Abstract
This study focused on O3 variations and the titration effects of NOx during nighttime at urban, industrial, sub-urban and background sites. Nighttime O3 concentration variations and the presence of high particles with an aerodynamic diameter of less than 10 μm (PM10) were examined because haze disturbs the photochemical reactions of O3. Hourly data on O3, NO2, NO and PM10 concentrations provided by the Air Quality Division of the Department of Environment were divided into two groups of daytime and nighttime and analysed. The maximum O3 concentrations during daytime were generally observed during noon. At nighttime, the concentration of O3 decreased, indicating that destruction activities occurred mainly via titration. The retention of O3 during daytime caused the nighttime O3 during haze events to be higher than that during normal days. Apparent fluctuations in nighttime O3 concentrations were observed in the urban site (20 ± 13 ppb) during haze events. The NO2/NO ratio in the urban site during haze was higher than that on normal days; amongst the sites, the urban one had the highest value (6.6). Results indicated that during haze, the reactions between NO and O3 were enhanced at nighttime, leading to low nighttime NO concentrations. The low nighttime NO concentrations led to low nighttime NO titration rates, which enabled O3 to persist in ambient air. Nighttime O3 was not completely absent due to anthropogenic sources. This condition accelerated NO titration to NO2, thus promoting O3 production even during haze.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
O3 exists as a secondary pollutant in the lower atmosphere, where its formation and destruction highly depend on UV radiation and the intensity of its precursors, such as NOx (Ainsworth et al. 2012; Hassan et al. 2013; Alghamdi et al. 2014). Aside from being a secondary pollutant that requires UV light to complete its photochemical reactions, O3 is a noxious air pollutant and recognised as the second most significant air pollutant in Malaysia (Rahman 2013). O3 is toxic to humans and vegetation at the ground level due to its capability to oxidise biological tissues (Brimblecombe 2009; Pugliese et al. 2014).
The transformational characteristics of O3 during haze are crucial for understanding the role of this air pollutant in such an event. Haze conditions may trigger high O3 photochemical reactions, which intensify the effects of high particulate events resulting from large increments in ambient particulates and O3. McNaught and Wilkinson (1997) explained that haze is a phenomenon where the readings of an environmental application programme interface consecutively exceed 100 within a 72-h period or longer. They measured the level of visibility interference in the atmosphere by using the coefficient of haze. Quan et al. (2014) reported that stagnation weather, which is generally characterised by low wind speeds and decreased planetary boundary layer height, is the dominant factor in haze events, followed by high particulate emission. Wildfires or biomass burning from the open burning of agricultural residues and forest fires are the major sources of haze, especially in Southeast Asia (Velasco and Rastan 2015; Ahmed et al. 2016). Haze also originates from anthropogenic sources, which are mainly contributed by growing urbanisation and expanding economic activities (Rahman 2013).
In Malaysia, the high frequency of large-scale biomass burning adversely affects air quality. Several researchers have reported that the long-range transport of air pollution from Sumatera (Ashfold et al. 2017; Kusumaningtyas and Aldrian 2016; Dominick et al. 2015; Khan et al. 2016; Latif et al. 2012) and the prolonged dry weather in Southeast Asia reduce the air quality in Peninsular Malaysia and other neighbouring countries; for example, Singapore experienced extensive haze in June 2013 (DOE 2013; Rahman 2013). Awang et al. (2018) found that light scattering influences the amount of sunlight that penetrates the Earth and the formation of ground-level ozone. Given that ozone requires precursors for their formation, the frequent occurrence of haze as indicated by elevated air pollution index (API) levels essentially represents the rate of transformation of ozone in the atmosphere (Awang et al. 2018).
Theoretically, the O3 concentration in daytime is higher than that in nighttime because photochemical reactions could occur with the availability of insolation (Alghamdi et al. 2014; Kumar et al. 2015; Reddy and Pfister 2016). The absence of solar radiation during nighttime decreases the O3 photochemical reaction rate and O3 concentrations due to continuous chemical destruction by NO and titration (Ghosh et al. 2013). NO2 does not photolyse at night; as a result, the chemistry of the NOx family at night is entirely different from that during daytime. The steady state of O3 concentration is directly proportional to the NO2/NO ratio (Clapp and Jenkin 2001; Han et al. 2011). Han et al. (2011) reported that O3 concentration build-up is high in the presence of small values of [NO2]/[NO] and reaches stable conditions when the O3 concentration exceeds 90 ppb. However, during nighttime, NO2 and NO cease to be O3 precursors and become O3 reduction agents (Awang and Ramli 2017). Any NO present at night reacts rapidly with O3 (Eq. (1)). As a result, almost all NOx at night is converted to NO2, which reacts with O3 to produce a nitrate radical (NO3) (Eq. (2)) (Brown and Stutz 2012) and subsequently removes nighttime O3 concentration from ambient air.
