Abstract
Developmental dyslexia is a common neurodevelopmental disorder that is associated with alterations in the behavioral and neural processing of speech sounds, but the scope and nature of that association is uncertain. It has been proposed that more variable auditory processing could underlie some of the core deficits in this disorder. In the current study, magnetoencephalography (MEG) data were acquired from adults with and without dyslexia while they passively listened to or actively categorized tokens from a /ba/-/da/ consonant continuum. We observed no significant group difference in active categorical perception of this continuum in either of our two behavioral assessments. During passive listening, adults with dyslexia exhibited neural responses that were as consistent as those of typically reading adults in six cortical regions associated with auditory perception, language, and reading. However, they exhibited significantly less consistency in the left supramarginal gyrus, where greater inconsistency correlated significantly with worse decoding skills in the group with dyslexia. The group difference in the left supramarginal gyrus was evident only when neural data were binned with a high temporal resolution and was only significant during the passive condition. Interestingly, consistency significantly improved in both groups during active categorization versus passive listening. These findings suggest that adults with dyslexia exhibit typical levels of neural consistency in response to speech sounds with the exception of the left supramarginal gyrus and that this consistency increases during active versus passive perception of speech sounds similarly in the two groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyslexia is an inherited neurobiological disorder leading to significant struggles in reading acquisition, despite adequate schooling and intelligence, in approximately 10–15% of the population (Peterson & Pennington, 2015). Auditory perception deficits have been widely reported in dyslexia (Hari & Kiesilä, 1996; Helenius et al., 1999; Lehongre et al., 2011; Tallal, 1980; Vandermosten et al., 2010), and a majority of individuals with dyslexia demonstrate a phonological deficit (Vellutino et al., 2004). The link between abnormal auditory processing and phonological impairments has often been investigated through the lens of categorical speech perception; however, evidence of reduced categorical perception has been found in some dyslexia samples but not others (Noordenbos et al., 2013; Noordenbos & Serniclaes, 2015; Ramus et al., 2003; Ozernov-Palchik et al., 2021a, 2021b; Vandermosten et al., 2010; Werker & Tees, 1987), perhaps due to underlying differences in general language ability among samples (Joanisse et al., 2000).
When a speech-sound continuum is perceived categorically, tokens at each endpoint are consistently labeled as members of their category, and there is a sharp discontinuity in labeling responses at the category boundary. This pattern of behavioral responses suggests that perception is stable across trials and that category representations are well-defined and non-overlapping. The opposite is suggested by an identification function with a shallower slope, as multiple presentations of the same stimulus have yielded different responses across trials. A shallow slope, as has been reported in some prior studies of dyslexia (Noordenbos & Serniclaes, 2015; Vandermosten et al., 2010, 2011), may suggest a deficit in categorical perception. A shallow slope on speech sound continuum tasks could be due to trial-by-trial variability in neural encoding and/or overlapping category representations. Importantly, underspecified long-term representations could also be the result of inconsistent neural responses to speech, as the development of robust phoneme categories relies upon the extraction of acoustic–phonetic regularities from the spoken-language environment, and nosier encoding could reasonably be expected to disrupt such patterns. Therefore, interrogating purported neural inconsistency in dyslexia is critical to both interpreting patterns of behavioral performance and understanding its etiology. To this end, we measured the trial-by-trial consistency of evoked neural responses to speech in the context of categorical perception in a sample of adults with and without dyslexia.
Less-consistent neural responses to multiple presentations of the same speech sounds and tones have been observed in the auditory brainstem (Hornickel & Kraus, 2013; Neef et al., 2016) and in cortex (Centanni et al., 2018) in children with dyslexia. The neural noise hypothesis (Hancock et al., 2017) posits that genetic variants commonly associated with dyslexia may increase spontaneous firing in the brain (Centanni et al., 2018, 2014ab; Neef et al., 2017), potentially leading to inconsistent neural representation of phonemes and reduced categorical perception. Neural noise could negatively impact speech sound perception and/or reading by impacting any number of cortical regions, including early processing by subcortical auditory areas (Hornickel & Kraus, 2013; Neef et al., 2017) and primary auditory cortex (Centanni et al., 2018), as well as higher level processing by cortical language and reading networks. Precise temporal cues are especially important for speech sound perception (Engineer et al., 2008; Neef et al., 2016; Perez et al., 2012), which increases the negative impact of neural noise on auditory processing of speech and the mapping between speech sounds and letter symbols. Thus, increased neural noise in any of these regions could have a notable impact on reading ability.
While the presence of increased neural noise in many of these regions of interest has been documented in children with dyslexia, it is currently unknown whether decreased consistency is also present in adults or whether years of practice, and perhaps intervention, lead to typical neural consistency in adults with dyslexia. The possibility that years of experience with speech sounds improve the consistency of neural responses to those sounds is suggested by research with a rat model of dyslexia. In this model, suppression of the dyslexia-susceptibility gene Kiaa0319 initially results in increased neural inconsistency in response to human speech sounds (Centanni, et al., 2014a). Following 4 months of behavioral training using speech-sound stimuli, however, rats with this genetic modification exhibited significantly increased consistency in neural responses to speech sounds and were no longer different from controls (Centanni, et al., 2014b). This finding suggests that extended experience with speech can enhance neural consistency.
One fMRI study to date utilized multivariate pattern analysis to investigate the consistency of neural responses to speech sounds in adults with dyslexia and found no differences across a variety of language and reading regions (Boets et al., 2013). The authors interpreted this as evidence that neural representations of spoken language in dyslexia are intact and suggested that those with dyslexia instead exhibited deficits in retrieval of otherwise intact representations. However, the neural activity in that study was measured in the context of a monitoring and discrimination task, while prior research in children reporting neural inconsistency in dyslexia involved passive exposure to language sounds (Centanni et al., 2018; Hornickel & Kraus, 2013; Lam et al., 2017; Neef et al., 2017). Thus, it is unclear whether typical brain responses to speech in the adults with dyslexia in that study reflected the attentional task demands, and whether greater neural inconsistency would have been observed with a fully passive task as it has been observed in children with dyslexia.
Attention influences the perception of and neural response to sound. In primary auditory cortex, the response amplitude to attended stimuli is significantly increased compared with ignored stimuli (Picton & Hillyard, 1974; Poghosyan & Ioannides, 2008). Since much of this prior work was conducted using EEG, it is difficult to attribute the higher amplitudes in active tasks to a recruitment of additional neurons, an increase in the firing of the neurons encoding the stimulus (Cohen & Maunsell, 2009; Reynolds et al., 2000), or an increase in the consistency with which the relevant neurons fire, a relationship that has been demonstrated in rat models (Centanni, et al., 2014a, b). One prior study in macaques found that neural firing rates in visual cortex increased during an active task compared with passive exposure, but that attention did not alter the consistency of those responses (McAdams & Maunsell, 1999). Prior research, however, has not specifically determined how attention influences trial-by-trial neural consistency in evoked auditory responses and whether the relationship between attention and neural consistency differs in dyslexia. Thus, we hypothesized that tasks which require attention to auditory stimuli will enhance trial-by-trial consistency in key brain regions and may provide insight into speech sound processing deficits in dyslexia.
In addition, prior reports of neural consistency deficits in dyslexia used imaging techniques with millisecond precision, both in human participants (Centanni et al., 2018; Hornickel & Kraus, 2013; Lam et al., 2017; Skeide et al., 2015) and in animal models (Centanni, et al., 2014a, b). The perception of some speech sounds, such as stop consonants, relies on precise millisecond timing. Microelectrode recordings in rats demonstrate that a single consonant can be identified by the subcortical or primary auditory cortex responses (Centanni et al., 2013; Engineer et al., 2008). In rodent work, neural responses recorded using microelectrodes yield millisecond precision. When that millisecond precision is maintained, a classifier based on Euclidean distance accurately predicts the identity of the evoking speech sound stimulus but accuracy falls rapidly when neural responses are binned in larger intervals (Engineer et al., 2008; Perez et al., 2012). It is therefore possible that equivalent temporal resolution in human neural imaging is required to detect decreased consistency of responses to such stimuli and that techniques with poor temporal precision, such as fMRI, may be unable to accurately measure differences in neural consistency across individuals.
