Abstract
Purpose of Review
Hymenoptera anaphylaxis is one of the leading causes of severe allergic reactions and can be fatal. Venom-specific immunotherapy (VIT) can prevent a life-threatening reaction; however, confirmation of an allergy to a Hymenoptera venom is a prerequisite before starting such a treatment. Component resolved diagnostics (CRD) have helped to better identify the responsible allergen.
Recent Findings
Many new insect venom allergens have been identified within the last few years. Commercially available recombinant allergens offer new diagnostic tools for detecting sensitivity to insect venoms. Additional added sensitivity to nearly 95% was introduced by spiking yellow jacket venom (YJV) extract with Ves v 5. The further value of CRD for sensitivity in YJV and honey bee venom (HBV) allergy is more controversially discussed. Recombinant allergens devoid of cross-reactive carbohydrate determinants often help to identify the culprit venom in patients with double sensitivity to YJV and HBV. CRD identified a group of patients with predominant Api m 10 sensitization, which may be less well protected by VIT, as some treatment extracts are lacking this allergen.
Summary
The diagnostic gap of previously undetected Hymenoptera allergy has been decreased via production of recombinant allergens. Knowledge of analogies in interspecies proteins and cross-reactive carbohydrate determinants is necessary to distinguish relevant from irrelevant sensitizations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the data of the anaphylaxis registry of German-speaking countries, allergy to Hymenoptera venom is the most common cause of anaphylaxis in adult patients and was reported in 50.1% of all reactions, of which were 70.4% wasps, 19.9% bees, 4.5% hornets, and 0.2% bumblebees [1]. Similarly in a European multicenter study, insect venom was in 48.2% identified as the eliciting cause of anaphylaxis with a similar distribution of the culprit insects [2]. In the population, sensitization to Hymenoptera venom has been detected in 15 to 26.5% of subjects by positive skin test or detection of IgE antibodies to insect venoms; however, only 0.3–7.5% have a history of a systemic reaction [3, 4, 5•, 6, 7]. In patients with systemic mast cell disorder, the proportion of insect venom allergy is much higher than in the normal population and ranges between 22 and 53% [8,9,10]. In addition, these patients have more severe symptoms and experience more often systemic adverse events during specific venom immunotherapy (VIT), and this therapy is slightly less effective in the prevention of further systemic reactions [11]. Furthermore, in patients with professions such as beekeepers, gardeners, farmers, truck drivers, and masons, venom allergy is considered as an occupational allergy and its occurrence exceeds that of the general population due to higher exposure to the respective insect [12, 13].
The causative insects belong to the order of Hymenoptera (in English: membrane wings) which is separated into the suborder Apocrita comprising bees, wasps, and ants. The different species are individually distributed on the globe and the occurrence of venom allergies differs regionally. Whereas in Western and Central Europe allergies to honeybees (Apis melifera) and yellow jackets (Vespula vulgaris) dominate, allergy to paper wasps (Polistinae) are additionally frequent in Southern Europe as well as in Northern America [14]. Allergic reactions to ants play a role in Australia (Myrmecia pilosula) [15] and the USA (Solenopsis invicta) [16].
In patients who have suffered from a systemic reaction after a Hymenoptera sting, the severity of the next reaction cannot be predicted from the previous reaction, as the amount of venom can differ. In addition, cofactors, such as intake of drugs (beta-blockers and ACE inhibitors), illness, stress, or alcohol, can increase the severity of allergic symptoms [17,18,19]. A specific VIT with the proper Hymenoptera venom is the only available causative treatment [20]. Subcutaneous applications of the respective venom induce a tolerance in allergic patients [21•]. In patients with elevated serum tryptase and/or systemic mastocytosis, VIT is recommended to be performed lifelong and is associated with a higher risk of side effects that can hamper reaching maintenance dose. In some cases, a concomitant pretreatment with antihistamines or anti-IgE antibody omalizumab is necessary [9].
The indication of a VIT requires diagnosis of a Hymenoptera venom allergy including a patient history of systemic reaction after an insect sting and detection of a sensitization to Hymenoptera venom by either skin testing or demonstration of specific IgE antibodies. This may be complicated by the fact that in many cases the causative insect was either not seen by the patients or they were not able to identify the insect because of the inability to differentiate between different similar-looking insects [22, 23]. Whereas in the case of detection of an unequivocal monosensitization to a Hymenoptera venom it is easy to choose a proper therapeutic venom extract, sensitization to multiple venoms after a convincing systemic reaction to a sting of an unknown insect poses problems for the selection of the venom(s) for VIT. Diagnostic sting challenges are not recommended due to lack of reproducibility and risk involved [24,25,26]. In earlier times, in those cases, VIT against more than one venom was performed, if the culprit insect was not identified [27]. More recently, addition of the basophil activation test to Hymenoptera venom [28, 29] and availability of component resolved diagnosis (CRD) have vastly augmented our ability to discriminate between clinically significant and irrelevant sensitizations in Hymenoptera venom anaphylaxis. In addition, these methods increased the sensitivity to diagnose Hymenoptera venom allergy. There is some evidence that compound resolved diagnostic may also be helpful in the prediction of VIT efficacy.