Nighttime processes considerably influence the next day’s photochemistry and refine our understanding of the role of heterogeneous processes (reaction between gas- and particulate-phase constituents) occurring at night (Brown and Stutz 2012). Studies have found that up to 50% of freshly emitted NOx within a 24-h period in urban areas can be removed through nighttime processes (Stutz et al. 2010). NOx from anthropogenic sources, especially vehicle exhaust, is broken down during nighttime and form NO3, which is naturally destroyed at night (IDA 2010). Awang et al. (2015) recorded high nighttime O3 concentrations in Kemaman, Terengganu (Malaysia), due to inefficient removal mechanisms. Ineffective removal or depletion mechanisms cause high O3 concentrations to remain in the atmosphere and could exert negative effects. Awang and Ramli (2017) emphasized that NO and NO2 play essential roles in O3 elimination. Their results confirmed that high nighttime NO concentrations further enhance the destruction of nighttime O3 concentrations. Dey et al. (2017) found that the diurnal variation of O3 during nighttime is higher than that during daytime in an industrial site in Golgapur, India. According to Kulkarni et al. (2013), increased nighttime O3 concentration is associated with prevailing meteorological conditions and transport processes because the photochemical production of O3 ceases with the absence of sunlight. This explanation could be valid only when the supply of NO2 at night is constant.
Studies on nighttime O3 fluctuation during high particulate events (haze) are scarce in Malaysia. Previous studies have confirmed that aerosol particles affect the surface O3 concentration in Tianjin (Gu et al. 2010; Han et al. 2011). Recently, Awang et al. (2018) reported that haze disturbs the photochemical reactions of O3. The present study aims to assess variations in nighttime O3 concentrations during normal days and haze events in Malaysia.
Material and methods
Sampling sites
This study was conducted in four continuous air quality monitoring stations in Malaysia; these stations represent different categories of urban (Seremban (SB)), industrial (Petaling Jaya (PJ)), sub-urban (Muar (MR)) and background (Jerantut (JT)) sites located in Peninsular Malaysia, as shown in Fig. 1. The occurrence of haze for over 24 h was the primary criterion for selecting the study areas because haze is considered to occur once the API value exceeds 100 for 24 h. Figure 2 shows the differences in the trends of PM10 concentrations during normal days and haze events.
Sampling measurements
The data for this study, including hourly O3, NO2, NO and PM10 concentrations, were obtained from the Air Quality Division of the Department of Environment, Ministry of Natural Resources and Environment of Malaysia. These variables (NO2, NO and PM10 concentrations) were selected because of their relationship with O3 production (Clapp and Jenkin 2001; Ahamad et al. 2014). The haze events in three consecutive years (2013, 2014 and 2015) were regarded as the baseline of nighttime ground-level ozone variations during normal days and haze events. The occurrence of haze for over 24 h was the main criterion for selecting the study areas. Meanwhile, data for normal days refer to the remaining data for a particular month of the year. Daylight hours (daytime) were defined as the complete hours that fall between sunrise and sunset (Clapp and Jenkin 2001). Nighttime hours ranged from 7 p.m. to 7 a.m. (12 h), as used by Mohammed et al. (2013), Awang et al. (2015) and Awang and Ramli (2017) for the observation of nighttime O3 diurnal characteristics in Malaysia.
Hourly O3 concentration was measured with a UV absorption ozone analyser (Model 400E). The device was operated using the Beer–Lambert Law, which is based on the internal electronic resonance of O3 molecules with the absorption of 254 nm UV light for measuring low ranges of O3 concentration in ambient air (Ghazali et al. 2010; Mohammed et al. 2013). NO2 and NO concentrations were determined using a NO/NO2/NOx analyser (Model 200A) (Ghazali et al. 2010; Latif et al. 2014). PM10 concentration was determined with a BAM-1020 continuous particulate monitor (Met One Instruments, Inc., USA), a high-resolution equipment that can measure PM10 concentrations that are as low as 4.8 and 1.0 μg m−3 for 1 and 24 h, respectively (Latif et al. 2014).