In the current study, we investigated responses to a ten-step /ba/-/da/ continuum in adults with and without dyslexia using behavioral and neural imaging techniques. We first evaluated behavioral responses to test the hypothesis that abnormal categorical perception is present in adults with dyslexia. Second, we quantified the consistency of neural responses evoked by hearing tokens from this continuum. We hypothesized that neural inconsistency would be higher in those with dyslexia and that the degree of inconsistency observed would correlate with participants’ reading skills (Centanni et al., 2018; Hornickel & Kraus, 2013). Third, we investigated whether attention influences the precision of neural responses by comparing consistency measured during passive exposure and active categorization conditions. We hypothesized that attention would improve consistency in both groups. Finally, because the perception of consonants such as /b/ and /d/ requires fine-grained temporal processing, we evaluated how varying the temporal bin size for neural data analysis affects the sensitivity of the consistency metric. We hypothesized that larger temporal bins would mask any group differences in neural consistency.
Methods
Participants
We recruited and screened potential participants between the ages of 18 and 45 from the greater Boston area as part of a larger study on auditory processing in adults with dyslexia (Beach et al., 2021; Ozernov-Palchik et al., 2021a, b). All individuals were required to have been exposed to English from birth with no exposure to a second language prior to age 2 and have normal hearing. Hearing was measured by pure-tone audiometry in which participants responded to the presence of a tone by raising their hand. The level began at 30 dB and was reduced by 5 dB until the participant no longer detected the tone. Six frequencies were tested in each ear: 8000, 4000, 2000, 1000, 500, and 250 Hz, and all eligible participants had thresholds at or below 30 dB. Participants were also required to have no history of neuropsychological conditions, including ADHD and autism and be right-handed. Inclusion criterion included a score of 85 or above on the KBIT Matrices subtest, which measures non-verbal IQ (Kaufman & Kaufman, 2004). To qualify for the typical-reader group, participants needed a standard score of 90 or greater on four single-word and single pseudoword reading measures: Sight Word Efficiency (SWE; Test of Word Reading Efficiency/TOWRE-2; Torgesen et al., 1999), Phonemic Decoding Efficiency (PDE; TOWRE-2), Word Identification (WID; Woodcock Reading Mastery Test/WRMT-3; Woodcock et al., 2001), and Word Attack (WA; WRMT-3). Participants were placed in the dyslexia group if they scored below 90 on at least two of those four reading measures. To calculate standard scores on certain measures with limited age-based normative data (e.g., the TOWRE, which is normed up to 24 years, 11 months), the oldest age group was used to calculate standard scores for participants whose age was outside the measure’s range.
A total of 95 individuals were screened. Of these, seven were exposed to a language other than English prior to the age of two, two were not neurotypical, two were left-handed, seven withdrew partway through the study, two yielded unusable imaging data, two did not have normal hearing, one could not complete imaging due to metal in the spine, 11 had a history of dyslexia but did not meet our testing criteria, 10 had one reading score below 90 (thus not qualifying for either group), three had a low KBIT score, and one participant exceeded our age range. We were left with a sample of 47 adults, of which 23 were typical readers (10 female) and 24 had dyslexia (15 female), 18 of whom had self-reported an external diagnosis of dyslexia. Characteristics of the finalized groups are summarized in Table 1. All participants provided informed consent prior to participating in study activities and all behavioral assessment and neural imaging procedures were approved by the Institutional Review Board of the Massachusetts Institute of Technology.
Additional reading and language assessments
In addition to the assessments described above that were used for inclusion purposes, all participants completed a background survey probing their language and reading backgrounds as well as the following measures: rapid automatized naming of letters and rapid alternating stimuli (RAN/RAS; (Wolf & Denckla, 2005)), phonological awareness (Elision and Blending Words subtests of the CTOPP; (Wagner et al., 1999)), listening comprehension (WRMT-3; (Woodcock, 2011)), and reading comprehension (subscores for Rate, Accuracy, Fluency, and Comprehension: Gray Oral Reading Test/GORT; (Wiederholt & Bryant, 2012)).
Categorical perception and stimuli
Following the behavioral assessments, participants completed a computerized categorical-perception task. They were presented with items from a ten-step /ba/-/da/ continuum and asked to categorize them as either /ba/ or /da/ using a button press. Each stimulus was presented ten times each over the course of the task in pseudorandom order. The stimuli, each 310 ms in duration, were created by morphing natural speech (see Stephens & Holt, 2011 for full details). We used the ten odd-numbered items from the original continuum. To quantify categorical perception, we fit a logistic function to each participant’s proportion of /da/ responses at each of the ten continuum steps. The slope parameter indicated the degree to which the continuum was perceived categorically.
Magnetoencephalography data acquisition
On a separate day, brain activation measurements were obtained using an Elekta Neuromag Triux system equipped with a whole-brain sensor array comprising 102 magnetometers and 204 planar gradiometers (306 total magnetic sensors). Magnetoencephalography (MEG) recordings were obtained at a sampling rate of 1000 Hz and filtered between 0.03 and 330 Hz. The position of the head was measured continuously during the recordings using a set of five head position indicator coils.
MEG tasks
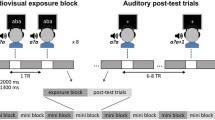
While in the MEG device, participants first completed a passive task intended to provide exposure to the continuum stimuli (described above) without attention. Participants pressed a button when a picture of a landscape appeared on the screen in place of a fixation cross while consonant continuum stimuli were played one at a time through insert earphones (Etymotic, Oak Grove Village, IL). Inter-stimulus intervals were randomized across trials to be between 1 and 1.5 s. Participants were told to make sure not to miss any of the pictures and that they would hear sounds but could ignore them. In the second, active task, participants listened to the same stimuli and classified each as “ba” or “da” with a delayed button-press once response options (a cartoon ball for “ba” and a cartoon dog for “da”) appeared on the screen. The program waited for a button press before advancing to the next stimulus. In both tasks, each of the ten stimuli was presented 40 times in pseudorandom order. Button-press responses were made with the left hand so as not to create motor artifacts in the left hemisphere. Together, these tasks were completed in approximately 25 min. Custom Matlab programs (The Mathworks, Natick, MA) were used to control stimulus presentation and timing. Visual stimuli were presented using Psychtoolbox (www.psychtoolbox.org).
Anatomical MRI acquisition
Following their MEG session, all participants completed a short MRI session to acquire anatomical data. Imaging was performed using a Siemens 3 T MAGNETOM Trio, A Tim System (Siemens Medical Solutions, Erlangen, Germany), and a commercial Siemens 32 channel head coil. A high resolution T1-weighted image was acquired (128 slices; 256 mm FOV; 1.33 mm slice thickness; TR = 2530msl TE = 3.39, 7° flip angle). Before each scan, 4 dummy volumes were acquired and discarded to reach equilibrium, and online prospective acquisition correction was applied throughout the scan. After acquisition, anatomical images were processed using FreeSurfer Version 5.3.0 (Fischl, 2012). Custom individual anatomy files for each participant were then used to analyze MEG source data as described below.
Magnetoencephalography data processing
Raw MEG data were preprocessed using Maxfilter software (Elekta Neuromag, Stockholm) to compensate for head movements and perform noise reduction with spatiotemporal filters (Taulu & Simola, 2006; Taulu et al., 2004). We used default parameters (harmonic expansion origin in head frame = [0 0 40] mm; expansion limit for internal multipole base = 8; expansion limit for external multipole base = 3; bad channels automatically excluded from harmonic expansions = 7 s.d. above average; temporal correlation limit = 0.98; buffer length = 10 s). MEG data were then analyzed using Brainstorm software (Tadel et al., 2011). First, a notch filter was applied to remove electrical artifacts (60 and 120 Hz notch). Heartbeat and eye-blink artifacts were identified by an experienced researcher and projected out of the signal. We then extracted trials and performed baseline correction (− 200 to 0 ms) to remove the mean from each channel. Trials with excessive movement (peak-to-peak value greater than 10,000 fT) were labeled and removed from the database. The time series were then temporally smoothed with a 40-Hz low-pass filter. For each trial, the MEG data were mapped on the cortical mantle derived from the Freesurfer automatic segmentation of the participant’s custom anatomy (Fischl, 2012). This was accomplished by first calculating an overlapping-spheres head model (Huang et al., 1999) and then computing an inverse model using a dynamic statistical parametric mapping approach (dSPM) (Dale et al., 2000).
