Abstract
Purpose of Review
Recent studies have attempted to identify interactions among the causes of otitis media with effusion (OME). This review discusses the interaction between allergy and infection with regard to host and environmental factors in terms of the development of OME.
Recent Findings
Protection of the upper airway against microbial invasion requires active interaction between the defense mechanisms of the respiratory epithelium, including innate and adaptive immunity, and mechanical factors. The impairment of these defenses due to allergy and/or increased bacterial resistance may lead to increased susceptibility to infectious organisms in the respiratory tract and middle ear mucosa. Recent genetic studies have provided valuable information about the association of Toll-like receptor signaling variations with clinical phenotypes and the risk of infection in the middle ear.
Summary
Among the causal factors of OME, allergy not only induces an inflammatory reaction in the middle ear cavity but also facilitates the invasion of infectious pathogens. There is also evidence that allergy can affect the susceptibility of patients to infection of the upper respiratory tract, including the middle ear cavity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Otitis media, one of the most common disorders of childhood, can cause conductive hearing loss, which potentially has adverse effects on speech and language development. Its estimated economic burden exceeds US$5 billion annually. Acute otitis media (AOM) is defined as an acute infectious (bacterial and/or viral) process, whereas otitis media with effusion (OME) is a chronic inflammatory condition that is characterized by the presence of middle ear effusion (MEE) and several mediators in the absence of symptoms and signs of acute inflammation [1, 2].
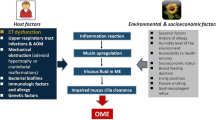
Because OME is a multifactorial disease arising from complex interactions among viral and/or bacterial pathogens, environmental risk factors (e.g., parental smoking), and host genetic factors (e.g., presence of allergy), it is particularly important to understand the mechanisms by which these factors regulate the inflammatory responses in the middle ear, either alone or in combination. However, the precise relationships among the causal contributors to OME remain unclear, although each factor has been proven to individually contribute to the development of this condition. Most epidemiological studies have shown that OME patients have an increased prevalence of atopic conditions, including eczema, asthma, and allergic rhinitis compared with non-OME controls [3].
Recently, many studies have attempted to reveal the interactions among the individual causal factors of OME. In particular, allergy not only induces an inflammatory reaction in the middle ear cavity but also facilitates the invasion of infectious pathogens. There is also evidence that allergy can affect the susceptibility of patients to infection at different sites in the upper respiratory tract. For example, a significantly increased risk of pneumococcal diseases has been reported in patients with asthma compared with those without asthma [4, 5]. Even without apparent infection, asthma has been found to be associated with increased colonization by Streptococcus pneumoniae and Staphylococcus aureus in the nasopharynx [6, 7]. A similar increase in colonization of the skin in patients with atopic dermatitis has also been reported [8, 9].
Epidemiologically, the prevalence of allergic rhinitis in patients with OME is higher than that in the general population [10], but it varies widely, ranging from 16.3 to 89 %, depending on the cohort and diagnostic criteria considered [11, 12•]. One potential reason for the wide range of prevalence is uncontrolled study designs, including the criteria used for diagnosing allergy and the different populations studied [13–15]. Bentdal et al. found that atopic eczema early in life was significantly associated with the risk of AOM at 10 years of age [16]. This study showed that a predisposition to atopic eczema might contribute to subsequent infection in the middle ear. It has also been reported that children with asthma or other atopic conditions had higher rates of ventilation tube insertion than non-asthmatics [17]. Because ventilation tube insertion is one of the most common procedures for the treatment of OME, this finding may suggest that the presence of an allergy can affect the natural course of chronic inflammation in the middle ear.
OME involves two different mechanisms leading to the accumulation of fluid in the middle ear cavity: (1) transduction from the mucosal capillaries and (2) active secretion (exudation) from the middle ear mucosa. Inflammatory cells and molecules in the fluid have been used to evaluate the pathogenesis of OME. Elevated levels of eosinophil and neutrophil mediators have been identified in middle ear fluid, which suggests that middle ear inflammation is the result of infection, allergy, or both [18, 19]. Interleukin (IL)-2 appears to be involved in T cell proliferation and induces other cytokines, including IL-4, IL-5, IL-13, and granulocyte macrophage colony-stimulating factor [12]. These cytokines participate in the regulation of molecular and cellular immunity involved in different types of chronic inflammation. Thus, the causal relationships between allergic conditions and infection or colonization have been studied.