Results and discussion
Nighttime and daytime ozone measurements
During haze, SB had the highest mean daytime and nighttime O3 concentrations with a mean value ± standard deviation of 41 ± 16 ppb and 20 ± 13 ppb, respectively, as shown in Table 1. During normal days, given that photochemical reactions occur during daytime, the O3 concentration was higher than that at nighttime. The nighttime O3 concentrations observed at this site are higher than the values reported for other cities, such as Kemaman (Awang et al. 2015, 2016), Kolkata (Ghosh et al. 2013) and Portugal (Kulkarni et al. 2013) on a typical day (14, 12 and 10 ppb, respectively). Usually, the lack of human activities at night reduces NO, which eventually leads to low titration (Yusoff et al. 2019).
During haze, the nighttime O3 concentrations were higher than those on normal days because the efficiency rate of NOx photochemical reactions increased due to daytime O3 retention. The variations in nighttime O3 concentrations in all the sites suggested that the O3 concentrations during haze were higher than those during normal days. High O3 concentrations are likely to be found in cities due to high emissions of O3 precursors (Dufour et al. 2010; Ran et al. 2012) and high occurrence frequency of haze (Awang et al. 2018).
The mean nighttime O3 concentrations ranged from 10 to 20 ppb. The minimum concentrations were observed from 4 to 6 a.m. Similar to the findings of He et al. (2012), the mean concentrations ranged from 6 to 12 ppb. Saxena and Ghosh (2011) reported that the lifetime of tropospheric O3 with minor amounts of NOx is approximately 4–5 days to 1–2 weeks, and hydrocarbons act as an O3 sink. Meanwhile, at the atmosphere, the lifetime of O3 is very short (typically hours), especially at high NOx concentrations (Young et al. 2013). These low concentrations of O3 are due to the absence of photochemical reactions that directly convert O3 precursors into O3 (Awang et al. 2015). In the current study, JT (background site) showed the lowest concentrations during normal days and haze, with mean nighttime O3 concentrations of 10 ± 7 ppb and 12 ± 7 ppb, respectively.
Diurnal variation in O3, NO, NO2 and PM10
Sunlight induces O3 photochemical reactions at daytime. Hence, the maximum O3 concentrations are recorded at noontime in all the sites, as shown in Fig. 3. Meanwhile, during nighttime, O3 concentrations are theoretically supposed to be zero due to the absence of photochemistry reactions and other destruction processes. However, in this study, high nighttime O3 concentrations were observed early at night (7 p.m.) and began to decrease until 10 p.m., suggesting that destruction activities occurred mainly through titration. Particles influenced the interaction of O3 and solar radiation, similar to the results of previous modelling experiments (Li et al. 2011; Péré et al. 2015) that showed that particles decrease daytime O3 concentrations by 2–17%. The O3 nighttime chemistry is a new piece of the air quality puzzle. According to Brown and Stutz (2012), nighttime cleansing is slowed down by 7% by the effects of light pollution in Los Angeles, thus increasing O3 formation the next day by up to 5%. Similarly, nighttime O3 variation was observed in the current study during haze, and the concentration recorded during haze was higher than that during normal days at all sites. The nighttime O3 concentrations during haze were influenced by the rate of removal reactions but were unchanged even under high NOx reactions.
During daytime, the NO2 peaks occurred about 1–2 h after the NO peak, and the O3 peak emerged 6 h after the NO peak (Han et al. 2011; Banan et al. 2013). At nighttime, the NO2 peaks usually occurred 3 h before the NO peak and about 2 h after the O3 peak in all the sites. This pattern may be due to the nocturnal planetary boundary layer, where the surface emissions of NO are retained under this inversion and cause the hourly NOx concentration to increase during nighttime. In many instances, these variations depend on site location, local emission of precursor gases (An et al. 2015; Wang et al. 2017; Zhao et al. 2018) and meteorological conditions (Yusoff et al. 2019; Reddy and Pfister 2016; Li et al. 2017).
