Abstract
Food allergies have increased in prevalence over the past 20 years, now becoming an important public health concern. Although there are no therapies currently available for routine clinical care, recent reports have indicated that immunotherapies targeting the mucosal immune system may be effective. Oral immunotherapy is conducted by administering small, increasing amounts of food allergen; it has shown promise for desensitizing individuals with peanut, egg, or milk allergies. Sublingual immunotherapy also desensitizes allergic patients to foods—two major studies have examined the effects of sublingual immunotherapy in subjects with peanut allergies. We review the complex nature of IgE-mediated food allergies and the therapies being evaluated in clinical trials. We focus on the diagnosis and management of food allergies and investigational therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food allergies affect an estimated 15 million Americans and 17 million Europeans and carry a high risk of life-threatening allergic reactions. According to the Centers for Disease Control and Prevention, food allergies among children increased approximately 50 % between 1997 and 2011, but there is no clear answer as to why and no effective therapy. Thus, strict avoidance of food allergens and early recognition and management of allergic reactions to food are important measures to prevent serious health consequences. Even with appropriate dietary avoidance, however, half of peanut allergic children reported accidental ingestion in the prior year. Therefore, the need for food allergy treatments is of critical importance. In this review, we seek to highlight key areas of innovation and emerging approaches for the treatment of food allergies as well as discuss the clinical implications.
Investigational Therapies
Proactive therapies for food allergies are needed because avoidance is not a long-term solution for the millions of individuals at risk for accidental reactions. Subcutaneous immunotherapy (SCIT) is effective and safe for the treatment of allergies to environmental factors and insect stings, so this therapy was tested for peanut allergy in the 1980s. While trials did show some efficacy of SCIT for peanut allergy, there was an unacceptably high rate of severe allergic reactions [1]. SCIT has not been tested for food allergies since these trials. Instead, researchers have turned to other routes of administration for immunotherapy. It is important to note that the therapies discussed here are investigational and not ready for routine clinical practice except where indicated [2].
A key concept in immunotherapy for food allergies is desensitization vs sustained unresponsiveness (also referred to as tolerance). Desensitization means increasing the allergen reactivity threshold in subjects receiving daily immunotherapy. Sustained unresponsiveness means retention of an increased reactivity threshold after immunotherapy has been discontinued for weeks or months [3•].
Oral Immunotherapy
Oral immunotherapy (OIT) typically is conducted in three phases with allergens in a flour form and ingested with a food vehicle. Phase 1 is a modified rush desensitization, starting with minute quantities of allergen, which increases in dose several times during a single day. Phase 2 is a buildup dosing period in which subjects ingest daily doses of the allergens at home. Doses increase approximately every 2 weeks under clinical observation. Phase 3 is the maintenance dosing period in which subjects ingest the target dose of allergen daily, at home, for months or years. OIT was reported to induce desensitization in some subjects in a preliminary case series of various food allergies, but rigorous studies were needed to show safety, efficacy, and mechanism [4]. OIT trial outcomes now have been reported for peanut, egg, and milk allergies—we will focus on peanut allergy.
Peanut OIT
In 2009, the first findings from an open-label study of peanut OIT performed in children in the USA were reported [5]. Subjects began taking 0.1 mg of peanut protein; the dose increased for several months to a maintenance dose of 300 mg peanut protein daily. Twenty-seven of 29 subjects subsequently were able to accept a peanut challenge of 3900 mg of peanut protein (approximately 13 whole peanut kernels). Side effects occurred most often during the modified rush and buildup phases [6]. Skin, gastrointestinal, and upper respiratory symptoms were most common. Other open-label studies since have reported findings from the UK and Germany [7, 8]. Results from these trials and subsequent others confirm that reactivity thresholds can be increased via OIT (summarized in Table 1).
A multicenter, double-blind, randomized, placebo-controlled trial of peanut OIT provided strong evidence that this approach can desensitize children with peanut allergies [9]. In subjects receiving peanut OIT, the peanut protein was increased to a maintenance level of 4000 mg. After 12 months of dosing, subjects underwent a double-blind, placebo-controlled food challenge (DBPCFC) to 5000 mg of peanut protein. All 16 of the subjects continuing on peanut OIT passed the challenge, compared with none of the subjects given placebo (they could ingest only a median of 280 mg peanut protein).