We extracted the time series from seven cortical regions of interest (ROIs) derived from the Desikan-Killiany atlas (Desikan et al., 2006). These ROIs comprised bilateral primary auditory cortex (i.e., the transverse temporal mask) and five left-hemisphere brain areas involved in reading and language: inferior frontal gyrus (IFG), inferior parietal lobule (IPL), superior temporal gyrus (STG), supramarginal gyrus (SMG), and fusiform gyrus (FFG) (Destrieux et al., 2010; Hickok & Poeppel, 2007). The superior temporal gyrus (Turkeltaub & Coslett, 2010), the supramarginal gyrus (Raizada & Poldrack, 2007), and the inferior parietal lobule (Norton et al., 2014) have all been associated with phonological processing; activity in the inferior frontal gyrus is correlated with categorical perception (Lee et al., 2012); and a region of the fusiform gyrus serves as the hub for visual print processing (Cohen et al., 2002). To our knowledge, there is no evidence to suggest the FFG plays a role in categorical perception; however we included it in our analyses because of its role in reading and for comparison to prior work on neural consistency in dyslexia (Centanni et al., 2018, 2019a, b).
Imaging data analysis and statistical methods
The primary outcome measure of interest was neural response consistency across trials (Centanni et al., 2018; Hornickel & Kraus, 2013; Lam et al., 2017; Neef et al., 2017; Fig. 1). We analyzed consistency within two time windows: 50–100 ms for basic sensory processing and 150–300 ms for syllable-level processing (Centanni et al., 2018; Poeppel, 2003; Travis et al., 2013). Within each participant and ROI, we first calculated the average response separately across the even-numbered trials and across the odd-numbered trials of a single stimulus (Centanni et al., 2018; Hornickel & Kraus, 2013; Neef et al., 2017). This step was implemented in order to minimize any effects of fatigue across the recording session (Figs 1a and b). We then calculated consistency by correlating the even and odd trials within the above time windows of interest using Spearman’s rho, with the data binned in 1 ms increments (Hornickel & Kraus, 2013; Figs. 1c and d).
To calculate neural consistency, single trial responses were binned into even numbered trials (black lines) and odd numbered trials (blue lines) (a–b). Even and odd trials were then averaged separately and Spearman’s rho was calculated between the two averages within 150–300 ms (marked with vertical lines) relative to stimulus onset at 0 ms (c–d). Example single trial responses (a) and averaged responses (c) in the left SMG of a typical reader. Example single trial responses (b) and averaged responses (d) in the left SMG of a reader with dyslexia. Overall, even vs odd trials were more similar in the typical readers than in those with dyslexia
To evaluate the effect of temporal bin size on the neural consistency metric, we also evaluated Spearman’s rho across the average of even-numbered trials vs. average of odd-numbered trials using progressively larger temporal bins. This analysis was conducted only on passive responses in the left SMG, as this ROI/condition combination was the only one to exhibit a significant group difference. Within the time window of interest (150–300 ms for syllable-level processing), we binned the neural responses in a range of intervals (5, 10, 50, and 100 ms). For example, within the 150 ms time window of interest, neural data were averaged within successive non-overlapping bins of 5 ms, 10 ms, 50 ms, or 100 ms. We then re-ran the correlations to determine the effect of reduced temporal precision on the neural consistency metric.
Results are reported as the mean of the correlation coefficient and the standard error of that mean (sem) across participants; t tests were paired or unpaired, as appropriate, and post-hoc tests were two-tailed unless otherwise noted. Bonferroni correction for multiple comparisons was applied as indicated.
Results
Categorical perception
Participants actively categorized ten speech sounds from a /ba/-/da/ continuum (Stephens & Holt, 2011) during their initial assessment session and again on a separate day while MEG data were acquired. During the initial assessment session, there was no main effect of group on response rates to each continuum step (F (1441) = 2.51, p = 0.12; Fig. 2a). Similarly, during the MEG session, there was no main effect of group on response rates to each continuum step (F (1, 387) = 0.04, p = 0.85; Fig. 2b). Thus, our hypothesis was not supported, as intact categorical perception was observed across two sessions in this group of adults with dyslexia.
Identification responses for the steps of the /ba/-/da/ continuum measured during the behavioral assessment session (a) and again on a separate day in the MEG device (b). On both days, each of the ten steps was presented multiple times (10 each in the first session and 40 each in the MEG session) in pseudorandom order. There were no differences between typical readers (black) and individuals with dyslexia (blue) in response rate across the steps of the continuum on either day (ps > 0.8)
To probe this null effect, we next investigated whether other aspects of communication, such as language processing, may relate to categorical perception. Understanding the degree to which abnormal categorical perception is characteristic of dyslexia versus related to broader language abilities has been somewhat confounded by the comorbidity between dyslexia and specific language impairment (SLI) (Catts et al., 2005; Visscher et al., 2012). While we did not specifically test our participants for SLI, we did measure oral listening comprehension. To determine whether oral language processing was associated with categorical perception, we correlated the slope of each participant’s continuum identification function (from the behavioral session) with their standard score on the listening comprehension measure. In the typical readers, there was no relationship between these two variables (r = − 0.19, p = 0.40). However, there was a significant positive relationship between oral listening comprehension and continuum slope in the dyslexia group (r = 0.48, p = 0.02), such that worse oral listening comprehension was associated with a shallower slope.
Early sensory processing
Because the same auditory stimuli were presented in two different tasks, with and without attention, we were able to evaluate the potential influence of attention on neural consistency in bilateral primary auditory cortices with a three-way repeated-measures ANOVA (hemisphere x attention x group; Fig. 3). There was a significant main effect of attention (F (1180) = 80.12, p < 0.0001), such that attention lowered consistency. There were no main effects of hemisphere (F (1180) = 1.94, p = 0.17) or group (F (1180) = 0.87, p = 0.35).
Syllable-level processing
To evaluate neural consistency in the language and reading network more broadly, we ran a three-way ANOVA (attention x ROI x group) using neural consistency values in the five left-hemisphere ROIs (Fig. 4). There were significant main effects of attention (F (1450) = 115.2, p < 0.0001), ROI (F (4450) = 76.92, p < 0.0001), and group (F (1450) = 8.2, p = 0.004). There was also a significant interaction between ROI and attention (F (4450) = 3.43, p = 0.009). There were no other interactions (ps > 0.30).
The main effect of attention reflected increased consistency in the active versus passive conditions (one-tailed paired t test: t (46) = 10.51, p < 0.0001). The main effect of group was driven by significantly higher consistency in typical readers during passive exposure compared with those with dyslexia in the left SMG (one-tailed independent t test: t (45) = 3.55, p = 0.0005) and a trend in the same direction in the left STG (one-tailed independent t test: t (45) = 1.42, p = 0.08). There were no group differences in the other ROIs (ps > 0.21). In the active condition, there were trends in the group comparison in two ROIs: left IFG (one-tailed independent t-test: t (45) = 1.36, p = 0.089) and left SMG (one-tailed independent t-test: t (45) = 1.49, p = 0.071), such that consistency was higher in typical readers compared with those with dyslexia. There were no group differences in the other ROIs (ps > 0.12).
Visual inspection of the data suggested that the main effect of ROI was driven by increased consistency in STG compared with the other ROIs. Post hoc t tests confirmed this hypothesis, with greater consistency in the STG than in all other regions in both the passive condition (one-tailed, paired t-tests; ps < 0.01) and the active condition (one-tailed, paired t tests; ps < 0.01). Both groups exhibited significantly higher consistency during the active task compared with the passive task (ps < 0.001).