Characterization of Hymenoptera Venom Allergens
Hymenoptera venoms are a complex mixture of biogenic amines, peptides, toxins, enzymes, and low-weight proteins. Among different Hymenoptera venoms, honey bee venom (HBV) is currently the best characterized. Recently, 83 new compounds have been discovered via peptide ligand library approach combined with mass spectrometry analysis leading to a total of 102 compounds [30]. In addition, variable glycosylation may further increase the complexity. Actually, out of these compounds, 12 allergens have been identified in HBV. Phospholipase A2 (Api m 1) [31], hyaluronidase (Api m 2) [32], acid phosphatase (Api m 3) [33], dipeptidyl peptidase IV (Api m 5) [34], and/or icarapin (Api m 10) [35] are those allergens of high abundance in HBV and specific IgE against those compounds have been identified in 94.4% of allergic patients [36••]. Allergens of low abundance identified in the last years are melittin (Api m 4) [37], serine protease inhibitor (Api m 6) [38], CUB serine protease 1 (Api m 7) [39], carboxylesterase (Api m 8) [40], serine carboxypeptidase (Api m 9) [41], major royal jelly proteins 8/9 (isoallergens Api m 11.0101 and Api m 11.0201) [42], and vitellogenin (Api m 12) [43•].
Hyaluronidase of honey bees (Api m 2) and hyaluronidase of yellow jacket wasps (Ves v 2) [44] as well as dipeptidyl peptidase of honey bees (Api m 5) and yellow jackets (Ves v 3) [34] show cross-reactivity due to structural homologies. In addition, by analyzing the high molecular weight protein vitellogenin, a protein with 200 kDa, a 40% congruency between honey bee vitellogenin (Api m 12) and yellow jacket vitellogenin (Ves v 6) has been found, explaining cross-reactivity in some patients [43•]. Hence, Api m 1, Api m 3, and Api m 10, all having no analogue protein in yellow jacket venom (YJV), are more appropriate to identify genuine HBV sensitization in patients with double sensitization to HBV and YJV [45•].
Proteomic analysis of bumblebee (Bombus terrestris) venom revealed 57 compounds with 72% homologies to HBV [46]. Two allergens have been identified, phospholipase A2 (Bom t 1) and a protease (Bom t 4) [46]. A systemic reaction to a bumblebee sting can either result from extensive sequence identity between honey bee phosphatase A2 with Bom t1 leading to cross-reactivity after primary sensitization by a honey bee sting or due to a genuine specific sensitization to bumblebee venom after a bumblebee sting. In spite of their peaceful behavior, bumblebee stings are often observed as occupational allergies in green houses, where they are used for pollination [47].
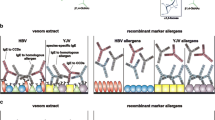
In yellow jacket (V. vulgaris) venom, phospholipase A 1 (Ves v 1) [48] and antigen 5 (Ves v 5) [49] have been identified as major allergens. Ninety-four percent of genuine YJV-sensitized patients were correctly identified by combined use of specific IgE to Ves v 1 and Ves v 5 [50]. Allergens of low abundance are hyaluronidase (Ves v 2), a glycoprotein, which exists in two isoforms (Ves v 2.01 and Ves v 2.02, the latter being the predominant) [51, 52], dipeptidylpeptidase IV (Ves v 3) and vitellogenin (Ves v 6). Those allergens show cross-reactivity to the correspondent proteins of Apis mellifera (Api m 2, Api m 5, and Api m 12, respectively) [34, 43•]. For an overview of HBV and YJV specific and cross-reactive allergens, see Fig. 1.
Venoms of the Vespidae family (comprising yellow jackets, paper wasps, and hornets) contain phospholipase A1, hyaluronidases, and antigens 5 and show a high interindividual degree of similarity. [53,54,55] A detailed overview about the presently known Hymenoptera venom allergens is shown in Table 1.
Recombinant Hymenoptera Allergens Available for Diagnosis
For many years, specific IgE to the whole HBV extract and YJV extract have been the only diagnostic tools to identify sensitization to Hymenoptera venom in patient serum. Later, the quality of these extracts was increased by purification and better isolation of natural allergens. A further step towards better recognition of Hymenoptera venom allergy came by the introduction of recombinant allergens into the diagnostic procedure. Venom allergenic proteins were cloned and expressed using bacteria, mainly Escherichia coli. However, this prokaryotic systemic failed to reproduce several proteins correctly [56]. Proper posttranslational folding and glycosylation are mandatory for immunologic identity in order to be recognized by allergic patients. Recently, a new system, Spodoptera frugiperda Sf9 insect cells, was used and produced properly folded proteins containing all specific IgE epitopes and lacking alpha-1,3-linked fucose molecules [57, 58]. Currently available Hymenoptera venom recombinant allergens for specific IgE, but not for skin tests, are phospholipase A1 (Ves v 1) and antigen 5 (Ves v 5) of YJV, antigen 5 (Pol d 5) of paper wasp venom and phospholipase A2 (Api m 1), hyaluronidase (Api m 2), acid phosphatase (Api m 3), dipeptidyl peptidase IV (Api m 5), and icarapin (Api m 10) of HBV.