NO and NO2 during nighttime in the current study exhibited the same trend as those in the work of Han et al. (2011), Awang and Ramli (2018) and Awang et al. (2018). They began increasing with the destruction of O3. The variations during normal days and haze were similar, and NO2 was always higher than NO. Two peaks (i.e. 8–9 a.m. and 9–10 p.m.) were observed in all the sites, and the maximum NO2 mean concentrations ranged from 5 to 47 ppb. The fluctuation in the urban site was clearer than that in the industrial, sub-urban and background sites during haze. This finding is similar to that obtained by Awang et al. (2018), who reported that urban sites have higher PM10 concentrations than other sites.
Table 2 shows the Pearson correlation results of nighttime O3 concentrations with NO, NO2, PM10, temperature, relative humidity and wind speed. During nighttime, temperature exerted a direct effect on O3 formation (positively correlated). During haze, O3 and PM10 were positively correlated. The highest association was recorded in the background site (JT with 0.86), and the urban site (SB) exhibited the lowest association of 0.52. Meanwhile, a negative correlation between O3 concentration and NO, NO2 and relative humidity was found during normal days and haze in all the sites. Thus, the production of O3 during nighttime could be associated with the decrease in NO, NO2 and relative humidity. High relative humidity could enhance O3 cleansing through wet deposition. Similar findings were obtained by Toh et al. (2013) and Awang et al. (2015).
Nighttime NO2/NO and O3/NO ratio
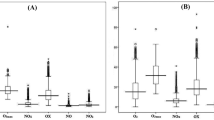
Figure 4 shows the mean ratios of NO2/NO and O3/NO for all the sites. The concentrations of O3 increased as the ratio of NO2/NO increased. All sites showed higher NO2/NO ratios during haze than during normal days, and the urban site (SB) had the highest value (6.6). These results suggest that haze enhanced the reactions between NO and O3, resulting in reduced nighttime NO in air. Atkinson (2000) indicated that during nighttime, NO reacts with O3 and produces NO2, which in turn produces NO3 and N2O5. Evidently, net production of O3 still occurs after these conversions.
In the present study, a high NO2 was recorded in the urban site (SB) due to the contribution of anthropogenic sources, particularly from the downtown site, during haze. Given that NO titration is limited during haze (Awang et al. 2018), the conversion of NO into NO2 is also limited. This observation is supported by the findings of Khalil et al. (2018). They studied nighttime O3 concentrations in Makkah and found that nighttime activities in all cities cause high NO2 and NO concentrations and contribute to the high O3 concentration at night. Low nighttime NO titration rates could reduce nighttime O3 (Banan et al. 2013), thus enabling O3 to persist in ambient air.
Conclusions
This study revealed fluctuations in nighttime O3 concentrations, especially in urban sites (SB). The mean nighttime O3 concentrations ranged from 10 ± 7 ppb to 20 ± 13 ppb. The minimum levels during daytime were observed from 4 to 6 a.m., with the mean levels ranging from 6 to 12 ppb. The daytime O3 level affected the nighttime O3 level even during haze but at different magnitudes; the value during haze was higher than that during normal days in all the sites. All of the sites showed higher NO2/NO ratio during haze than during normal days, with the urban site (SB) having the highest recorded value (6.6). Increased NO may result in high levels of nighttime O3. During haze, the enhanced reactions between NO and O3 at nighttime lead to low nighttime NO concentrations. Thus, we suspect that additional anthropogenic sources accelerate NO titration to NO2 and directly increase the O3 concentrations during nighttime in haze events.
References
Ahamad F, Latif MT, Tang R, Juneng L, Dominick D, Juahir H (2014) Variation of surface ozone exceedance around Klang Valley, Malaysia. Atmos Res 139:116–127. https://doi.org/10.1016/j.atmosres.2014.01.003
Ahmed M, Guo X, Zhao XM (2016) Determination and analysis of trace metals and surfactant in air particulate matter during biomass burning haze episode in Malaysia. Atmos Environ 141:219–229
Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu Rev Plant Biol 63:637–661
Alghamdi M, Khoder M, Harrison RM, Hyvärinen AP, Hussein T, Al-Jeelani H, Almehmadi F (2014) Temporal variations of O3 and NOx in the urban background atmosphere of the coastal city Jeddah, Saudi Arabia. Atmos Environ 94:205–214. https://doi.org/10.1016/j.atmosenv.2014.03.029
An J, Zou J, Wang J, Lin X, Zhu B (2015) Differences in ozone photochemical characteristics between the megacity Nanjing and its suburban surroundings, Yangtze River Delta, China. Environ Sci Poll Res 22(24):19607–19617
Ashfold M, Latif M, Samah A, Mead M, Harris N (2017) Influence of northeast monsoon cold surges on air quality in Southeast Asia. Atmos Environ 166:498–509
Atkinson R (2000) Atmospheric chemistry of VOCs and NOx. Atmos Environ 34(12):2063–2101
Awang NR, Ramli NA (2017) Preliminary study of ground-level ozone nighttime removal process in an urban area. J Trop Resour Sustain Sci 5:83–88
Awang NR, Ramli NA (2018) Ground-level ozone in Malaysia: fluctuational and transformational characteristics. School of Civil Engineering. Universiti Sains Malaysia. ISBN: 978-967-0167-21-3.