The aim of this review is to summarize the roles of infection and allergy in the pathogenesis of OME and to discuss the effects of allergy on the risk of infection. This will focus on reports of studies that evaluated interactions among the risk factors of otitis media.
Role of Bacterial Infection in the Pathogenesis of OME
OME often occurs after AOM; however, it may also arise without preceding symptomatic infection. The pathophysiological mechanism of OME without preceding symptomatic infection is still not fully understood. Most studies using conventional culture have identified bacteria in less than half the effusions from OME patients, and commonly used antibiotics have had limited treatment effects on this condition. Specifically, bacteria have been cultured from 20 to 40 % of the effusions from OME patients, with Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis as the most frequently detected pathogens [20, 21]. However, recent studies have identified bacterial DNA in more than 80 % of effusions using polymerase chain reaction (PCR) [22–24]. Furthermore, in children recovering from acute pneumococcal otitis media, pneumolysin DNA was persistently detected in MEE by PCR months after the original infection [25]. These studies suggest that latent bacterial infection may be present even without any signs of infection in the middle ear. However, the presence of bacterial nucleic acids does not necessarily mean that the bacteria are viable; rather, it reflects the mere existence of bacterial RNA or DNA [26].
One possible explanation for the discrepancy between high PCR-positive and culture-negative rates in OME is the involvement of biofilms [27–29]. Biofilms are structured, specialized communities of adherent micro-organisms encased in a complex extracellular polymeric substance [30]. Bacteria in biofilms are more resistant to host defenses because the extracellular matrix of a biofilm serves to protect the bacteria against antibodies, phagocytosis, antibiotic penetration, and complement binding. There is also a decreased need for oxygen and nutrients when bacteria exist in a biofilm, further reducing their susceptibility to certain antimicrobials [31]. The enhanced persistence associated with biofilms also correlates well with the delayed emergence of opaque colony variants within the bacterial population and a decrease in systemic infection [32]. Another possible explanation for the persistent infection in OME is the intracellular persistence of bacteria in the middle ear mucosa [33]. For example, using transmission electron microscopy, Coates et al. found gram-positive cocci in the middle ear mucosal epithelial cells of 36 % of 11 children with OME [20].
Although single pathogen infection is typical, coinfections involving more than one type of bacterial pathogen, as well as bacterial and viral coinfections, also commonly occur [34]. Bacterial pathogens can also act synergistically to induce host responses in several ways. For example, Streptococcus pneumoniae synergistically enhances non-typeable Haemophilus influenzae (NTHi)-induced inflammation by upregulating TLR2 expression, one of the major host receptors for the NTHi pathogen [35], and Moraxella catarrhalis can increase the incidence of otitis media by Streptococcus pneumoniae [36]. Additionally, it has been shown that coinfection with NTHi facilitates pneumococcal biofilm formation and increases its persistence on middle ear mucosa.
Kania et al. used double staining to visualize both the bacterial matrix and bacterial cells, which demonstrated the presence of mucosal biofilm in adenoid tissue. Among adenoids from children with chronic and/or recurrent otitis media, 54 % showed evidence of mucosal biofilms [37].