Ves v 1 and Ves v 5 identify 94% of true YJV-sensitized patients [50]. After increasing the variety of recombinant allergens of HBV in the last few years, the IgE diagnostic is now able to identify 94.4% of HBV patients compared to 58 to 80% when only Api m 1 was available [31, 36••, 59,60,61,62].
Added Value for Detecting Double Sensitivity Due to Cross-Reactivity
Between 30 and 59% of patients who had a systemic reaction after Hymenoptera sting show double sensitization to HBV and YJV [63, 64]. One reason for double reactivity is the existence of specific IgE directed against allergens, which show a high congruency between honey bees and yellow jackets: hyaluronidases (Api m 2 and Ves v 2) [44], dipeptidyl peptidases (Api m 5 and Ves v 3) [34], and vitellogenins (Api m 12 and Ves v 6) [43•]. It is believed that in these cases, the sensitization against the other species is clinically non-relevant.
On the contrary, it has been speculated that sensitization to Api m 3 and Api m 10 identifies genuine HBV allergy in Api m 1-negative sera of patients with double sensitization [45•]. Whereas Api m 1 was detected in 66.3% of those patients, addition of Api m 3 and Api m 10 raised the sensitivity to 78.6%, which resulted in an increased number of “genuine” double-positive patients that would receive VIT to both venoms from 43.9 to 65.9%. These figures may raise doubt, if all of these double sensitizations after a clinical reaction to one sting really are clinically relevant and further studies are necessary. Nevertheless, for the actually recommended diagnostic approach in Hymenoptera venom double-positive patients, see Fig. 2.
Allergens in Hymenoptera venom are glycoproteins with potential additional glycosylation by alpha-1,3-linked fucose at N-acetylglucosamine of the carbohydrate core structure. These structures, called cross-reactive carbohydrate determinants, are highly immunogenic and can be another reason for double sensitization. In up to 72% of the serum of insect venom allergic patients, IgE to cross-reactive carbohydrate determinants were measured. Although clinically not relevant, they can hamper unambiguous diagnosis [65]. In addition, alcohol consumption is associated with high levels of IgE to glycosylated allergens [66]. For the detection of cross-reactive carbohydrate determinants, bromelain, horseradish peroxidase, ascorbic acid oxidase, and isolated N-glycan chains from bromelain, called MUXF, are used. Their occurrences do not automatically expulse a true double sensitization [67]. In paper wasp venom, a reactivity to cross-reactive carbohydrate determinants has not been seen [68].
Added Sensitivity
In patients with a reliable history of systemic reaction to a sting but lacking reactivity in skin test and specific IgE to venom extract, CRD has been reported to help to identify the culprit insect. Underrepresentation of certain allergens of low abundance in venom extracts has been reported, which may lead to the loss of detection of specific IgE to the whole venom extract in spite of Hymenoptera venom sensitization [69•, 70•]. This does not lead to problems concerning HBV Api m 1 and Api m 4, which represent together 62% of the dry weight. However, a sensitization of allergens of low abundance, such as Api m 3 or Api m 10, may not lead to reactivity against the whole venom extract and direct measurement of specific IgE against recombinant allergens may be necessary to define the culprit venom.
For YJV allergy, a study in a cohort of YJV allergic patients reported that detection rate of specific IgE against Ves v 5 and Ves v 1 is higher than specific IgE against venom extract [71••]. The reason for this is unclear, but possible explanations given included shortage of Ves v 5 protein in the venom extract, inefficient coupling of Ves v 5 to the assay’s solid phase, and sterical shielding of Ves v 5 epitopes by endogenous ligands of Ves v 5 or its attachment to the solid phase. This has led to the introduction of rVes v 5 spiked YJV which increased the detection rate from 83% up to 96%. Although in another group of patients only minor non-significant augmentation of sensitivity has been confirmed by spiking the YJV extract with Ves v 5, this assay is now considered to be the new standard assay for the detection of YJV allergy [72].
One study reported an added sensitivity by the use of recombinant allergens in a group of 19 patients with YJV allergy and 8 patients with HBV allergy, who despite a positive history and a positive skin test result to the culprit insect showed negative specific IgE to the respective insect venom. Specific IgE were detected in 15 of 19 (84%) of YJV allergic patients and 8 of 8 (100%) HBV-allergic patients by the use of recombinant allergens rApi m 1, rApi m 2, rApi m 3, rApi m 5, rVes v 1, rVes v 2, rVes v 3, and rVes v 5. However, for this study, in YJV allergic patients, probably the old and not the rVes v 5-spiked YJV assay has been used, which may explain differences in that patient group. In a different study, none of 27 patients with a convincing history of YJV anaphylaxis but with specific IgE level below 0.35 kU/L to the currently available rVes v 5-spiked YJV assay showed IgE reactivity above 0.35 kU/L to the recombinant YJV allergens rVes v 1, 2, or 5, and only one patient displayed IgE reactivity to the Api m 5 homolog Ves v 3. Also in 22 HBV-allergic patients with specific IgE to HBV below 0.35 kU/L, none of the patients displayed IgE reactivity above 0.35 kU/L to any of the HBV-specific allergens (Api m 1, 3, 4, and 10), with the exception of one cross-reactive patient with YJV sensitization and hornet sting anaphylaxis sharing specific IgE to the cross-reactive recombinant allergen Api m 5 [70•].