The largest trial to date was the STOP II trial, a cross-over study in the UK of 99 children with a peanut allergy (age, 7–16 years) [12]. In phase 1, the subjects received OIT with peanut protein or continued to avoid peanuts (standard of care, controls). Then, in phase 2, subjects from the control group in phase 1 were reallocated and received peanut OIT. Subjects underwent 6 months of peanut OIT and then underwent a peanut challenge. After this period, 84 % of subjects who received OIT in phase 1 were able to ingest 800 mg of peanut (the daily maintenance dose), as were 91 % of those who received OIT in phase 2. In the food challenge outcome at the end of phase 1, 62 % of subjects who received OIT could tolerate 1400 mg of peanut without symptoms, whereas none of the controls could tolerate 1400 mg of peanut. These findings indicate that peanut OIT can desensitize most patients within 6 months, but there was no assessment of sustained unresponsiveness. Importantly, side effects were deemed mild for most subjects.
Although in most studies of peanut OIT desensitization is achieved, it was only recently reported that OIT also can lead to sustained unresponsiveness. Vickery et al. reported findings from an open-label study of 24 children who received daily doses of peanut for up to 5 years, then stopped the OIT for 1 month and were challenged [3•]. After abstaining from peanut OIT for 1 month, 50 % of subjects were still able to pass a DBPCFC and were considered tolerant. It is important to note that the patients who did not pass the tolerance challenge still tolerated a median challenge dose of 3750 mg—which is much greater than would be expected if the OIT effect had subsided completely. This study shows that peanut OIT can lead to sustained unresponsiveness, indicating that long-term use of daily maintenance doses may not be necessary for all subjects. In the future, it will be important to include a placebo arm in order to determine the rate of subjects who spontaneously develop tolerance during the trial period.
Combination Therapies
Oral immunotherapy poses an inherent risk associated with food allergic patients intentionally ingesting proteins that cause reactions. Therefore, it has been proposed that anti-IgE therapies, such as omalizumab, could be given to bind free circulating IgE before OIT, to increase safety and dose. The highest dose of the humanized monoclonal antibody TNX-901, given without concurrent OIT, was shown to reduce reactions to peanut protein in 75 % of subjects with a peanut allergy [16]. Findings from a study of omalizumab plus peanut OIT showed that all 13 subjects were able to achieve a 500 mg dose of peanut on the first day of desensitization [17•]. Twelve of 13 subjects reached a 4000 mg maintenance dose, in a median time of only 8 weeks. Administration of omalizumab before OIT therefore appears to allow subjects to ingest large quantities of allergen faster than peanut OIT protocols without omalizumab. Further studies, especially randomized, placebo-controlled trials, are needed to better assess this strategy.
Sublingual Immunotherapy
Sublingual immunotherapy (SLIT) is performed by placing allergen extract, in a liquid solution, under the tongue for as long as several minutes; then, it is spit out or swallowed. Langerhans cells in the oral mucosa take up the allergens and are thought to induce tolerance. SLIT has been used to reduce allergies to environmental allergens in Europe for several decades and recently was approved in the USA for treatment of grass pollen allergy. SLIT is thought to be safer than OIT because smaller quantities of allergen are administered.
Kim and colleagues performed a randomized, double-blind, placebo-controlled trial of SLIT for pediatric patients with a peanut allergy [10]. The dose increased from 0.25 mg peanut protein to 2000 mg in approximately 6 months. Subjects then continued to receive 2000 mg of peanut protein per day for the next 6 months. Twelve months after the trial started, all subjects participated in a DBPCFC. The SLIT group (n = 11) tolerated a median of 1710 mg of peanut protein in the DBPCFC, whereas the placebo subjects (n = 7) tolerated only 85 mg.
The Consortium of Food Allergy Research conducted a multicenter, double-blind, randomized, placebo-controlled trial of SLIT in 40 subjects with a peanut allergy [11]. After 44 weeks, 70 % of subjects given SLIT could tolerate increased levels of peanut protein, compared with only 15 % of subjects given placebo. The median tolerated dose was 496 mg in subjects who received SLIT, compared with 3.5 mg at enrollment. After 68 weeks of SLIT, the median tolerated dose among SLIT recipients increased to 996 mg, which was much lower than the final doses tolerated after OIT (typically several grams of allergen).