Role of Immunity in the Pathogenesis of OME
Allergic rhinitis may contribute to the development of OME. Nasal challenge tests have shown that Eustachian tube (ET) dysfunction may occur immediately after exposure to or within a few hours of the late phase of this condition [38–41]. The pathogenesis of OME is clearly related to ET dysfunction [15, 42, 43]. ET facilitates communication between the middle ear cavity and the nasopharynx, nasal cavity, and nasal mucosa. The basic functions of ET, which plays a pivotal role in some middle ear diseases, are (1) protecting the middle ear from nasopharyngeal secretions and sound pressure; (2) draining the middle ear secretions into the nasopharynx; and (3) ventilating the middle ear cavity to regulate the air pressure there with the atmospheric pressure, replenishing oxygen that has been absorbed and allowing the gases produced in the middle ear cavity to escape [44]. If the control of the opening and closing of the ET is suboptimal, these basic functions may be affected. Several mechanisms that explain ET dysfunction due to an inflammatory process have been proposed: intrinsic mechanical obstruction from the inflammatory swelling of the ET mucosa, altered mucociliary activity, and hypersecretion by seromucous glands. As a result, any condition affecting the normal function of the ET can cause the retrograde invasion of the nasopharyngeal flora into the middle ear. The respiratory epithelium lining the mucosa of the ET, middle ear, and nasal cavity has several types of defense mechanism, including mechanical (e.g., mucociliary apparatus), innate (e.g., inflammatory mediators), and acquired (e.g., antigen-specific), against viral or bacterial invasion [45]. When viruses or bacteria adhere to airway epithelial cells, antimicrobial agents, such as interferons, lactoferrin, β-defensins, and nitric oxide, and chemical mediators, such as cytokines and chemokines, are induced as part of the innate immune response and influence the adaptive immune system [46, 47]. Although these defense mechanisms are intended to facilitate rapid microbial clearance, bacteria and viruses have evolved to evade these protective systems. The mucosal-associated lymphoid tissue of the upper airways is arranged in a circular pattern around the pharyngeal wall, known as Waldeyer’s ring. It consists of the adenoid, the palatine, and the lingual tonsils. Adenoids are organized in deep crypts lined with a specialized lympho-epithelium, which consists not only of epithelial cells but also of lymphocytes, macrophages, and dendritic cells. Follicular germinal centers and inter-follicular areas are present at a deeper level, and they are populated predominantly by T lymphocytes [48]. The adenoids, which play an important role in the generation of T helper (Th)1 and Th2 cells and IgA-committed B cells, develop postnatally. They are not apparent in early infancy but gradually become hypertrophic and hyperplasic, acquiring their greatest dimensions between 2 and 5 years of age [49]. Recurrent adenoidal infection is usually secondary to adenoid hypertrophy due to lymphoid tissue hyperplasia [50]. Adenoids may cause ET dysfunction due to their proximity and may even do so in the middle ear through the release of chemical mediators. Furthermore, adenoids and ET may act as potential target organs of allergic inflammation, inducing prolonged dysfunction of ET resulting in bacterial or viral regurgitation and development of otitis media. Because experiments with intranasal and intratympanic allergen challenges have induced allergic reaction in the cavity but not OME, the middle ear is not regarded as an “allergic shock organ” [51]. Zelazowska-Rutkowska et al. examined the proportions of CD4+, CD8+, and CD19+ cells with the Bcl-2 protein (anti-apoptotic protein) and CD95+ antigen (acting in apoptosis induction) in adenoid tissue in cases with or without OME [52]. Children with adenoid hypertrophy and OME were found to have lower rates of CD4+Bcl-2+, CD8+Bcl-2+, and CD19+Bcl-2+ lymphocytes but higher rates of CD4+, CD8+, and CD19+ cells with the CD95+ antigen than children with adenoid hypertrophy without OME. Lower proportions of CD4+ and CD8+ lymphocytes with Bcl-2 expression in hypertrophic adenoid in children with OME may indicate enhanced apoptosis of the naïve and effector T cells in adenoid tissue, which suggests that the adenoid plays an immunological role in local immunological disorders. In another study, the population of dendritic cells and the lymphocyte subpopulations of adenoid and peripheral blood in patients with adenoid hypertrophy and OME showed differences between children with adenoid hypertrophy with coexisting OME and those without OME in the adenoids but not in the blood [53]. Additionally, Bernstein et al. reported that lymphocytes from the adenoids showed decreased Th1 cytokine (IL-2 and interferon-γ) but normal Th2 cytokine (IL-4 and IL-5) secretions compared with peripheral blood lymphocytes among children with recurrent otitis media, suggesting that the underlying immune profile associated with atopy might play a role in determining the risk of recurrent otitis media [54]. These results suggest that the adenoids act as an “allergic shock organ” in the development of atopy-related otitis media.