We next evaluated whether neural consistency was associated with behavior. Within the dyslexia group, there was a significant positive correlation between neural consistency in left SMG and performance on a timed pseudoword reading task (r = 0.49, p = 0.018) and a trend in the positive relationship with rapid letter naming (r = 0.41, p = 0.05; Table 2). Neither of these correlations survived correction. We also measured the relationship between left SMG consistency during the active task and the variability in participant response to each continuum step across repeats. There was no relationship between variability in the SMG during the task and variability in participant response across the entire sample (r = − 0.09, p = 0.55) or in the dyslexia group alone (r = − 0.26, p = 0.21).
As was observed in our prior study of neural variability in children (Centanni et al., 2018), there was no relationship between neural consistency and age in dyslexia (r = 0.07, p = 0.76) or across the entire sample (r = − 0.06, p = 0.68).
Temporal precision is necessary for detecting neural inconsistency
To determine whether millisecond precision is important for detecting increased neural variability, we evaluated neural consistency in left SMG during the passive task when the neural response was binned using successively larger temporal windows. When temporal bins were smaller than 100 ms, the neural consistency group difference remained significant (ps < 0.03; Fig. 5). However, with bins of 100 ms, the group difference was no longer significant (t (45) = 0.77, p = 0.45), suggesting that the use of a technique with millisecond precision and the use of small temporal bins is critical for detection of neural consistency differences.
Influence of temporal bin size on neural consistency metric during passive task. There was a significant group difference in neural consistency in left SMG when neural activity was binned at 1, 5, 10, and 50 ms, but not at 100 ms. Thus, temporal precision is likely needed to detect neural consistency deficits. * ps < 0.05
Discussion
In the present study, we investigated neural consistency in relation to categorical perception of speech sounds in adults with and without dyslexia and asked (1) whether greater neural inconsistency is present in adults with dyslexia compared to typically reading adults, (2) whether the performance of an active task versus passive exposure alters neural consistency, and (3) whether millisecond temporal precision is needed to detect neural consistency differences in dyslexia. We found that adults with dyslexia exhibited significantly less neural consistency compared with their typically reading peers in one ROI (the left supramarginal gyrus) and only in the passive condition. Adults with dyslexia exhibited intact neural consistency in all other regions and in both passive and active conditions. This largely intact brain response was consistent with their unimpaired behavioral performance on a categorical perception task. Neural consistency increased in both groups for the active versus passive task, except in primary auditory cortex, where attention decreased consistency in both groups. Finally, high temporal resolution was necessary for measuring neural consistency differences, as bins of 100 ms were already too large to detect group differences in the left supramarginal gyrus.
No behavioral deficit in identifying tokens from a stop-consonant continuum in dyslexia
Identifying items from a continuum as members of distinct categories provides a measure of how distinct one’s category representations are. Noisy or degraded representations of speech sounds are hypothesized to impede letter-to-sound mapping during reading acquisition (Centanni et al., 2018; Hornickel & Kraus, 2013; Schulte-Körne et al., 1999; Snowling, 1998). Thus, reduced categorical perception might be associated with dyslexia and/or dyslexia risk. However, findings of categorical perception deficits in dyslexia have been inconsistent, with some researchers finding shallower slopes in dyslexia and some not (for review and meta-analysis, see Noordenbos & Serniclaes, 2015). In the current study, we saw no evidence of a categorical perception deficit in adults with dyslexia, and this result was consistent across two test administrations on two different days for each participant. Thus, in this sample of adults with dyslexia, categorical perception was reliably intact. One potential explanation for the null group difference is the choice of endpoint phonemes for the continuum. The spectral, temporal, and spectrotemporal acoustic cues that distinguish consonants are encoded by different neural firing patterns (Centanni et al., 2013; Chang et al., 2010; Engineer et al., 2008). If dyslexia is associated with specific alterations in basic auditory processing abilities important for the perception of certain speech sounds but not others, then a categorical perception deficit might be observed with some continua but not others. A second potential explanation for the lack of group difference is that we used an identification task, whereas discrimination tasks are more sensitive to differences in categorical perception in dyslexia (Noordenbos & Serniclaes, 2015).
It has also been suggested that categorical perception differences are observed in some dyslexia samples but not others because these differences may be associated with language disability and not dyslexia per se. Dyslexia and specific language impairment are frequently comorbid (Catts et al., 2005; McCarthy et al., 2012). Children with dyslexia and comorbid language deficits exhibited less precise categorical boundaries while children with dyslexia and normal language skills performed more similarly to typically-developing readers (Joanisse et al., 2000). In the current study, lower scores on a measure of oral language comprehension correlated with less-categorical perception in the adults with dyslexia. Thus, our findings are consistent with the idea that reduced categorical perception is not a consistent hallmark of dyslexia (Blomert & Mitterer, 2004; Noordenbos & Serniclaes, 2015; Vandermosten et al., 2010) but rather that it is associated with language difficulties that often, but not always, co-occur with dyslexia (Joanisse et al., 2000).
STG and SMG as regions of interest for categorical perception
In the current study, we report significant effects in two main regions of interest. The superior temporal gyrus (STG) drove the significant main effect of ROI by exhibiting the strongest consistency in both participant groups. The supramarginal gyrus (SMG) drove the main effect of group such that typical readers exhibited significantly increased consistency compared to the dyslexia group. There were also significant relationships between consistency in SMG and reading measures in the dyslexia group, suggesting a functional consequence of decreased neural consistency, as was previously observed in children with reading difficulties (Centanni et al., 2018; Hornickel & Kraus, 2013; Neef et al., 2017). These regions of interest are commonly identified as relevant in studies of phonological perception (Raizada & Poldrack, 2007; Ruff et al., 2003; Simos et al., 1998; Turkeltaub & Branch Coslett, 2010). The current results should be interpreted with caution, however, as the correlations in this sample of adults did not survive correction.
A model of the language network proposed bilateral STG as a hub for sublexical phonological processing (Hickok & Poeppel, 2007). The STG is specialized for processing acoustics and sublexical phonological information (Turkeltaub and Branch Coslett 2010). A number of studies utilizing intracranial electrode recordings in STG have demonstrated specific encoding of individual phonemes as well as classes of phonemes (Chan et al., 2014; Chang et al., 2010; Hamilton et al., 2018; Mesgarani et al., 2014; Yi et al., 2019).
The left SMG has been implicated in both categorical speech perception and functional and structural brain differences in dyslexia. The left SMG was found to selectively amplify key stimulus features to support categorical speech perception (Raizada & Poldrack, 2007), and a meta-analysis suggested that this region exhibited the highest activation likelihood during phonological analysis tasks with no significant recruitment of its right-hemisphere counterpart (Turkeltaub and Branch Coslett 2010). With respect to this region’s importance in the study of dyslexia, left SMG is hypoactivated in dyslexia during auditory phoneme processing (Zuk et al., 2018). Further, individuals with a polymorphism (variant) of the DCDC2 dyslexia-associated gene exhibit both increased cortical thickness in left SMG and lower reading scores (Darki et al., 2014). It is possible that, despite relatively inconsistent neural responses in the left SMG (although the difference between the groups was only significant in the passive condition), our group of adults with dyslexia were still able to achieve typical patterns of categorical perception by relying on representations in other cortical regions where speech-evoked responses are reliable.
Possible role of experience on neural consistency
Across seven cortical regions and two tasks, we observed one instance of decreased neural consistency to speech sounds in adults with dyslexia. However, in our previous study of children with dyslexia (Centanni et al., 2018), we observed neural inconsistency in multiple regions of interest in response to several types of stimuli in the auditory (tones and speech sounds) and visual (letters and nonsense objects) domains. This neural consistency deficit was associated with a single nucleotide polymorphism (SNP) on the gene KIAA0319. The minor allele is present in approximately 33% of the Caucasian population, as verified by our prior work in children (Centanni et al., 2018). In our current sample of adults, 9 out of 24 individuals with dyslexia exhibited neural consistency in left SMG that was more than one standard deviation below the mean of the control group. While one standard deviation is not enough to classify this group as outliers, this number of adults aligns with the expected percentage of people with the KIAA0319 SNP. We did not, however, acquire DNA samples from the participants in this study, so future research is needed to determine whether this gene influences neural consistency in adulthood. Given the possibility that at least a portion of the adults in our sample possessed the candidate consistency SNP, the discrepancy between the large effect found in children (Centanni et al., 2018) and the muted effect found in the current study in adults is more likely due to the increased years of intervention, practice, and compensation in adults.