Awang NR, Ramli NA, Yahaya AS, Elbayoumi M (2015) Multivariate methods to ground-level ozone during the daytime, nighttime, and critical conversion time in urban areas. Atmos Pollut Res 6(5):726–734. https://doi.org/10.5094/APR.2015.081
Awang NR, Elbayoumi M, Ramli NA, Yahaya AS (2016) Diurnal variations of ground-level ozone in three port cities in Malaysia. Air Qual Atmos Health 9(1):25–39
Awang NR, Ramli NA, Shith S, Zainordin NS, Manogaran H (2018) Transformational characteristics of ground-level ozone during high particulate events in the urban area of Malaysia. Air Qual Atmos Health 11(6):715–727
Banan N, Latif MT, Juneng L, Ahamad F (2013) Characteristics of surface ozone concentrations at stations with different backgrounds in the Malaysian Peninsula. Aerosol Air Qual Res 13(3):1090–1106. https://doi.org/10.4209/aaqr.2012.09.0259
Brimblecombe P (2009) Transformation in understanding the health impacts of air pollutants in the 20th century. In: Paper Presented at the EPJ Web of Conferences.
Brown SS, Stutz J (2012) Nighttime radical observations and chemistry. Chem Soc Rev 41(19):6405–6447. https://doi.org/10.1039/C2CS35181A
Clapp LJ, Jenkin ME (2001) Analysis of the relationship between ambient levels of O3, NO2 and NO as a function of NOx in the UK. Atmos Environ 35:6391–6405. https://doi.org/10.1016/S1352-2310(01)00378-8
Dey S, Sibanda P, Gupta S, Chakraborty A (2017) Occurrences of high nocturnal surface ozone at a tropical urban area. Inter J Environ Eco Eng 11(2):97–105. https://doi.org/10.5281/zenodo.1339752
DOE (2013) Department of environment, Malaysia. Malaysia environmental quality report 2013. In: M. O. S. Department of Environment, Technology and the Environment, Malaysia
Dominick D, Latif MT, Juneng L, Khan MF, Amil N, Mead MI, Nadzir MSM, Moi PS, Samah AA, Ashfold MJ, Sturges WT, Harris NRP, Robinson AD, Pyle JA (2015) Characterisation of particle mass and number concentration on the east coast of the Malaysian Peninsula during the Northeast monsoon. Atmos Environ 117:187–199
Dufour G, Fremenko M, Orphan J, Flaud J (2010) IASI observations of seasonal and day-to-day variations of tropospheric ozone over three highly populated areas of China: Beijing, Shanghai, and Hong Kong. Atmos Chem Phys 10:3787–3801. https://doi.org/10.5194/acp-10-3787-2010
Ghazali NA, Ramli NA, Yahaya AS, Md Yusof NFF, Sansuddin N, Al Madhoun W (2010) Transformation of nitrogen dioxide into ozone and prediction of ozone concentrations using multiple linear regression techniques. Environ Monit Assess 165(1):475–489. https://doi.org/10.1007/s10661-009-0960-3
Ghosh D, Lal S, Sarkar U (2013) High nocturnal ozone levels at a surface site in Kolkata, India: trade-off between meteorology and specific nocturnal chemistry. Urban Clim 5:82–103. https://doi.org/10.1016/j.uclim.2013.07.002
Gu JX, Bai ZP, Liu AX, Wu LP, Xie YY, Li WF, Dong HY, Zhang X (2010) Characterization of atmospheric organic carbon and element carbon of PM2.5 and PM10 at Tianjin, China. Aerosol Air Qual Res 10:167–176. https://doi.org/10.4209/aaqr.2009.12.0080