Patients with elevated serum tryptase or mastocytosis have lower total IgE levels compared to the general population and hence, in case of additional Hymenoptera allergy, may have a higher risk of negative skin tests and negative IgE to venom extracts [73, 74]. In 49 of patients with a history of YJV allergy, specific IgE against the YJV extract did not diagnose six patients (12%). Of those, additional use of specific IgE against Ves v 1 and Ves v 5 allowed the diagnosis in 2/6 of these patients and a combination with lowering of the threshold of specific IgE level to 0.1 kU/L was needed to avoid otherwise undetectable specific IgE to YJV in the remaining 4/6 of the patients [75••].
Thus, there still is controversy about a limited benefit of adding recombinant allergens to improve the detection of Hymenoptera venom allergic patients in addition to spiking YJV extract with Ves v 5. However, in the vast majority of patients, specific IgE to YJV and HBV extracts is sufficient to allow the diagnosis of Hymenoptera venom allergy.
Prediction of Therapy Success
Currently, there is no pattern of sensitization of allergens known which can predict safety or efficacy of a specific immunotherapy. Authors of a small study cohort with 31 patients treating patients either with native or purified aqueous HBV extracts according to IgE levels of Api m 4< or ≥0.98 kUA/L, respectively, reported a higher rate of side effects and less success after insect sting challenge in the group of patients with higher IgE to Api m 4, which may be followed up by further studies [76]. In a large cohort of 115 HBV-allergic patients after HBV immunotherapy, sensitization profiles were analyzed in those with versus without systemic reactions in controlled honey bee sting challenges [77••]. With an odds ratio of 8.4, predominant Api m 10 sensitization (>50% of specific IgE to HBV) was the best discriminator for treatment failure. One explanation for this difference was that some therapeutic HBV preparations displayed a lack of Api m 10 and significant Api m 10 sIgG4 induction was observed only in those patients who were treated with HBV in which Api m 10 was detectable. Thus, Api m 10 is underrepresented in some venom treatment formulations, which may lead to treatment failure in those patients with dominant Api m 10 sensitization [78].
Conclusions
With the introduction of CRD in Hymenoptera allergy, the diagnostic tools for detecting sensitivity have vastly increased over the last years. Spiking of the YJV extract with Ves v 5 has increased the sensitivity of sIgE determination to YJV so that nearly 95% of insect venom allergic patients can be diagnosed by the commercially available HBV and spiked YJY extracts. Some publications suggest that in patients with no detectable sensitization to any venom extract and negative skin tests further use of recombinant allergens could be helpful, although this is discussed controversially and still has to be confirmed by further studies. In patients with sensitization to two different venom extracts, the use of recombinant allergens, which are devoid of cross-reactive carbohydrate determinants, is often capable to identify the culprit venom and therefore prevents unnecessary double VIT. For a correct interpretation of the results, the knowledge of congruent analogue proteins in the different Hymenoptera species, as well as the impact of cross-reactive carbohydrate determinants, is important. One problem still to be solved is the specificity of specific IgE to recombinant insect venom allergens. Even the non-cross-reactive insect-specific allergens (Fig. 1) have not a 100% specificity for relevant insect venom allergy and using a combination of several allergens cumulatively lowers the specificity furthermore and has shown to increase the number of patients who would be considered to be “genuinely” double sensitive. It remains unclear if these patients would react even to a sting of the insect that was not the culprit of their previous reaction. If different sensitization profiles in CRD will have the potency to predict the course or efficacy of VIT or even lead to personalized treatment will have to be demonstrated. First evidence suggest that patients with predominant Api m 10 sensitization may be less well protected by VIT when the treatment extracts are lacking this allergen.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
Worm M, Eckermann O, Dolle S, Aberer W, Beyer K, Hawranek T, et al. Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany. Austria and Switzerland Dtsch Arztebl Int. 2014;111:367–75. doi:10.3238/arztebl.2014.0367.
Worm M, Moneret-Vautrin A, Scherer K, Lang R, Fernandez-Rivas M, Cardona V, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69:1397–404. doi:10.1111/all.12475.
Mosbech H, Tang L, Linneberg A. Insect sting reactions and specific IgE to venom and major allergens in a general population. Int Arch Allergy Immunol. 2016;170:194–200. doi:10.1159/000448399.
Golden DB, Marsh DG, Kagey-Sobotka A, Freidhoff L, Szklo M, Valentine MD, Lichtenstein LM. Epidemiology of insect venom sensitivity. JAMA. 1989;262:240–4.