A previous study analyzing the relationship between antibody levels and the presence of bacteria in MEE reported no correlation between immunoglobulin concentrations in MEE and the presence of bacteria [55]. Instead, the presence of effusion bacteria was found to be related to the serum immunoglobulin concentration. The higher serum antibody concentration in bacterium-positive OME patients may be related to a systemic immune reaction in response to a local infection in the middle ear or vice versa. In our study evaluating the correlation between the presence of bacteria in the middle ear and the presence of allergy according to the results of multiple allergosorbent tests, we could not find any difference between allergic and non-allergic children [56]. Thus, the relationship between systemic immune reaction and local infection in MEE should be evaluated in further studies.
The impairment of the defense mechanisms of the respiratory epithelium due to an allergy and/or an increase in bacterial resistance may lead to the colonization of the respiratory mucosa by infectious organisms. Protection of the upper airway against microbial invasion requires active interaction between the defense systems of innate and adaptive immunity and mechanical factors. The first step in the activation of the defense mechanism against an infectious organism is the recognition of the pathogen by innate immunity. Many functions of innate immunity in the respiratory mucosa are controlled by pattern recognition receptors (PRRs), which can recognize specific pathogen-associated molecular patterns of the microorganism. PRRs including Toll-like receptors (TLRs) have been considered to perform a crucial immunologic function in upper respiratory tract diseases. Recently, it has been revealed that TLR signaling is involved in both innate immunity in response to infection and the development of acquired immune responses. Hirano et al. reported that TLR4 may play an important role in enhancing mucosal and systemic immune responses [57]. They showed that immune responses, including the mucosal IgA, systemic IgG, and Th1 cell responses against the outer membrane protein from NTHi elicited in both TLR4-mutant and wild-type mice, were reduced in the former group compared with in the latter. This suggests that TLR4 plays an important role related to Th1 function for the optimal development of acquired immune responses. Additionally, Leichtle et al. demonstrated that TLR2−/− and TLR4−/− mice failed to induce tumor necrosis factor-α after NTHi challenge in the early phase of otitis media [58]. Moreover, TLR2−/− mice showed an increase in IL-10 expression and impaired bacterial clearance. In TLR4−/− mice, loss of TLR2 induction occurred in the early phase of otitis media. It was concluded that TLR2 activation induced by TLR4 signaling is critical for bacterial clearance and timely resolution of otitis media. There is some evidence that allergy is associated with developmental variations in TLR-mediated immune function. It has been suggested that TLR-mediated activation of both innate and acquired immunity plays an important role in reducing the risk of Th2-mediated allergic responses [59, 60]. Recent genetic studies have provided supporting evidence that functional variations of TLR signaling are associated with clinical phenotypes and disease. For example, studies of polymorphisms in the genes encoding TLR2, TLR4, and CD14 demonstrated a possible relationship between the immunoresponse genes and middle ear infections [61]. Various other genotypes are expected to interact with one another and numerous environmental and host factors, and the increasing availability of data on otitis media-related bacterial and viral gene expression and activity is improving our understanding of the involvement of these interactions in the pathogenesis of otitis media.
Conclusion
Children with allergic rhinitis or other allergic conditions have a significantly increased risk of OME. This association is probably due to impairment in both innate and acquired immunity and alterations in the integrity of respiratory mucosa. Protection of the upper airway against microbial invasion or colonization requires active interaction among the defense mechanisms. Therefore, additional studies are required to provide more in-depth information about interactions between the host and environmental factors to develop treatment strategies for otitis media.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur Cytokine Netw. 2002;13(2):161–72.
Kubba H, Pearson JP, Birchall JP. The aetiology of otitis media with effusion: a review. Clin Otolaryngol Allied Sci. 2000;25(3):181–94.
Alles R, Parikh A, Hawk L, Darby Y, Romero JN, Scadding G. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatric allergy and immunology. 2001;12(2):102–6.
Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352(20):2082–90. doi:10.1056/NEJMoa044113.
Juhn YJ, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, et al. Increased risk of serious pneumococcal disease in patients with asthma. The Journal of allergy and clinical immunology. 2008;122(4):719–23. doi:10.1016/j.jaci.2008.07.029.
Cernelc D, Gerbec M, Cernelc P. Comparative study of virological infections in asthmatic and nonasthmatic children. Acta Allergol. 1975;30(6):423–33.