Prior research with rats with suppression of the dyslexia-susceptibility gene Kiaa0319 revealed that extensive behavioral training improved neural response consistency in auditory cortex (Centanni, et al., 2014a, b). A training effect has also been demonstrated in the human auditory brainstem: children with a developmental language disorder who received auditory training (“Earobics”) exhibited significantly more consistent auditory brainstem responses than children in the waitlist control group (Russo et al., 2005). Adults, of course, have many more years of auditory experience and those with a prior diagnosis have likely received significantly more intervention than the children studies in prior investigations. This may explain why we observed a smaller group difference compared with our prior work and why only one region of interest was significant in the current study.
There are few studies that directly compare neural response patterns between adults and children with dyslexia, with all comparisons to date conducted in fMRI datasets. One meta-analysis of nine studies in children and nine studies in adults reported at least one difference in activation patterns across age groups: while both age groups exhibited hypoactivation in the left ventral occipitotemporal (vOT) region, hypoactivation in the left temporoparietal area was found only in children, with hyperactivation present in some subcortical regions in adults (Richlan et al., 2011). The authors suggest that the hypoactivation result in children may be due to this region exhibiting a task-related deactivation compared with baseline and may therefore relate to some unknown compensatory mechanism (Richlan et al., 2011). A second meta-analysis reviewed 20 studies in each age group and replicated the shared overlap in the left vOT cortex (Martin et al., 2015). Additionally, while children exhibited greater activation than adults in supplementary motor areas and left superior temporal gyrus, adults exhibited greater activation than children in several regions, including cerebellum, middle occipital gyrus, and precentral gyrus. These findings provide precedent for different findings in adults and children, and the current study adds to this body of work by suggesting that neural consistency improves over time, perhaps in line with other compensatory processes. Future research is needed to determine when and how neural consistency improves with age and whether such improvement is related to intervention and/or behavioral improvement.
Interestingly, some suggest that the presence of abnormal auditory representations may not be causal of dyslexia in and of itself. A longitudinal study of beginning readers at various levels of familial risk were scanned using fMRI and evaluated for future reading outcomes. While children at high familial risk exhibited less distinct activation patterns to speech sounds compared to children without familial risk, there were no group differences based on future dyslexia diagnosis (Vandermosten et al., 2020). This suggests that auditory perception of speech sounds may indicate susceptibility to dyslexia rather than predict it directly. Predictive neural metrics may exist in the functioning of other regions of interest such as the visual word form area, in which print sensitivity is reduced in children at risk who go on to a diagnosis compared to children at risk who become typical readers (Centanni et al., 2019a, b). Longitudinal research is necessary to characterize the developmental trajectory of neural consistency in dyslexia and whether this feature is casual or indicates a degree of susceptibility only.
Role of attention on neural responses
To date, most studies reporting poor neural consistency in dyslexia have employed passive listening (Centanni et al., 2018; Hornickel & Kraus, 2013; Lam et al., 2017; Neef et al., 2017). One fMRI study that required active responses to speech sounds reported intact neural consistency in adults with dyslexia (Boets et al., 2013). This pattern of findings raised the possibility that neural inconsistency in dyslexia occurs during passive exposure to speech sounds, but not during active listening. Such a dissociation could occur if attention critically affects neural consistency to speech sounds in dyslexia. Therefore, we performed the first analysis of neural consistency for both active and passive exposure to the same speech syllables within the same individuals with dyslexia. The absence of any interaction between group, attention condition (active vs. passive), and neural consistency contradicts the idea that attention is a critical factor in explaining differences in neural inconsistency in dyslexia.
The lack of group difference was not due to the absence of an effect of attention on neural consistency because such an effect was significant in all brain regions of interest. In primary auditory cortex, attention was associated with lower neural consistency almost immediately after stimulus onset. In higher-order language and reading areas, we observed the opposite pattern: attention was associated with greater consistency in the neural response in both groups. It is perhaps surprising that attention decreased consistency in primary auditory cortex while increasing it elsewhere in the network. However, a growing body of work suggests that increased noise early in a sensory pathway may be beneficial for signal detection. In the visual system, increased noise corresponds with increased stimulus identification in early visual cortex of macaques (Cohen & Maunsell, 2009; McAdams & Maunsell, 1999). In the auditory system, this phenomenon is known as stochastic resonance and is proposed to serve an optimization role by altering neural noise to improve hearing thresholds in certain conditions (Krauss et al., 2018; Schilling et al., 2020). Artificial increases in neural noise generated by transcranial random noise stimulation (tRNS) in one study led to increased detection of near-threshold auditory stimuli in a gap detection paradigm (Rufener et al., 2017). It is therefore possible that variability early in the auditory signal is important, especially when the system is expecting a signal as part of a perceptual task. Under this assumption, primary sensory areas would increase neural noise to improve perception of a stimulus. In the context of speech perception, a stochastic resonance in low-level systems could ensure that accurate speech-sound representations are passed to syllable- and word-level processing regions. Future research should test this hypothesis using a task with more distinct “correct” vs. “incorrect” responses, as opposed to a continuum-perception task in which stimulus labels are somewhat subjective.
While stochastic resonance may be useful in an active task, it is likely that increased variability is detrimental when stimuli are passively presented or unexpected. If the brain responds inconsistently to passive input, this could lead to abnormalities in automatic attentional shift processes such as habituation. For example, in the oddball paradigm, the brain habituates to the repeated or standard stimulus such that the deviant stimulus draws attention and generates the mismatch negativity response. However, this canonical response is significantly reduced in dyslexia (Maurer et al., 2003; Perrachione et al., 2016; Renvall & Hari, 2002). This deficit may reflect an abnormality in the subconscious attention shifting mechanism that is specific to passive processing. We did not assess visual or auditory attentional shift in this study and so cannot directly evaluate this link. However, future research should determine whether neural inconsistency to passive stimuli predicts attentional shift deficits.
Importance of temporal precision for measuring neural variability
Speech sounds are rapidly changing, complex acoustic stimuli. Within 40 ms after stimulus onset, primary auditory cortex encodes all the information necessary to determine the identity of a single consonant (Centanni et al., 2013; Engineer et al., 2008; Perez et al., 2012). When neural activity recorded using microelectrodes is binned into large windows, consonant classification no longer matches behavioral discrimination ability in rats (Engineer et al., 2008; Perez et al., 2012). Within that initial 40 ms time window, variability in the timing of individual neurons has a dramatic impact on the perceived identity of the stimulus. Neural activity in primary auditory cortex of rats with suppression of the dyslexia-candidate gene Kiaa0319 was significantly less reliable for decoding the speech-sound stimulus than neural activity in control animals (Centanni, et al., 2014a, b), and this variability was significantly correlated with animals’ ability to behaviorally discriminate between various speech-sound stimuli (Centanni, et al., 2014a, b).
In the current study, we demonstrated that using temporal bins of 5, 10, and 50 ms produced similar neural consistency results, while bins of 100 ms eliminated the group difference in neural consistency to sound. While prior EEG studies of neural consistency binned their data with 1 ms precision (Hornickel & Kraus, 2013; Neef et al., 2016, 2017), these studies utilized thousands of trials per stimulus. It is possible that calculation of neural consistency follows an inverted-U function, such that the most precise bin and larger bins all yield less reliable calculations and that there may be an optimal bin size for calculating neural consistency. Further, for the most precise time window to yield reliable measurements, a large number of trials may be needed, while fewer trials require a slightly larger bin size to capture the effect clearly. Future work is needed to clarify how number of trials interacts with optimal bin size.
fMRI inherently records with significantly longer temporal bins. Although fast-sampling methods exist, the minimum sampling window for fMRI is approximately 100 ms (Lin et al., 2012). Thus, we hypothesize that the low temporal resolution of fMRI effectively eliminates the ability to detect a variability difference and those interested in measuring this metric should carefully consider their choice of neural imaging technique. Future research should combine these methods in the same subject pool to evaluate this possibility.