Han S, Bian H, Feng Y, Liu A, Li X, Zeng F, Zhang X (2011) Analysis of the relationship between O3, NO and NO2 in Tianjin China. Aerosol Air Qual Res 11:128–139. https://doi.org/10.4209/aaqr.2010.07.0055
Hassan IA, Basahi JM, Ismail IM, Habeebullah TM (2013) Spatial distribution and temporal variation in ambient ozone and its associated NOx in the atmosphere of Jeddah City, Saudi Arabia. Aerosol Air Qual Res 13:1712–1722
He J, Wang Y, Hao J, Shen L, Wang L (2012) Variations of surface O3 in August at a rural site near Shanghai: influences from the West Pacific subtropical high and anthropogenic emissions. Environ Sci Poll Res 19(9):4016–4029
IDA (2010) International Dark-sky Association: City light pollution affects air pollution. Available on http://banbillboardblight.org/wp-content/uploads/2010/12/Light-Pollution-Effects-Air-Pollution.pdf. 15th December 2010, Access on April 2018, 2010
Khalil MAK, Butenhoff CL, Harrison RM (2018) Ozone balances in urban Saudi Arabia. Climate and Atmospheric Science 1(1):27. https://doi.org/10.1038/s41612-018-0034-8
Khan M, Latif M, Saw W, Amil N, Nadzir M, Sahani M, Tahir N, Chung J (2016) Fine particulate matter in the tropical environment: monsoonal effects, source apportionment, and health risk assessment. Atmos Chem Phys 16:597–617
Kulkarni PS, Bortoli D, Silva AM (2013) Nocturnal surface ozone enhancement and trend over urban and suburban sites in Portugal. Atmos Environ 71:251–259. https://doi.org/10.1016/j.atmosenv.2013.01.051
Kumar A, Singh D, Singh BP, Singh M, Anandam K, Kumar K, Jain VK (2015) Spatial and temporal variability of surface ozone and nitrogen oxides in urban and rural ambient air of Delhi-NCR, India. Air Qual Atmos Health 8(4):391–399
Kusumaningtyas SDA, Aldrian E (2016) Impact of the June 2013 Riau province Sumatera smoke haze event on regional air pollution. Environ Res Lett 11(7):075007
Latif MT, Huey LS, Juneng L (2012) Variations of surface ozone concentration across the Klang Valley, Malaysia. Atmos Environ 61:434–445
Latif MT, Dominick D, Ahamad F, Khan MF, Juneng L, Hamzah FM, Nadzir MSM (2014) Long term assessment of air quality from a background station on the Malaysian Peninsula. Sci Total Environ 482:336–348. https://doi.org/10.1016/j.scitotenv.2014.02.132.
Li G, Bei N, Tie X, Molina LT (2011) Aerosols effects on the photochemistry in Mexico City during the MCMA-2006/MILAGRO campaign. Atmos Chem Phys 11:5169–5182. https://doi.org/10.5194/acp-11-5169-2011.
Li G, Bei N, Cao J, Wu J, Long X, Feng T, Dai W, Liu S, Zhang Q, Tie X (2017) Wide-spread and persistent ozone pollution in eastern China during the non-winter season of 2015: observations and source attributions. Atmos Chem Phys 17:2759–2774. https://doi.org/10.5194/acp-17-2759-2017.
McNaught AD, Wilkinson A (1997) IUPAC. In: Compendium of chemical terminology, 2nd edn. (the BGold book^). Blackwell Scientific Publications, Oxford.