Halablab MA, Hijazi SM, Fawzi MA, Araj GF. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol Infect. 2010;138(5):702–6. doi:10.1017/S0950268809991233.
Warner JA, McGirt LY, Beck LA. Biomarkers of Th2 polarity are predictive of staphylococcal colonization in subjects with atopic dermatitis. Br J Dermatol. 2009;160(1):183–5. doi:10.1111/j.1365-2133.2008.08905.x.
Leung AD, Schiltz AM, Hall CF, Liu AH. Severe atopic dermatitis is associated with a high burden of environmental Staphylococcus aureus. Clin Exp Allergy. 2008;38(5):789–93. doi:10.1111/j.1365-2222.2008.02964.x.
Newacheck PW, Stoddard JJ. Prevalence and impact of multiple childhood chronic illnesses. J Pediatr. 1994;124(1):40–8.
Luong A, Roland PS. The link between allergic rhinitis and chronic otitis media with effusion in atopic patients. Otolaryngologic clinics of North America. 2008;41(2):311–23. doi:10.1016/j.otc.2007.11.004.vi.
Hurst DS. The role of allergy in otitis media with effusion. Otolaryngologic clinics of North America. 2011;44(3):637–54. doi:10.1016/j.otc.2011.03.009.viii-ix. Up-to-date review on the relationship between allergy and eustachian tube dysfunction.
Chantzi FM, Kafetzis DA, Bairamis T, Avramidou C, Paleologou N, Grimani I, et al. IgE sensitization, respiratory allergy symptoms, and heritability independently increase the risk of otitis media with effusion. Allergy. 2006;61(3):332–6. doi:10.1111/j.1398-9995.2006.00971.x.
Miceli Sopo S, Zorzi G, Calvani Jr M. Should we screen every child with otitis media with effusion for allergic rhinitis? Arch Dis Child. 2004;89(3):287–8.
Tewfik TL, Mazer B. The links between allergy and otitis media with effusion. Curr Opin Otolaryngol Head Neck Surg. 2006;14(3):187–90. doi:10.1097/01.moo.0000193190.24849.f0.
Bentdal YE, Nafstad P, Karevold G, Kvaerner KJ. Acute otitis media in schoolchildren: allergic diseases and skin prick test positivity. Acta oto-laryngologica. 2007;127(5):480–5. doi:10.1080/00016480600895128.
Bjur KA, Lynch RL, Fenta YA, Yoo KH, Jacobson RM, Li X, et al. Assessment of the association between atopic conditions and tympanostomy tube placement in children. Allergy Asthma Proc. 2012;33(3):289–96. doi:10.2500/aap.2012.33.3529.
Hurst DS. Association of otitis media with effusion and allergy as demonstrated by intradermal skin testing and eosinophil cationic protein levels in both middle ear effusions and mucosal biopsies. Laryngoscope. 1996;106(9 Pt 1):1128–37.
Hurst DS, Venge P. Evidence of eosinophil, neutrophil, and mast-cell mediators in the effusion of OME patients with and without atopy. Allergy. 2000;55(5):435–41.
Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(8 Suppl):S7–11.
Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, Anderson KW, et al. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995;273(20):1598–604.
Matar GM, Sidani N, Fayad M, Hadi U. Two-step PCR-based assay for identification of bacterial etiology of otitis media with effusion in infected Lebanese children. J Clin Microbiol. 1998;36(5):1185–8.
Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279(4):296–9.
Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ. Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. International journal of pediatric otorhinolaryngology. 2001;60(1):49–54.
Palmu AA, Saukkoriipi PA, Lahdenkari MI, Kuisma LK, Makela PH, Kilpi TM, et al. Does the presence of pneumococcal DNA in middle-ear fluid indicate pneumococcal etiology in acute otitis media? J Infect Dis. 2004;189(5):775–84. doi:10.1086/381765.
Peizhong L, Whatmough K, Birchall JP, Wilson JA, Pearson JP. Does the bacterial DNA found in middle ear effusions come from viable bacteria? Clin Otolaryngol Allied Sci. 2000;25(6):570–6.
Fergie N, Bayston R, Pearson JP, Birchall JP. Is otitis media with effusion a biofilm infection? Clin Otolaryngol Allied Sci. 2004;29(1):38–46.
