, 54]. Another drug, belimumab, targets the cytokine BAFF and has recently been approved for use in systemic lupus erythematosus. As discussed above, BAFF has previously been reported to be elevated in CRSwNP tissue, and its expression levels are correlated with increased local CD20 and IgA expression [34]. Importantly, safety studies of belimumab to date demonstrate comparable rates of sinopulmonary infections as placebo-treated groups [55]. Together, as increased experience with anti-B cell therapies is gained, safe options with potential efficacy in CRS may be identified for future study in CRS.
Conclusions
CRS is a complex and very heterogeneous disease, and it is becoming clear that defining patient subsets based solely on differences in clinical presentations may not be sufficient to adequately treat all patients. A better understanding the different inflammatory patterns within the tissue and sinuses of these patients may help to pave the way for new definitions of patient groups or endotypes. This information could then be used to more specifically target inflammatory pathways that contribute to disease pathogenesis. There are currently several different drugs that have been approved for the treatment of diseases other than CRS but which target specific aspects of the adaptive immune response that may contribute to CRS pathogenesis. Moving forward, new studies are needed to determine whether any of these available treatments could be beneficial to specific groups of CRS patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12.
Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017;72:274–81.
Lidder AK, Detwiller KY, Price CP, Kern RC, Conley DB, Shintani-Smith S, et al. Evaluating metrics of responsiveness using patient-reported outcome measures in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:128–34.
Hulse KE. Immune mechanisms of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2016;16:1.
Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209.
Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin N Am. 2013;33:195–210.
Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27:387–95.
Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123:57–63.
Banerji A, Piccirillo JF, Thawley SE, Levitt RG, Schechtman KB, Kramper MA, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21:19–26.
Thompson CF, Price CP, Huang JH, Min JY, Suh LA, Shintani-Smith S, et al. A pilot study of symptom profiles from a polyp vs an eosinophilic-based classification of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:500–7.
Soler ZM, Hyer JM, Ramakrishnan V, Smith TL, Mace J, Rudmik L, et al. Identification of chronic rhinosinusitis phenotypes using cluster analysis. Int Forum Allergy Rhinol. 2015;5:399–407.
Soler ZM, Hyer JM, Rudmik L, Ramakrishnan V, Smith TL, Schlosser RJ. Cluster analysis and prediction of treatment outcomes for chronic rhinosinusitis. J Allergy Clin Immunol. 2016;137:1054–62.
Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90.
Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70:995–1003.
•• Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–79. First multicenter randomized trial using widely used patient reported outcome measures demonstrating efficacy of targeted therapy
• Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, Hellings P, Brusselle G, De Bacquer D, van Cauwenberge P, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 2013; 131:110–116 e111. First study showing that omalizumab can be effective in treating CRSwNP patients, regardless of their atopic status.
Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192:682–94.
•• Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017;139:699–703. In-depth analysis of inflammatory patterns of CRSsNP
•• Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–53. In-depth analysis of inflammatory patterns in tissues from CRS patients from different countries
Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8.
Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–84.
Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–56.
Morinaka S, Nakamura H. Inflammatory cells in nasal mucosa and nasal polyps. Auris Nasus Larynx. 2000;27:59–64.
Sanchez-Segura A, Brieva JA, Rodriguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol. 1998;102:953–60.
• Ryan MW, Davis LS. T cells in chronic rhinosinusitis with nasal polyposis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:200–5. Comprehensive review of T cells in CRSwNP
Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9.
• Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014;9:e97581. First comprehensive analysis of T cell cytokine profiles from cells isolated from sinus tissues of CRS patients and controls
Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41.
Ma J, Shi LL, Deng YK, Wang H, Cao PP, Long XB, et al. CD8(+) T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2016;46:1162–75.
Pant HHA, Schembri M, Miljkovic D, Krumbiegel D. CD4(+) and CD8(+) regulatory T cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy. 2014;28:e83–9.
Miljkovic D, Psaltis A, Wormald PJ, Vreugde S. T regulatory and Th17 cells in chronic rhinosinusitis with polyps. Int Forum Allergy Rhinol. 2016;6:826–34.
Edward JASM, Le W, Soudry E, Ramakrishnan VR, Bravo DT, Nguyen AL, et al. Selective expansion of human regulatory T cells in nasal polyps, and not adjacent tissue microenvironments, in individual patients exposed to steroids. Cin Immunol. 2017;179:66–76.
•• Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–46. Comprehensive review of mechanisms contributing CRSwNP pathogenesis
Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92.
Chan TD, Gardam S, Gatto D, Turner VM, Silke J, Brink R. In vivo control of B-cell survival and antigen-specific B-cell responses. Immunol Rev. 2010;237:90–103.
Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83.
Feldman S, Kasjanski R, Popowski J, Hernandez D, Chen J, Norton JE, et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin Exp Allergy. 2017;47:457–66.
Miljkovic D, Ou J, Kirana C, Hulse KE, Hauben E, Psaltis A, et al. Discordant frequencies of tissue-resident and circulating CD180-negative B cells in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; doi:10.1002/alr.21924.
Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63.
Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–7.
Chen JB, James LK, Davies AM, Wu YB, Rimmer J, Lund VJ, et al. Antibodies and superantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139:1195–204.
Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206.
Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123:2104–11.
• Van Roey GA, Vanison CC, Wu J, Huang JH, Suh LA, Carter RG, et al. Classical complement pathway activation in the nasal tissue of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2016; doi:10.1016/j.jaci.2016.11.015. Comprehensive review of mechanisms of complement activation in CRSwNP
Min JYKR, Hulse KE, Chandra R, Conley D, Suh L, Carter R, et al. Evidence for immunoglobulin D in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133:AB236.
Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131:933–57.
Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2012;131:110–6.
Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010;48:318–24.
Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41.
Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95.
De Schryver E, Calus L, Bonte H, Natalie de R, Gould H, Donovan E, et al. The quest for autoreactive antibodies in nasal polyps. J Allergy Clin Immunol. 2016;138:893–5.
Faurschou M, Jayne DR. Anti-B cell antibody therapies for inflammatory rheumatic diseases. Annu Rev Med. 2014;65:263–78.
Hirsch AG, Yan XS, Sundaresan AS, Tan BK, Schleimer RP, Kern RC, et al. Five-year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy. 2015;70:1613–21.
Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–60.
Merrill JT, Ginzler EM, Wallace DJ, McKay JD, Lisse JR, Aranow C, et al. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:3364–73.
Acknowledgement
The authors would like to thank Ms. Jacqueline Schaffer for her artwork contained in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
NIH Grants: U19 AI106683, R56 AI131239, and the Ernest S. Bazley Trust
Additional information
This article is part of the Topical Collection on Rhinosinusitis
Rights and permissions
About this article
Cite this article
Tan, B.K., Min, JY. & Hulse, K.E. Acquired Immunity in Chronic Rhinosinusitis. Curr Allergy Asthma Rep 17, 49 (2017). https://doi.org/10.1007/s11882-017-0715-0
Published:
DOI: https://doi.org/10.1007/s11882-017-0715-0