Limitations
There are three main limitations in the current study. The first limitation is the low number of trials in each task. As discussed above, prior research measuring neural consistency in EEG utilized thousands of trials, while we only collected 40 trials for each of 10 stimuli. While the use of many more trials may yield higher consistency values by reducing the impact of natural trial by trial variability, this approach may also mask any group effect for the same reason. Future research is needed to determine whether additional trials provide more reliable estimates of neural consistency or whether additional trials attenuate the effect. The second limitation is that while we did perform a bin-size analysis with our MEG data, we did not directly compare data collected with a temporally precise technique (EEG/MEG) with a less temporally precise technique (fMRI). Although prior studies reporting the absence of neural consistency may have failed to find a deficit due to either their use of an active task or longer temporal bins (Boets et al., 2013), such a conclusion cannot be drawn without a direct comparison across approaches. Finally, we did not account for any effects of intervention in the dyslexia group. As discussed above, behavioral training can increase neural consistency and it is likely that prior and variable experiences with intervention in the dyslexia group interacted with our measures. Future work should be careful to quantify prior intervention experiences in participants for use as a covariate.
Data availability
De-identified data is available upon request to the corresponding author.
Code availability
Analysis code is available upon request to the corresponding author.
References
Beach, S. D., Ozernov-Palchik, O., May, S. C., Centanni, T. M., Gabrieli, J. D. E., & Pantazis, D. (2021). Neural decoding reveals concurrent phonemic and subphonemic representations of speech across tasks. Neurobiology of Language, 2(2), 254–279. https://doi.org/10.1162/nol_a_00034
Blomert, L., & Mitterer, H. (2004). The fragile nature of the speech-perception deficit in dyslexia: Natural vs. synthetic speech. Brain and Language, 89(1), 21–26. https://doi.org/10.1016/S0093-934X(03)00305-5
Boets, B., de Beeck, H., Vandermosten, M., Scott, S., Gillebert, C., Mantini, D., … Ghesquiere, P. (2013). Intact but less accessible phonetic representations in adults with dyslexia. Science, 342(6163), 1251–1254. Retrieved from http://www.sciencemag.org/content/342/6163/1251.short
Catts, H. W., Adlof, S. M., Hogan, iffany P., & Weismer, S. E. (2005). Are specific language impairment and dyslexia distinct disorders? Journal of Speech, Language and Hearing Research, 48(6), 1378. Retrieved from http://jslhr.pubs.asha.org/article.aspx?articleid=1783909
Centanni, T. M., Booker, A. B., Sloan, A. M., Chen, F., Maher, B. J., Carraway, R. S., … Kilgard, M. P. (2014a). Knockdown of the dyslexia-associated gene Kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cerebral Cortex, 24(7), 1753–1766. https://doi.org/10.1093/cercor/bht028
Centanni, T. M., Chen, F., Booker, A. B., Engineer, C. T., Sloan, A. M., Rennaker, R. L., … Kilgard, M. P. (2014b). Speech sound processing deficits and training-induced neural plasticity in rats with dyslexia gene knockdown. PloS One, 9(5), e98439. https://doi.org/10.1371/journal.pone.0098439
Centanni, T. M., Engineer, C. T., & Kilgard, M. P. (2013). Cortical speech-evoked response patterns in multiple auditory fields are correlated with behavioral discrimination ability. Journal of Neurophysiology, 110(1), 177–189. https://doi.org/10.1152/jn.00092.2013
Centanni, T. M., Norton, E. S., Ozernov-Palchik, O., Park, A., Beach, S. D., Halverson, K., … Gabrieli, J. D. E. (2019). Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. NeuroImage: Clinical, 22. https://doi.org/10.1016/j.nicl.2019.101715
Centanni, T., Norton, E., Ozernov-Palchik, O., Park, A., Beach, S., Halverson, K., … Gabrieli, J. (2019). Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. NeuroImage. Clinical, 22, 101715. Retrieved from https://www.sciencedirect.com/science/article/pii/S2213158219300658
Centanni, T., Pantazis, D., Truong, D., Gruen, J., Gabrieli, J., & Hogan, T. (2018). Increased variability of stimulus-driven cortical responses is associated with genetic variability in children with and without dyslexia. Developmental Cognitive Neuroscience, 34, 7–17. https://doi.org/10.1016/j.dcn.2018.05.008
Chan, A. M., Dykstra, A. R., Jayaram, V., Leonard, M. K., Travis, K. E., Gygi, B., … Cash, S. S. (2014). Speech-specific tuning of neurons in human superior temporal gyrus. Cerebral Cortex (New York, N.Y. : 1991), 24(10), 2679–2693. https://doi.org/10.1093/cercor/bht127
Chang, E., Rieger, J., Johnson, K., Berger, M. S., Barbaro, N. M., & Knight, R. T. (2010). Categorical speech representation in human superior temporal gyrus. Nature Neuroscience, 13(11), 1428–1432. Retrieved from http://www.nature.com/articles/nn.2641
Cohen, L., Lehéricy, S., Chochon, F., Lemer, C., Rivaud, S., & Dehaene, S. (2002). Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain : A Journal of Neurology, 125(Pt 5), 1054–1069.
Cohen, M., & Maunsell, J. (2009). Attention improves performance primarily by reducing interneuronal correlations. Nature Neuroscience, 12(12), 1594. Retrieved from https://idp.nature.com/authorize/casa?redirect_uri=https://www.nature.com/articles/nn.2439.pdf%3Forigin%3Dppub&casa_token=FXd9XF89RAkAAAAA:pPYlOgcBX7Fofxb-pCR4GdrGxXSgR5_j0jKSFnLLg5rMNAfnWrbmqvvOlG59DA0KdAlVu_ReSehFXw
Dale, A. M., Liu, A. K., Fischl, B. R., Buckner, R. L., Belliveau, J. W., Lewine, J. D., & Halgren, E. (2000). Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron, 26(1), 55–67.
Darki, F., Peyrard-Janvid, M., Matsson, H., Kere, J., & Klingberg, T. (2014). DCDC2 Polymorphism is associated with left temporoparietal gray and white matter structures during development. The Journal of Neuroscience, 34(43), 14455–14462. Retrieved from http://www.jneurosci.org/content/34/43/14455.short
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., ... & Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980.
Destrieux, C., Fischl, B., Dale, A., & Halgren, E. (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. https://doi.org/10.1016/j.neuroimage.2010.06.010
Engineer, C. T., Perez, C. A., Chen, Y. T. H., Carraway, R. S., Reed, A. C., Shetake, J. A., … Kilgard, M. P. (2008). Cortical activity patterns predict speech discrimination ability. Nature Neuroscience, 11(5), 603–608
Fischl, B. (2012). FreeSurfer. Neuroimage, 62(2), 774–781. Retrieved from https://www.sciencedirect.com/science/article/pii/S1053811912000389
Hamilton, L. S., Edwards, E., & Chang, E. F. (2018). A spatial map of onset and sustained responses to speech in the human superior temporal gyrus. Current Biology, 28(12), 1860-1871.e4. https://doi.org/10.1016/J.CUB.2018.04.033
Hancock, R., Pugh, K. R., & Hoeft, F. (2017). Neural noise hypothesis of developmental dyslexia. Trends in Cognitive Sciences, 21(6), 434–448. https://doi.org/10.1016/j.tics.2017.03.008
Hari, R., & Kiesilä, P. (1996). Deficit of temporal auditory processing in dyslexic adults. Neuroscience Letters, 205(2), 138–140.
Helenius, P., Uutela, K., & Hari, R. (1999). Auditory stream segregation in dyslexic adults. Brain, 122(5), 907–913.
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. Retrieved from http://www.nature.com/nrn/journal/v8/n5/abs/nrn2113.html
Hornickel, J., & Kraus, N. (2013). Unstable Representation of Sound: A Biological Marker of Dyslexia. The Journal of Neuroscience, 33(8), 3500–3504.