Hoa M, Syamal M, Schaeffer MA, Sachdeva L, Berk R, Coticchia J. Biofilms and chronic otitis media: an initial exploration into the role of biofilms in the pathogenesis of chronic otitis media. American journal of otolaryngology. 2010;31(4):241–5. doi:10.1016/j.amjoto.2009.02.015.
Tawfik SA, Ibrahim AA, Talaat IM, El-Alkamy SS, Youssef A. Role of bacterial biofilm in development of middle ear effusion. Eur Arch Otorhinolaryngol. 2016. doi:10.1007/s00405-016-4094-2.
Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13(1):7–10. doi:10.1016/j.tim.2004.11.004.
Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31.
Weimer KE, Armbruster CE, Juneau RA, Hong W, Pang B, Swords WE. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis. 2010;202(7):1068–75. doi:10.1086/656046.
Daniel M, Imtiaz-Umer S, Fergie N, Birchall JP, Bayston R. Bacterial involvement in otitis media with effusion. International journal of pediatric otorhinolaryngology. 2012;76(10):1416–22. doi:10.1016/j.ijporl.2012.06.013. Study demonstrating live bacteria in more than 90% of middle ear effusions in children.
Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010;87(2):213–22. doi:10.1189/jlb.0709518.
Lim JH, Ha U, Sakai A, Woo CH, Kweon SM, Xu H, et al. Streptococcus pneumoniae synergizes with nontypeable Haemophilus influenzae to induce inflammation via upregulating TLR2. BMC Immunol. 2008;9:40. doi:10.1186/1471-2172-9-40.
Krishnamurthy A, McGrath J, Cripps AW, Kyd JM. The incidence of Streptococcus pneumoniae otitis media is affected by the polymicrobial environment particularly Moraxella catarrhalis in a mouse nasal colonisation model. Microbes Infect. 2009;11(5):545–53. doi:10.1016/j.micinf.2009.03.001.
Kania RE, Lamers GE, Vonk MJ, Dorpmans E, Struik J, Tran Ba Huy P, et al. Characterization of mucosal biofilms on human adenoid tissues. Laryngoscope. 2008;118(1):128–34. doi:10.1097/MLG.0b013e318155a464.
Ebert Jr CS, Pollock HW, Dubin MG, Scharer SS, Prazma J, McQueen CT, et al. Effect of intranasal histamine challenge on Eustachian tube function. International journal of pediatric otorhinolaryngology. 2002;63(3):189–98.
Skoner DP, Doyle WJ, Boehm S, Fireman P. Effect of terfenadine on nasal, eustachian tube, and pulmonary function after provocative intranasal histamine challenge. Ann Allergy. 1991;67(6):619–24.
Doyle WJ, Ingraham AS, Fireman P. The effects of intranasal histamine challenge on eustachian tube function. The Journal of allergy and clinical immunology. 1985;76(4):551–6.
Hardy SM, Heavner SB, White DR, McQueen CT, Prazma J, Pillsbury HC. Late-phase allergy and eustachian tube dysfunction. Otolaryngology--head and neck surgery. 2001;125(4):339–45. doi:10.1067/mhn.2001.119140.
Fireman P. Otitis media and eustachian tube dysfunction: connection to allergic rhinitis. The Journal of allergy and clinical immunology. 1997;99(2):S787–97.
Pelikan Z. The role of nasal allergy in chronic secretory otitis media. Ann Allergy Asthma Immunol. 2007;99(5):401–7. doi:10.1016/S1081-1206(10)60563-7.
Bernstein JM. Role of allergy in eustachian tube blockage and otitis media with effusion: a review. Otolaryngology--head and neck surgery. 1996;114(4):562–8.
Murphy TF, Chonmaitree T, Barenkamp S, Kyd J, Nokso-Koivisto J, Patel JA, et al. Panel 5: microbiology and immunology panel. Otolaryngology--head and neck surgery. 2013;148(4 Suppl):E64–89. doi:10.1177/0194599812459636. Up-to-date review of the progress on the virology, bacteriology, and immunology related to otitis media.
Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24(1):210–29. doi:10.1128/CMR.00014-10.
Ogra PL. Ageing and its possible impact on mucosal immune responses. Ageing Res Rev. 2010;9(2):101–6. doi:10.1016/j.arr.2009.07.007.
Van Kempen MJ, Rijkers GT, Van Cauwenberge PB. The immune response in adenoids and tonsils. Int Arch Allergy Immunol. 2000;122(1):8–19. 24354.
Casselbrant ML. What is wrong in chronic adenoiditis/tonsillitis anatomical considerations. International journal of pediatric otorhinolaryngology. 1999;49 Suppl 1:S133–5.
Marseglia GL, Poddighe D, Caimmi D, Marseglia A, Caimmi S, Ciprandi G, et al. Role of adenoids and adenoiditis in children with allergy and otitis media. Current allergy and asthma reports. 2009;9(6):460–4.
Skoner AR, Skoner KR, Skoner DP. Allergic rhinitis, histamine, and otitis media. Allergy Asthma Proc. 2009;30(5):470–81. doi:10.2500/aap.2009.30.3272.
Zelazowska-Rutkowska B, Wysocka J, Skotnicka B. Chosen factors of T and B cell apoptosis in hypertrophic adenoid in children with otitis media with effusion. International journal of pediatric otorhinolaryngology. 2010;74(6):698–700. doi:10.1016/j.ijporl.2010.02.024. Study suggesting the immunological role of the adenoid tissue with regulating the lymphocytes.
Kotowski M, Niedzielski A, Niedzielska G, Lachowska-Kotowska P. Dendritic cells and lymphocyte subpopulations of the adenoid in the pathogenesis of otitis media with effusion. International journal of pediatric otorhinolaryngology. 2011;75(2):265–9. doi:10.1016/j.ijporl.2010.11.014. Study explaining the influence of immunological status of adenoid on the development of OME.
Bernstein JM, Ballow M, Xiang S, O’Neil K. Th1/Th2 cytokine profiles in the nasopharyngeal lymphoid tissues of children with recurrent otitis media. The Annals of otology, rhinology, and laryngology. 1998;107(1):22–7.
Yeo SG, Park DC, Lee SK, Cha CI. Relationship between effusion bacteria and concentrations of immunoglobulin in serum and effusion fluid in otitis media with effusion patients. International journal of pediatric otorhinolaryngology. 2008;72(3):337–42. doi:10.1016/j.ijporl.2007.11.005.
Kim WJ, Kim BG, Chang KH, Oh JH. Detection of bacteria in middle ear effusions based on the presence of allergy: does allergy augment bacterial infection in the middle ear? J Otolaryngol Head Neck Surg. 2015;44:58. doi:10.1186/s40463-015-0111-5.
Hirano T, Kodama S, Moriyama M, Kawano T, Suzuki M. The role of Toll-like receptor 4 in eliciting acquired immune responses against nontypeable Haemophilus influenzae following intranasal immunization with outer membrane protein. International journal of pediatric otorhinolaryngology. 2009;73(12):1657–65. doi:10.1016/j.ijporl.2009.08.015.
Leichtle A, Hernandez M, Pak K, Yamasaki K, Cheng CF, Webster NJ, et al. TLR4-mediated induction of TLR2 signaling is critical in the pathogenesis and resolution of otitis media. Innate Immun. 2009;15(4):205–15. doi:10.1177/1753425909103170.
Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1(1):69–75. doi:10.1038/35095579.
Holt PG, Macaubas C, Prescott SL, Sly PD. Microbial stimulation as an aetiologic factor in atopic disease. Allergy. 1999;54 Suppl 49:12–6.
Emonts M, Veenhoven RH, Wiertsema SP, Houwing-Duistermaat JJ, Walraven V, de Groot R, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120(4):814–23. doi:10.1542/peds.2007-0524.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Oh and Kim declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Otitis
Rights and permissions
About this article
Cite this article
Oh, JH., Kim, W.J. Interaction Between Allergy and Middle Ear Infection. Curr Allergy Asthma Rep 16, 66 (2016). https://doi.org/10.1007/s11882-016-0646-1
Published:
DOI: https://doi.org/10.1007/s11882-016-0646-1