Sublingual and oral immunotherapy approaches both have important advantages and limitations. SLIT may ultimately represent a bridge to OIT to decrease side effects or as a safer method of immunotherapy for patients with a history of severe allergy who cannot tolerate OIT. Overall, it is difficult to envision SLIT as the most effective or reliable method to induce clinically meaningful desensitization or sustained unresponsiveness due to the lower dosing. However, if in the future, the allergen dose used in SLIT could be increased, then the role for a sublingual approach may need to be re-evaluated. Peanut oral immunotherapy, alternatively, has been shown in multiple studies to increase reaction thresholds and accumulating evidence suggests OIT protocols lead to desensitization and does so in a more efficacious manner than SLIT [15]. Although one might believe that desensitization would translate into protection from reactions due to accidental ingestion, there are no established data to prove this important parameter. Moreover, peanut OIT has not been thoroughly studied in subjects who have a history of severe anaphylaxis (typical exclusion criterion), yet these patients might have the most to benefit clinically.
Epicutaneous Immunotherapy
Epicutaneous immunotherapy (EPIT) is a new approach in which a circular disk that contains dried allergens is applied to intact skin. The allergen is solubilized by moisture from the skin and taken up by dendritic cells. One proprietary epicutaneous delivery system (EDS) has been successfully developed in animal models and is being tested in humans [18]. One advantage of EPIT over other forms of immunotherapy is that administration of small doses of allergen to the skin could decrease the likelihood of systemic reactions, which can occur after allergen ingestion. EPIT is effective in animal models of food allergy and is being investigated in clinical trials for food allergies. Findings from only one clinical trial of EPIT for food allergies have been published, and there are few peer-reviewed results published in the literature to date [19•]. Adverse events were mostly mild skin symptoms. Studies are underway in North America and Europe to investigate EPIT for peanut allergy, and results were reported in an abstract detailing that after 1 year of therapy, half of the patients treated with the 250 ug patch tolerated at least 1 g of peanut protein on food challenge [13]. Notably, the results that have been released from pilot studies suggest that EPIT is well-tolerated with very few systemic reactions and no reports of anaphylaxis. In fact, the most common reactions reported have been mild, cutaneous symptoms, and flares of atopic dermatitis [19•].
If the ongoing studies with EPIT are successful, a cutaneous approach may be a viable and safe treatment option for food allergy—particularly as part of a more comprehensive approach to treatment. A role for EPIT might exist within the early phase of antigen exposure to perform low-dose desensitization to young peanut allergic subjects who might not otherwise be able to tolerate (or cooperate for) daily dosing.
Adjuvant Vaccines
As mentioned above, subcutaneous immunotherapy (SCIT) for peanut allergy has been attempted in the past in 12 subjects [1]. While some efficacy was established, the high rate of systemic reactions was unacceptable. However, SCIT represents an effective approach for venom and environmental allergens, and further exploration of a treatment vaccine or even SCIT approach to peanut allergy would likely need to be based upon modification of the allergen to eliminate systemic reactions. Alternatively, an approach that does not target the IgE response could offer a safer method yet allow for subcutaneous treatment. A combination approach with non-specific immunotherapy (omalizumab, food allergy herbal formula, probiotics) may be feasible to reduce reactions to SCIT and permit an allergen targeted approach.
Early Introduction of Peanut
In 2015, DuToit et al. reported the results of single-center, open-label, randomized, controlled interventional study to test the effect of timing of peanut introduction on the rate of peanut allergy development [14••]. The authors found that 17.2 % of subjects in the avoidance group developed peanut allergy compared to 3.2 % of the early introduction group. Upon careful examination of the data, there were interesting within group differences related to the skin prick test (SPT) positive vs negative subjects. The SPT negative group had 13.7 % of the avoidance cohort develop a peanut allergy compared to 1.9 % in the early introduction group. In the SPT positive arm, 35.3 % of the avoidance group developed peanut allergy whereas only 10.6 % of the early introduction group developed peanut allergy. While formal consensus expert panel recommendations are forthcoming, results from the LEAP trial may suggest that early introduction of peanut in selected children may protect against the development of peanut allergy and represent a paradigm shift in the current approach to food allergy management.