• Sturm GJ, Kranzelbinder B, Schuster C, Sturm EM, Bokanovic D, Vollmann J, et al. Sensitization to Hymenoptera venoms is common, but systemic sting reactions are rare. J Allergy Clin Immunol. 2014;133:1635–43.e1. doi:10.1016/j.jaci.2013.10.046. This study shows that only a small amount of patients with hymenoptera venom sensitization shows systemic symptoms after a sting challenge.
Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JNG. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–49. doi:10.1111/j.1398-9995.2005.00963.x.
Golden DBK. Anaphylaxis to insect stings. Immunol Allergy Clin N Am. 2015;35:287–302. doi:10.1016/j.iac.2015.01.007.
Bonadonna P, Bonifacio M, Lombardo C, Zanotti R. Hymenoptera anaphylaxis and C-kit mutations: an unexpected association. Curr Allergy Asthma Rep. 2015;15:49. doi:10.1007/s11882-015-0550-0.
Schuch A, Brockow K. Mastocytosis and anaphylaxis. Immunol Allergy Clin N Am. 2017;37:153–64. doi:10.1016/j.iac.2016.08.017.
Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–32. doi:10.1111/j.1398-9995.2007.01569.x.
Bonadonna P, Bonifacio M, Lombardo C, Zanotti R. Hymenoptera allergy and mast cell activation syndromes. Curr Allergy Asthma Rep. 2016;16:5. doi:10.1007/s11882-015-0582-5.
Moscato G, Pala G, Crivellaro M, Siracusa A. Anaphylaxis as occupational risk. Curr Opin Allergy Clin Immunol. 2014;14:328–33. doi:10.1097/ACI.0000000000000066.
Siracusa A, Folletti I, van Gerth WR, Jeebhay MF, Moscato G, Quirce S, et al. Occupational anaphylaxis—an EAACI task force consensus statement. Allergy. 2015;70:141–52. doi:10.1111/all.12541.
Fernandez J. Distribution of vespid species in Europe. Curr Opin Allergy Clin Immunol. 2004;4:319–24.
Brown SGA, van Eeden P, Wiese MD, Mullins RJ, Solley GO, Puy R, et al. Causes of ant sting anaphylaxis in Australia: the Australian Ant Venom Allergy Study. Med J Aust. 2011;195:69–73.
Caplan EL, Ford JL, Young PF, Ownby DR. Fire ants represent an important risk for anaphylaxis among residents of an endemic region. J Allergy Clin Immunol. 2003;111:1274–7.
Munoz-Cano R, Picado C, Valero A, Bartra J. Mechanisms of anaphylaxis beyond IgE. J Investig Allergol Clin Immunol. 2016;26:73–82; quiz 2p following 83. doi:10.18176/jiaci.0046.
Nassiri M, Babina M, Dolle S, Edenharter G, Rueff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135:491–9. doi:10.1016/j.jaci.2014.09.004.
Wolbing F, Fischer J, Koberle M, Kaesler S, Biedermann T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy. 2013;68:1085–92. doi:10.1111/all.12193.
Kosnik M, Korosec P. Venom immunotherapy: clinical efficacy, safety and contraindications. Expert Rev Clin Immunol. 2015;11:877–84. doi:10.1586/1744666X.2015.1052409.
• Dhami S, Zaman H, Varga E-M, Sturm GJ, Muraro A, Akdis CA, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342–65. doi:10.1111/all.13077. This review indicates that venom immunotherapy reduces the risk of subsequent severe systemic sting reactions.
Baker TW, Forester JP, Johnson ML, Stolfi A, Stahl MC. The HIT study: hymenoptera identification test—how accurate are people at identifying stinging insects? Ann Allergy Asthma Immunol. 2014;113:267–70. doi:10.1016/j.anai.2014.05.029.
Baker TW, Forester JP, Johnson ML, Sikora JM, Stolfi A, Stahl MC. Stinging insect identification: are the allergy specialists any better than their patients? Ann Allergy Asthma Immunol. 2016;116:431–4. doi:10.1016/j.anai.2016.01.025.
Franken HH, Dubois AE, Kauffman HF, de Monchy JG. Hymenoptera sting challenge tests. Lancet. 1991;338:1344.
Rueff F, Przybilla B. Stichprovokation: Indikation und Durchfuhrung. Hautarzt. 2014;65:796–801. doi:10.1007/s00105-014-2779-2.
van der Linden PW, Hack CE, Struyvenberg A, van der Zwan JK. Insect-sting challenge in 324 subjects with a previous anaphylactic reaction: current criteria for insect-venom hypersensitivity do not predict the occurrence and the severity of anaphylaxis. J Allergy Clin Immunol. 1994;94:151–9.
Straumann F, Bucher C, Wuthrich B. Double sensitization to honeybee and wasp venom: immunotherapy with one or with both venoms? Value of FEIA inhibition for the identification of the cross-reacting ige antibodies in double-sensitized patients to honeybee and wasp venom. Int Arch Allergy Immunol. 2000;123:268–74.
Korosec P, Silar M, Erzen R, Celesnik N, Bajrovic N, Zidarn M, Kosnik M. Clinical routine utility of basophil activation testing for diagnosis of hymenoptera-allergic patients with emphasis on individuals with negative venom-specific IgE antibodies. Int Arch Allergy Immunol. 2013;161:363–8. doi:10.1159/000348500.
Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130:155–61. doi:10.1016/j.jaci.2012.02.008.
van Vaerenbergh M, Debyser G, Devreese B, de Graaf DC. Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J Proteome. 2014;99:169–78. doi:10.1016/j.jprot.2013.04.039.
Jakob T, Kohler J, Blank S, Magnusson U, Huss-Marp J, Spillner E, Lidholm J. Comparable IgE reactivity to natural and recombinant Api m 1 in cross-reactive carbohydrate determinant-negative patients with bee venom allergy. J Allergy Clin Immunol. 2012;130:276–278; author reply 278-9. doi:10.1016/j.jaci.2012.03.048.
Soldatova LN, Tsai C, Dobrovolskaia E, Markovic-Housley Z, Slater JE. Characterization of the N-glycans of recombinant bee venom hyaluronidase (Api m 2) expressed in insect cells. Allergy Asthma Proc. 2007;28:210–5.
Grunwald T, Bockisch B, Spillner E, Ring J, Bredehorst R, Ollert MW. Molecular cloning and expression in insect cells of honeybee venom allergen acid phosphatase (Api m 3). J Allergy Clin Immunol. 2006;117:848–54. doi:10.1016/j.jaci.2005.12.1331.
Blank S, Seismann H, Bockisch B, Braren I, Cifuentes L, McIntyre M, et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J Immunol. 2010;184:5403–13. doi:10.4049/jimmunol.0803709.
van Vaerenbergh M, de Smet L, Rafei-Shamsabadi D, Blank S, Spillner E, Ebo DG, et al. IgE recognition of chimeric isoforms of the honeybee (Apis mellifera) venom allergen Api m 10 evaluated by protein array technology. Mol Immunol. 2015;63:449–55. doi:10.1016/j.molimm.2014.09.018.
•• Kohler J, Blank S, Muller S, Bantleon F, Frick M, Huss-Marp J, et al. Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol. 2014;133:1383–9. doi:10.1016/j.jaci.2013.10.060. 1389.e1-6. This study shows that sensitivity of diagnosis of HBV-allergy has been improved with component resolved diagnosis up to 94% using new recombinant allergens.
King TP, Lu G, Agosto H. Antibody responses to bee melittin (Api m 4) and hornet antigen 5 (Dol m 5) in mice treated with the dominant T-cell epitope peptides. J Allergy Clin Immunol. 1998;101:397–403. doi:10.1016/S0091-6749(98)70254-4.
Kettner A, Hughes GJ, Frutiger S, Astori M, Roggero M, Spertini F, Corradin G. Api m 6: a new bee venom allergen. J Allergy Clin Immunol. 2001;107:914–20. doi:10.1067/mai.2001.113867.
Georgieva D, Greunke K, Betzel C. Three-dimensional model of the honeybee venom allergen Api m 7: structural and functional insights. Mol BioSyst. 2010;6:1056–60. doi:10.1039/b923127g.
Elieh Ali Komi D, Shafaghat F, Zwiener RD. Immunology of bee venom. Clin Rev Allergy Immunol. 2017; doi:10.1007/s12016-017-8597-4.
Ollert M, Blank S. Anaphylaxis to insect venom allergens: role of molecular diagnostics. Curr Allergy Asthma Rep. 2015;15:26. doi:10.1007/s11882-015-0527-z.
Blank S, Bantleon FI, McIntyre M, Ollert M, Spillner E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin Exp Allergy. 2012;42:976–85. doi:10.1111/j.1365-2222.2012.03966.x.
• Blank S, Seismann H, McIntyre M, Ollert M, Wolf S, Bantleon FI, Spillner E. Vitellogenins are new high molecular weight components and allergens (Api m 12 and Ves v 6) of Apis mellifera and Vespula vulgaris venom. PLoS One. 2013;8:e62009. doi:10.1371/journal.pone.0062009. This paper describes a new identified cross-reactive allergen.
Jin C, Focke M, Leonard R, Jarisch R, Altmann F, Hemmer W. Reassessing the role of hyaluronidase in yellow jacket venom allergy. J Allergy Clin Immunol. 2010;125:184–90.e1. doi:10.1016/j.jaci.2009.08.037.
• Frick M, Muller S, Bantleon F, Huss-Marp J, Lidholm J, Spillner E, Jakob T. rApi m 3 and rApi m 10 improve detection of honey bee sensitization in Hymenoptera venom-allergic patients with double sensitization to honey bee and yellow jacket venom. Allergy. 2015;70:1665–8. doi:10.1111/all.12725. This study points out the additional value of rApi m3 and rApi m 10 in diagnosing genuine HBV-allergic patients with double sensitizations to HBV and YJV.
van Vaerenbergh M, Debyser G, Smagghe G, Devreese B, de Graaf DC. Unraveling the venom proteome of the bumblebee (Bombus terrestris) by integrating a combinatorial peptide ligand library approach with FT-ICR MS. Toxicon. 2015;102:81–8. doi:10.1016/j.toxicon.2013.10.002.