Huang, M. X., Mosher, J. C., & Leahy, R. M. (1999). A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Physics in Medicine & Biology, 44(2), 423.
Joanisse, M. F., Manis, F. R., Keating, P., & Seidenberg, M. S. (2000). Language deficits in dyslexic children: Speech perception, phonology, and morphology. Journal of Experimental Child Psychology, 77(1), 30–60.
Kaufman, A., & Kaufman, N. (2004). Kaufman brief intelligence test. Retrieved from https://doi.org/10.1002/9781118660584.ese1325/summary
Krauss, P., Tziridis, K., Schilling, A., & Schulze, H. (2018). Cross-Modal Stochastic Resonance as a Universal Principle to Enhance Sensory Processing. Frontiers in Neuroscience, 12, 578. https://doi.org/10.3389/fnins.2018.00578
Lam, S. S. Y., White-Schwoch, T., Zecker, S. G., Hornickel, J., & Kraus, N. (2017). Neural stability: A reflection of automaticity in reading. Neuropsychologia, 103, 162–167. https://doi.org/10.1016/j.neuropsychologia.2017.07.023
Lee, Y. S., Turkeltaub, P., Granger, R., & Raizada, R. D. S. (2012). Categorical speech processing in Broca’s area: An fMRI study using multivariate pattern-based analysis. Journal of Neuroscience, 32(11), 3942–3948. https://doi.org/10.1523/JNEUROSCI.3814-11.2012
Lehongre, K., Ramus, F., Villiermet, N., Schwartz, D., & Giraud, A. L. (2011). Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron, 72(6), 1080–1090.
Lin, F. H., Tsai, K. W. K., Chu, Y. H., Witzel, T., Nummenmaa, A., Raij, T., … Belliveau, J. W. (2012). Ultrafast inverse imaging techniques for fMRI. NeuroImage, 62(2), 699–705. https://doi.org/10.1016/j.neuroimage.2012.01.072
Martin, A., Schurz, M., Kronbichler, M., & Richlan, F. (2015). Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping, 36(5), 1963–1981. https://doi.org/10.1002/hbm.22749
Maurer, U., Bucher, K., Brem, S., & Brandeis, D. (2003). Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. NeuroReport, 14(17), 2245–2250. Retrieved from http://journals.lww.com/neuroreport/Abstract/2003/12020/Altered_responses_to_tone_and_phoneme_mismatch_in.22.aspx
McAdams, C., & Maunsell, J. (1999). Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron, 23(4), 765–773. Retrieved from https://www.sciencedirect.com/science/article/pii/S0896627301800349
McCarthy, J. H., Hogan, T. P., & Catts, H. W. (2012). Is weak oral language associated with poor spelling in school-age children with specific language impairment, dyslexia or both? Clinical Linguistics & Phonetics, 26(9), 791–805. https://doi.org/10.3109/02699206.2012.702185
Mesgarani, N., Cheung, C., Johnson, K., & Chang, E. F. (2014). Phonetic feature encoding in human superior temporal gyrus. Science (New York, N.Y.), 343(6174), 1006–1010. Retrieved from https://www.sciencemag.org/content/343/6174/1006.full.pdf%5Cnhttps://www.ncbi.nlm.nih.gov/pubmed/24482117
Neef, N. E., Müller, B., Liebig, J., Schaadt, G., Grigutsch, M., Gunter, T. C., … Friederici, A. D. (2017). Dyslexia risk gene relates to representation of sound in the auditory brainstem. Developmental Cognitive Neuroscience, 24, 63–71. https://doi.org/10.1016/j.dcn.2017.01.008
Neef, N. E., Schaadt, G., & Friederici, A. D. (2016). Auditory brainstem responses to stop consonants predict literacy. Clinical Neurophysiology, 128(3), 484–494. https://doi.org/10.1016/j.clinph.2016.12.007
Noordenbos, M., & Serniclaes, W. (2015). The categorical perception deficit in dyslexia: A meta-analysis. Scientific Studies of Reading, 19(5), 340–359. https://doi.org/10.1080/10888438.2015.1052455
Noordenbos, M. W., Segers, E., Serniclaes, W., & Verhoeven, L. (2013). Neural evidence of the allophonic mode of speech perception in adults with dyslexia. Clinical Neurophysiology, 124(6), 1151–1162.
Norton, E. S., Black, J. M., Stanley, L. M., Tanaka, H., Gabrieli, J. D. E., Sawyer, C., & Hoeft, F. (2014). Functional neuroanatomical evidence for the double-deficit hypothesis of developmental dyslexia. Neuropsychologia, 61(1), 235–246. https://doi.org/10.1016/j.neuropsychologia.2014.06.015
Ozernov-Palchik, O., Centanni, T. M., Beach, S. D., May, S., Hogan, T., & Gabrieli, J. D. E. (2021). Distinct neural substrates of individual differences in components of reading comprehension in adults with or without dyslexia. NeuroImage, 226(November 2020), 117570. https://doi.org/10.1016/j.neuroimage.2020.117570
Ozernov-Palchik, Ola, Beach, S. D., Brown, M., Centanni, T., Gaab, N., Kuperberg, G., … Gabrieli, J. (2021). Speech-specific perceptual adaptation deficits in children and adults with dyslexia. Psyarxiv.Com. https://doi.org/10.31234/OSF.IO/4N5EC
Ramus, F., Rosen, S., Dakin, S. C., Day, B. L., Castellote, J. M., White, S., & Frith, U. (2003). Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain, 126(4), 841–865.
Perez, C. A., Engineer, C. T., Jakkamsetti, V., Carraway, R. S., Perry, M. S., & Kilgard, M. P. (2012). Different Timescales for the Neural Coding of Consonant and Vowel Sounds. Cerebral Cortex, 23(3), 670–683.
Perrachione, T. K., Tufo, S. Del, Winter, R., Murtagh, J., Cyr, A., Chang, P., … Gabrieli, J. (2016). Dysfunction of Rapid Neural Adaptation in Dyslexia. Neuron, 92(6), 1383–1397
Peterson, R. L., & Pennington, B. F. (2015). Developmental dyslexia. Annual Review of Clinical Psychology, 283–307.
Picton, T. W., & Hillyard, S. A. (1974). Human auditory evoked potentials. II: Effects of attention. Electroencephalography and Clinical Neurophysiology, 36(C), 191–200. https://doi.org/10.1016/0013-4694(74)90156-4
Poeppel, D. (2003). The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time.” Speech Communication, 41(1), 245–255. Retrieved from http://www.sciencedirect.com/science/article/pii/S0167639302001073
Poghosyan, V., & Ioannides, A. A. (2008). Attention modulates earliest responses in the primary auditory and visual cortices. Neuron, 58(5), 802–813. https://doi.org/10.1016/j.neuron.2008.04.013
Raizada, R. D. S., & Poldrack, R. A. (2007). Selective amplification of stimulus differences during categorical processing of speech. Neuron, 56(4), 726–740. https://doi.org/10.1016/J.NEURON.2007.11.001
Renvall, H., & Hari, R. (2002). Auditory cortical responses to speech-like stimuli in dyslexic adults. Journal of Cognitive Neuroscience, 14(5), 757–768. https://doi.org/10.1162/08989290260138654
Reynolds, J. H., Pasternak, T., & Desimone, R. (2000). Attention increases sensitivity of V4 neurons. Neuron, 26(3), 703–714. https://doi.org/10.1016/S0896-6273(00)81206-4
Richlan, F., Kronbichler, M., & Wimmer, H. (2011). Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage, 56(3), 1735–1742. Retrieved from http://www.sciencedirect.com/science/article/pii/S1053811911001960
Rufener, K. S., Ruhnau, P., Heinze, H.-J., & Zaehle, T. (2017). Transcranial random noise stimulation (tRNS) shapes the processing of rapidly changing auditory information. Frontiers in Cellular Neuroscience, 11, 162. https://doi.org/10.3389/fncel.2017.00162
Ruff, S., Marie, N., Celsis, P., Cardebat, D., & Démonet, J.-F. (2003). Neural substrates of impaired categorical perception of phonemes in adult dyslexics: An fMRI study. Brain and Cognition, 53(2), 331–334. https://doi.org/10.1016/S0278-2626(03)00137-4
Russo, N. M., Nicol, T. G., Zecker, S. G., Hayes, E. A., & Kraus, N. (2005). Auditory training improves neural timing in the human brainstem. Behavioural Brain Research, 156(1), 95–103.