Redirecting Antibody Specificity
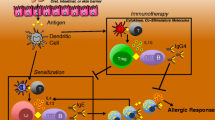
All immunocompetent humans have IgM and IgG antibodies that are specific for the carbohydrate epitope galactose-alpha-1, 3-galactose. The IgG antibody that recognizes the galactose-alpha-1,3- galactose (or alpha-gal) epitope is referred to as anti-Gal. Since anti-Gal antibodies are both ubiquitous and naturally occurring, these antibodies are an ideal target for modification (Fig. 1). In fact, data show that using synthetic peptides chemically linked to the alpha-gal epitope can redirect the anti-Gal response. The initial proof of concept was performed in vitro with HIV-1 infection using gp120 as the target. The results showed that HIV-1 activity was neutralized through the gp120-targeted, CD4-derived peptides in vitro [20]. Importantly, the binding of the CD4-alpha-gal peptides to HIV-1-infected cells conferred antibody-dependent cellular cytotoxicity after the addition of human sera. Taken together, these data demonstrate a proof of concept that the specificity of naturally occurring antibodies can be temporarily redirected. While we are not aware of any current trials using this technology for the treatment of peanut allergy, it is reasonable that a peptide array of modified peanut epitopes linked to alpha-gal could be used to redirect anti-Gal specificity, leading to opsonization and destruction of peanut IgE-bearing cells.
Conclusion
Immunotherapy in various forms remains a promising area for future clinical treatment and intervention of food allergy. Moreover, timing of dietary antigen exposure may also represent an important aspect to consider to prevent and/or treat food allergy. Continued investigation is likely to reveal that one approach will not suit all patients and that various algorithms may be needed based on the particular allergen and each patient’s molecular profile of sensitization. Given the number of unanswered questions regarding food allergy treatment, research into the production and biology of IgE, allergic mediators, and effector cells will provide the necessary understanding to move forward with novel therapies and new applications of existing techniques.
Abbreviations
- Ab:
-
Antibody
- Alpha-gal:
-
Galactose-alpha-1,3-galactose
- DBPCFC:
-
Double-blind, placebo-controlled food challenge
- EPIT:
-
Epicutaneous immunotherapy
- IgE:
-
Immunoglobulin E
- mAb:
-
Monoclonal antibody
- OFC:
-
Oral food challenge
- OIT:
-
Oral immunotherapy
- SCIT:
-
Subcutaneous immunotherapy
- sIgE:
-
Antigen-specific IgE
- SLIT:
-
Sublingual immunotherapy
- SPT:
-
Skin prick test
- Th:
-
T-helper
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51.
Sampson H. Peanut oral immunotherapy: is it ready for clinical practice? JACI: In Pract. 2013;1:15–21.
Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–75. This study was the first to show that peanut OIT can lead to sustained unresponsiveness, which suggests that long-term use of daily maintenance doses may not be necessary for all subjects.
Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003;17:459–65.
Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300.
Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124:286–91.
Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. 2011;41:1273–81.
Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91.
Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60.
Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6.
Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–27.
Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383:1297–304.
Sampson HA, Agbotounou W, Thébault C, Charles R, Martin L, Yang WH, et al. Epicutaneous immunotherapy (EPIT) is effective and safe to treat peanut allergy: a multi-national double-blind placebo-controlled randomized phase IIb trial. J Allergy Clin Immunol. 2015;135:390.
Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. While formal consensus expert panel recommendations are forthcoming, results from the LEAP trial may suggest that early introduction of peanut in selected children may protect against the development of peanut allergy and represent a paradigm shift in the current approach to food allergy management.
Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135:1275–82.
Leung DY, Sampson HA, Yunginger JW, Burks Jr AW, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–93.
Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132:1368–74. Although a small number of subject were treated, data from this study show that administration of omalizumab before OIT appears to allow subjects to ingest large quantities of allergen faster than peanut OIT protocols without omalizumab.
Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou PH. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy. 2010;40:659–67.
Dupont C, Kalach N, Soulaines P, Legoué-Morillon S, Piloquet H, Benhamou PH. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125:1165–7. One of the only clinical trials reporting data related to efficacy of epicutaneous treatment for food allergy. Results of other ongoing trials are eagerly awaited for further evaluation of the skin-based approach.
Perdomo MF, Levi M, Sällberg M, Vahlne A. Neutralization of HIV-1 by redirection of natural antibodies. Proc National Acad Sci. 2008;105:12515–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Commins reports receiving a research grant from NIH. Drs. Kim, Orgel, and Kulis declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Allergy and Immunology
Rights and permissions
About this article
Cite this article
Commins, S.P., Kim, E.H., Orgel, K. et al. Peanut Allergy: New Developments and Clinical Implications. Curr Allergy Asthma Rep 16, 35 (2016). https://doi.org/10.1007/s11882-016-0613-x
Published:
DOI: https://doi.org/10.1007/s11882-016-0613-x