Hoffman DR, El-Choufani SE, Smith MM, de Groot H. Occupational allergy to bumblebees: allergens of Bombus terrestris. J Allergy Clin Immunol. 2001;108:855–60. doi:10.1067/mai.2001.119029.
Seismann H, Blank S, Cifuentes L, Braren I, Bredehorst R, Grunwald T, et al. Recombinant phospholipase A1 (Ves v 1) from yellow jacket venom for improved diagnosis of hymenoptera venom hypersensitivity. Clin Mol Allergy. 2010;8:7. doi:10.1186/1476-7961-8-7.
Henriksen A, King TP, Mirza O, Monsalve RI, Meno K, Ipsen H, et al. Major venom allergen of yellow jackets, Ves v 5: structural characterization of a pathogenesis-related protein superfamily. Proteins. 2001;45:438–48.
Ebo DG, Faber M, Sabato V, Leysen J, Bridts CH, de Clerck LS. Component-resolved diagnosis of wasp (yellow jacket) venom allergy. Clin Exp Allergy. 2013;43:255–61. doi:10.1111/cea.12057.
Seppala U, Selby D, Monsalve R, King TP, Ebner C, Roepstorff P, Bohle B. Structural and immunological characterization of the N-glycans from the major yellow jacket allergen Ves v 2: the N-glycan structures are needed for the human antibody recognition. Mol Immunol. 2009;46:2014–21. doi:10.1016/j.molimm.2009.03.005.
Skov LK, Seppala U, Coen JJF, Crickmore N, King TP, Monsalve R, et al. Structure of recombinant Ves v 2 at 2.0 Angstrom resolution: structural analysis of an allergenic hyaluronidase from wasp venom. Acta Crystallogr D Biol Crystallogr. 2006;62:595–604. doi:10.1107/S0907444906010687.
Severino MG, Caruso B, Bonadonna P, Labardi D, Macchia D, Campi P, Passalacqua G. Cross reactivity between European hornet and yellow jacket venoms. Eur Ann Allergy Clin Immunol. 2010;42:141–5.
Monsalve RI, Vega A, Marques L, Miranda A, Fernandez J, Soriano V, et al. Component-resolved diagnosis of vespid venom-allergic individuals: phospholipases and antigen 5s are necessary to identify Vespula or Polistes sensitization. Allergy. 2012;67:528–36. doi:10.1111/j.1398-9995.2011.02781.x.
Schiener M, Eberlein B, Moreno-Aguilar C, Pietsch G, Serrano P, McIntyre M, et al. Application of recombinant antigen 5 allergens from seven allergy-relevant Hymenoptera species in diagnostics. Allergy. 2017;72:98–108. doi:10.1111/all.13000.
Perez-Riverol A, Justo-Jacomini DL. Zollner RdL, Brochetto-Braga MR. Facing Hymenoptera venom allergy: from natural to recombinant allergens. Toxins (Basel). 2015;7:2551–70. doi:10.3390/toxins7072551.
Spillner E, Blank S, Jakob T. Hymenoptera allergens: from venom to “venome”. Front Immunol. 2014;5:77. doi:10.3389/fimmu.2014.00077.
Seismann H, Blank S, Braren I, Greunke K, Cifuentes L, Grunwald T, et al. Dissecting cross-reactivity in hymenoptera venom allergy by circumvention of alpha-1,3-core fucosylation. Mol Immunol. 2010;47:799–808. doi:10.1016/j.molimm.2009.10.005.
Hofmann SC, Pfender N, Weckesser S, Huss-Marp J, Jakob T. Added value of IgE detection to rApi m 1 and rVes v 5 in patients with Hymenoptera venom allergy. J Allergy Clin Immunol. 2011;127:265–7. doi:10.1016/j.jaci.2010.06.042.
Sturm GJ, Hemmer W, Hawranek T, Lang R, Ollert M, Spillner E, et al. Detection of IgE to recombinant Api m 1 and rVes v 5 is valuable but not sufficient to distinguish bee from wasp venom allergy. J Allergy Clin Immunol. 2011;128:247–248; author reply 248. doi:10.1016/j.jaci.2011.02.021.
Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P, et al. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–1307.e3. doi:10.1016/j.jaci.2010.03.017.
Korosec P, Valenta R, Mittermann I, Celesnik N, Erzen R, Zidarn M, Kosnik M. Low sensitivity of commercially available rApi m 1 for diagnosis of honeybee venom allergy. J Allergy Clin Immunol. 2011;128:671–3. doi:10.1016/j.jaci.2011.03.012.
Muller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009;64:543–8. doi:10.1111/j.1398-9995.2008.01794.x.
Egner W, Ward C, Brown DL, Ewan PW. The frequency and clinical significance of specific IgE to both wasp (Vespula) and honey-bee (Apis) venoms in the same patient. Clin Exp Allergy. 1998;28:26–34.
Brehler R, Grundmann S, Stocker B. Cross-reacting carbohydrate determinants and hymenoptera venom allergy. Curr Opin Allergy Clin Immunol. 2013;13:360–4. doi:10.1097/ACI.0b013e328362c544.