Mohammed NI, Ramli NA, Yahya AS (2013) Ozone phytotoxicity evaluation and prediction of crops production in tropical regions. Atmos Environ 68:343–349. https://doi.org/10.1016/j.atmosenv.2012.09.010
Péré JC, Bessagnet B, Pont V, Mallet M, Minvielle F (2015) Influence of the aerosol solar extinction on photochemistry during the 2010 Russian wildfires episode. Atmos Chem Phys 15(19):10983–10998. https://doi.org/10.5194/acp-15-10983-2015
Pugliese SC, Murphy JG, Geddes JA, Wang JM (2014) The impacts of precursor reduction and meteorology on ground-level ozone in the greater Toronto area. Atmos Chem Phys 14:8197–8207
Quan J, Tie X, Zhang Q, Liu Q, Li X, Gao Y, Zhao D (2014) Characteristics of heavy aerosol pollution during the 2012–2013 winter in Beijing, China. Atmos Environ 88:83–89
Rahman HA (2013) Haze phenomenon in Malaysia: domestic or transboundary factor? In: Paper Presented at the 3rd International Journal Conference on Chemical Engineering and its Applications (ICCEA’13), Phuket (Thailand), 597–599
Ran L, Zhao C, Xu W, Han M, Lu X, Han S, Lin W, Xu X, Gao W, Yu Q, Geng F, Ma N, Deng Z, Chen J (2012) Ozone production in summer in the megacities of Tianjin and Shanghai, China: a comparative study. Atmos Chem Phys 12:7531–7542. https://doi.org/10.5194/acp-12-7531-2012
Reddy PJ, Pfister GG (2016) Meteorological factors contributing to the interannual variability of mid-summer surface ozone in Colorado, Utah, and other western U.S. states. J Geophys Res Atmos 121:2434–2456. https://doi.org/10.1002/2015JD023840
Saxena P, Ghosh C (2011) Variation in the concentration of ground-level ozone at selected sites in Delhi. Inter J Environ Sci 1(7):1899–1911
Stutz J, Wong KW, Lawrence L, Ziemba L, Flynn JH, Rappenglück B, Lefer B (2010) Nocturnal NO3 radical chemistry in Houston, TX. Atmos Environ 44(33):4099–4106. https://doi.org/10.1016/j.atmosenv.2009.03.004
Toh YY, Lim SF, von Glasow R (2013) The influence of meteorological factors and biomass burning on surface ozone concentrations at Tanah Rata, Malaysia. Atmos Environ 70:435–446. https://doi.org/10.1016/j.atmosenv.2013.01.018
Velasco E, Rastan S (2015) Air quality in Singapore during the 2013 smoke-haze episode over the Strait of Malacca: lessons learned. Sustain Cities Society 17:122–131
Wang T, Xue L, Brimblecombe P, Lam YF, Li L, Zhang L (2017) Ozone pollution in China: a review of concentrations, meteorological influences, chemical precursors, and effects. Sci Total Environ 575:1582–1596. https://doi.org/10.1016/j.scitotenv.2016.10.081
Young PJ, Archibald AT, Bowman KW, Lamarque JF, Naik V, Stevenson DS, Tilmes S, Voulgarakis A, Wild O, Bergmann D, Cameron-Smith P, Cionni I, Collins WJ, Dalsøren SB, Doherty RM, Eyring V, Faluvegi G, Horowitz LW, Josse B, Lee YH, MacKenzie IA, Nagashima T, Plummer DA, Righi M, Rumbold ST, Skeie RB, Shindell DT, Strode SA, Sudo K, Szopa S, Zeng G (2013) Pre-industrial to end 21st-century projections of tropospheric ozone from the atmospheric chemistry and climate model intercomparison project (ACCMIP). Atmos Chem Phys 13:2063–2090. https://doi.org/10.5194/acp-13-2063-2013
Yusoff MF, Latif MT, Juneng L, Khan MF, Ahamad F, Chung JX, Mohtar AAA (2019) Spatio-temporal assessment of nocturnal surface ozone in Malaysia. Atmos Environ 207:105–116
Zhao S, Yu Y, Yin D, Qin D, He J, Dong L (2018) Spatial patterns and temporal variations of six criteria air pollutants during 2015 to 2017 in the city clusters of Sichuan Basin, China. Sci Total Environ 624:540–557. https://doi.org/10.1016/j.scitotenv.2017.12.172
Acknowledgements
The authors would like to express their gratitude to Universiti Sains Malaysia, Universiti Malaysia Kelantan and the Department of Environment, Malaysia.
Funding
This study was funded by the Research University Individual Grant (1001/PAWAM/814278) and the Fundamental Research Grant Scheme (R/FRGS/10800/01525A/003/2018/00554).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shith, S., Awang, N.R., Latif, M.T. et al. Fluctuations in nighttime ground-level ozone concentrations during haze events in Malaysia. Air Qual Atmos Health 14, 19–26 (2021). https://doi.org/10.1007/s11869-020-00908-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-020-00908-5