Schilling, A., Tziridis, K., Schulze, H., & Krauss, P. (2020). The Stochastic Resonance model of auditory perception: A unified explanation of tinnitus development, Zwicker tone illusion, and residual inhibition. BioRxiv. https://doi.org/10.1101/2020.03.27.011163
Schulte-Körne, G., Deimel, W., Bartling, J., & Remschmidt, H. (1999). The role of phonological awareness, speech perception, and auditory temporal processing for dyslexia. European Child & Adolescent Psychiatry, 8, 28–34.
Simos, P., Diehl, R., Breier, J., Molis, M., Zouridakis, G., & Papanicolaou, A. (1998). MEG correlates of categorical perception of a voice onset time continuum in humans. Cognitive Brain Research, 7(2), 215–219. Retrieved from https://www.sciencedirect.com/science/article/pii/S0926641098000378?casa_token=9ZO1Gz2nAL4AAAAA:XheLRIg37t-lSyhspzsCqqG-I2kCQ7en0wtJoPPDR_mMjJONMes-pLrgEZh2br5AIdD-IvA
Skeide, M. A., Kirsten, H., Kraft, I., Schaadt, G., Müller, B., Neef, N., … Friederici, A. D. (2015). Genetic dyslexia risk variant is related to neural connectivity patterns underlying phonological awareness in children. NeuroImage, 118, 414–421. https://doi.org/10.1016/j.neuroimage.2015.06.024
Snowling, M. (1998). Dyslexia as a phonological deficit: Evidence and implications. Child Psychology and Psychiatry Review, 3(1), 4–11. https://doi.org/10.1111/1475-3588.00201/abstract
Stephens, J. D. W., & Holt, L. L. (2011). A standard set of American-English voiced stop-consonant stimuli from morphed natural speech. Speech Communication, 53(6), 877–888. https://doi.org/10.1016/J.SPECOM.2011.02.007
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D., & Leahy, R. M. (2011). Brainstorm: A user-friendly application for MEG/EEG analysis. Computational Intelligence and Neuroscience, 2011, 8.
Tallal, P. (1980). Auditory temporal perception, phonics, and reading disabilities in children. Brain and Language, 9(2), 182–198.
Taulu, S., Kajola, M., & Simola, J. (2004). Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topography, 16, 269–275.
Taulu, S., & Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51(7), 1759.
Torgensen, J., Wagner, R., & Rashotte, C. (1999). Test of word reading efficiency (TOWRE). Austin, TX: Pro-Ed. Retrieved from https://scholar.google.com/scholar?hl=en&q=towre&btnG=&as_sdt=1%2C22&as_sdtp=#1
Travis, K., Leonard, M., & Chan, A. (2013). Independence of early speech processing from word meaning. Cerebral Cortex, 23(10), 2370–2379. Retrieved from http://cercor.oxfordjournals.org/content/23/10/2370.short
Turkeltaub, P. E., & Branch Coslett, H. (2010). Localization of sublexical speech perception components. Brain and Language, 114(1), 1–15. https://doi.org/10.1016/J.BANDL.2010.03.008
Vandermosten, M., Boets, B., Luts, H., Poelmans, H., Golestani, N., Wouters, J., & Ghesquière, P. (2010). Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proceedings of the National Academy of Sciences, 107(23), 10389.
Vandermosten, M., Boets, B., Luts, H., Poelmans, H., Wouters, J., & Ghesquière, P. (2011). Impairments in speech and nonspeech sound categorization in children with dyslexia are driven by temporal processing difficulties. Research in Developmental Disabilities, 32(2), 593–603. https://doi.org/10.1016/j.ridd.2010.12.015
Vandermosten, M., Correia, J., Vanderauwera, J., Wouters, J., Ghesquière, P., & Bonte, M. (2020). Brain activity patterns of phonemic representations are atypical in beginning readers with family risk for dyslexia. Developmental Science, 23(1), 1–15. https://doi.org/10.1111/desc.12857
Vellutino, F. R., Fletcher, J. M., Snowling, M. J., & Scanlon, D. M. (2004). Specific reading disability (dyslexia): What have we learned in the past four decades?. Journal of Child Psychology and Psychiatry, 45(1), 2–40.
Visscher, P., Brown, M., McCarthy, M., & Yang, J. (2012). Five years of GWAS discovery. The American Journal Of, 90(1), 7–24. Retrieved from http://www.sciencedirect.com/science/article/pii/S0002929711005337
Wagner, R. K., Torgesen, J. K., Rashotte, C. A., & Pearson, N. A. (1999). Comprehensive test of phonological processing: CTOPP. Austin, TX: Pro-ed.
Werker, J. F., & Tees, R. C. (1987). Speech perception in severely disabled and average reading children. Canadian Journal of Psychology/revue Canadienne De Psychologie, 41(1), 48.
Wiederholt, J., & Bryant, B. (2012). Gray Oral Reading Test- Fifth Edition (GORT-5). Pro-Ed.
Wolf, M., & Denckla, M. (2005). Rapid automatized naming and rapid alternating stimulus tests (RAN/RAS). Austin, TX: Pro-Ed. Retrieved from http://www.asha.org/SLP/assessment/Rapid-Automatized-Naming-and-Rapid-Alternating-Stimulus-Tests-(RAN-RAS).htm
Woodcock, R. (2011). Woodcock Reading Mastery Tests - (3rd ed.). Pearson.
Woodcock, R., McGrew, K., & Mather, N. (2001). Woodcock-Johnson® III NU Tests of Achievement. Retrieved from http://www.v-psyche.com/doc/IQ/Woodcock Johnson Achievement Test.doc
Yi, H. G., Leonard, M. K., & Chang, E. F. (2019). The Encoding of Speech Sounds in the Superior Temporal Gyrus. Neuron, 102(6), 1096–1110. https://doi.org/10.1016/J.NEURON.2019.04.023
Zuk, J., Perdue, M. V., Becker, B., Yu, X., Chang, M., Raschle, N. M., & Gaab, N. (2018). Neural correlates of phonological processing: Disrupted in children with dyslexia and enhanced in musically trained children. Developmental Cognitive Neuroscience, 34, 82–91. https://doi.org/10.1016/J.DCN.2018.07.001
Acknowledgements
We thank our participants, the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research (MIT), and Atsushi Takahashi and Steve Shannon for data collection technical support. We also thank Marina G. Monsivais, Sehyr Khan, and Karolina Wade for scoring the behavioral data. This project was funded by the Halis Foundation for Dyslexia Research at MIT (to J.D.E.G.) and NIH Shared instrumentation grant (S10OD021569).
Funding
This project was funded by the Halis Foundation for Dyslexia Research at MIT (to J.D.E.G.), NIH Shared instrumentation grant (S10OD021569), and NIH F32-HD100064 (to OOP).
Author information
Authors and Affiliations
Contributions
TMC, SDB, OOP, and JDEG designed the study. JDEG and DP were responsible for overseeing the neuroimaging facility. TMC, SDB, OOP, and SM collected the data. TMC analyzed the data and wrote the manuscript. All authors were involved in data interpretation and manuscript editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Ethics approval
All behavioral assessment and neural imaging procedures were approved by the Institutional Review Board of the Massachusetts Institute of Technology.
Consent to participate
All participants provided informed consent prior to participating in study activities.
Consent for publication
All authors approved the final version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Centanni, T.M., Beach, S.D., Ozernov-Palchik, O. et al. Categorical perception and influence of attention on neural consistency in response to speech sounds in adults with dyslexia. Ann. of Dyslexia 72, 56–78 (2022). https://doi.org/10.1007/s11881-021-00241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11881-021-00241-1