Carballada FJ, Gonzalez-Quintela A, Nunez-Orjales R, Vizcaino L, Boquete M. Double (honeybee and wasp) immunoglobulin E reactivity in patients allergic to Hymenoptera venom: the role of cross-reactive carbohydrates and alcohol consumption. J Investig Allergol Clin Immunol. 2010;20:484–9.
Mertens M, Brehler R. Suitability of different glycoproteins and test systems for detecting cross-reactive carbohydrate determinant-specific IgE in hymenoptera venom-allergic patients. Int Arch Allergy Immunol. 2011;156:43–50. doi:10.1159/000322279.
Blank S, Neu C, Hasche D, Bantleon FI, Jakob T, Spillner E. Polistes species venom is devoid of carbohydrate-based cross-reactivity and allows interference-free diagnostics. J Allergy Clin Immunol. 2013;131:1239–42. doi:10.1016/j.jaci.2012.10.047.
• Rafei-Shamsabadi D, Muller S, Pfutzner W, Spillner E, Rueff F, Jakob T. Recombinant allergens rarely allow identification of Hymenoptera venom-allergic patients with negative specific IgE to whole venom preparations. J Allergy Clin Immunol. 2014;134:493–4. doi:10.1016/j.jaci.2014.05.035. This work demonstrates that using recombinant allergens in patients with negative specific IgE to HBV or YCV did not improve sensitivity in their study in contrast to [73].
• Cifuentes L, Vosseler S, Blank S, Seismann H, Pennino D, Darsow U, et al. Identification of Hymenoptera venom-allergic patients with negative specific IgE to venom extract by using recombinant allergens. J Allergy Clin Immunol. 2014;133:909–10. doi:10.1016/j.jaci.2013.09.047. This article shows positive data regarding added sensitivity of recombinant allergens in patients with negative specific IgE but positive skin test to venom extract, which is in contrast to [72].
•• Vos B, Kohler J, Muller S, Stretz E, Rueff F, Jakob T. Spiking venom with rVes v 5 improves sensitivity of IgE detection in patients with allergy to Vespula venom. J Allergy Clin Immunol. 2013;131:1225–7. doi:10.1016/j.jaci.2012.07.041. 1227.e1. This study shows the improved sensitivity of YJV extract spiked with rVes v 5 compared to YJV extract alone.
Bokanovic D, Schwarz I, Wutte N, Komericki P, Aberer W, Sturm GJ. Specificity of conventional and Ves v 5-spiked venom decreases with increasing total IgE. J Allergy Clin Immunol. 2014;134:739–41. doi:10.1016/j.jaci.2014.03.038.
Muller U, Helbling A, Hunziker T, Wuthrich B, Pecoud A, Gilardi S, et al. Mastocytosis and atopy: a study of 33 patients with urticaria pigmentosa. Allergy. 1990;45:597–603.
Potier A, Lavigne C, Chappard D, Verret JL, Chevailler A, Nicolie B, Drouet M. Cutaneous manifestations in Hymenoptera and Diptera anaphylaxis: relationship with basal serum tryptase. Clin Exp Allergy. 2009;39:717–25. doi:10.1111/j.1365-2222.2009.03210.x.
•• Michel J, Brockow K, Darsow U, Ring J, Schmidt-Weber CB, Grunwald T, et al. Added sensitivity of component-resolved diagnosis in hymenoptera venom-allergic patients with elevated serum tryptase and/or mastocytosis. Allergy. 2016;71:651–60. doi:10.1111/all.12850. This study demonstrates an improved sensitivity for the diagnosis of patients with mastocytosis/elevated serum tryptase by using recombinant hymenoptera venom allergens in combination with lowering the threshold forspecific IgE to hymenoptera venom extract to 0.1 kU A /L.
Ruiz B, Serrano P, Moreno C. IgE-Api m 4 is useful for identifying a particular phenotype of bee venom allergy. J Investig Allergol Clin Immunol. 2016;26:355–61. doi:10.18176/jiaci.0053.
•• Frick M, Fischer J, Helbling A, Rueff F, Wieczorek D, Ollert M, et al. Predominant Api m 10 sensitization as risk factor for treatment failure in honey bee venom immunotherapy. J Allergy Clin Immunol. 2016;138:1663–1671.e9. doi:10.1016/j.jaci.2016.04.024. This study implies that Api m 10 sensitization is a risk factor for treatment failure because of a underrepresentation in several therapeutic HBV preparation.
Blank S, Seismann H, Michel Y, McIntyre M, Cifuentes L, Braren I, et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66:1322–9. doi:10.1111/j.1398-9995.2011.02667.x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Brockow reports personal fees from Phadia Diagnostics. Dr. Tomsitz declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Anaphylaxis and Drug Allergy
Rights and permissions
About this article
Cite this article
Tomsitz, D., Brockow, K. Component Resolved Diagnosis in Hymenoptera Anaphylaxis. Curr Allergy Asthma Rep 17, 38 (2017). https://doi.org/10.1007/s11882-017-0707-0
Published:
DOI: https://doi.org/10.1007/s11882-017-0707-0