Abstract
Nanchang, the capital of Jiangxi Province, is a major constituent of the South China Acid Rain Zone. In this study, the chemical and sulfur isotopic compositions of precipitation in this region were investigated during 2015–2016 to clarify the origin of major chemicals. The pH was < 5.6 for 72.6% of precipitation events, reflecting the predominance of acid rain. SO42−, Ca2+, NH4+, and NO3− were the four main ions, occupying 77% of the total ions in precipitation. SO42− and NO3− were the dominant acidifying species while Ca2+ and NH4+ were the dominant neutralizing species. Overall, the concentrations of ions in precipitation were lower in the rainy months than in other months. Compared with foreign cities, the concentrations of ions in precipitation, particularly SO42− and NO3−, were significantly higher in Nanchang, indicating the effect of anthropogenic pollution. Factor analyses showed that in precipitation, anthropogenic pollutants controlled SO42−, NO3−, and NH4+ whereas K+, Mg2+, and Ca2+ originated from rock weathering, and Cl− was dominantly of marine origin. Based on the sulfur isotope data, it was further found that precipitation SO42− was associated with the use of northern Chinese coal in Nanchang and homogeneous oxidation was a major mechanism affecting δ34S fluctuations in precipitation SO42− with time. This work provides deep insights into the formation of acid rain and is helpful for guiding air quality protection in South China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With rapid economic growth and massive emissions of pollutants, acid rain has been a major environmental problem in China since the late 1980s (Kuribayashi et al. 2012). It was reported that precipitation pH values were < 4.5 for more than half of the cities of South China and less than 7 in many cities of northern China (Wu and Han 2015; Wu et al. 2016). Sulfur dioxide (SO2), which can be converted into sulfate (SO42−) dissolved in precipitation, is an important precursor of acid rain, originating from fossil fuel combustion, industrial emissions, and biomass burning (Yu et al. 2016). SO2 emissions in China have had a significantly increasing trend since 1980, reaching over 33 million tons in 2006 (Duan et al. 2016). To prevent acid pollution, the Chinese government has made great efforts in energy structure changes, energy savings, and emission reductions since the mid-1990s. As a result, SO2 emissions started to decrease in 2006 but still remained at historically higher levels (Kurokawa et al. 2013). Therefore, the provenance, transport, and deposition of air pollutants associated with acid rain are still topics of concern in China (Gu et al. 2014; Niu et al. 2017).
Precipitation constitutes a natural pathway for removing atmospheric pollutants and captures soluble components from gases and particles in the atmosphere (Lynam et al. 2014; Sinha et al. 2008; Han et al. 2019). Dissolved ions in precipitation are usually of natural or anthropogenic origin. For example, Ca2+ and Mg2+ are considered to be derived from soil dust while SO42− and NO3− are produced by human activities such as coal combustion and vehicle exhaust (Ciężka et al. 2016). Therefore, precipitation chemistry can help us understand the cycles of air pollutants. Moreover, it is fundamental for assessing air quality. Sulfate (SO42−) is a dominant acidic component in acid rain and usually has different sources with different δ34S values (Novák et al. 2001; Mukai et al. 2001; Norman et al. 2004). Furthermore, sulfur isotope fractionation occurs during atmospheric oxidation of SO2 to SO42− (Harris et al. 2013). Therefore, the use of sulfur isotopes can provide information on the provenance and formation of sulfate in precipitation. Precipitation chemistry with sulfur isotopes has been widely used to trace the provenance, transport, and deposition of air pollutants in the world, including China (Sinha et al. 2008; Wu and Han 2015; Górka et al. 2017; Han et al. 2019). Nevertheless, sulfur isotopic and chemical investigations of precipitation are still incipient in China, considering its large area affected by acid rain.
Nanchang, the capital of Jiangxi Province, lies in the South China Acid Rain Zone (Duan et al. 2016). Chemical and isotopic studies on air pollutants dissolved in precipitation in Nanchang are limited (Xiao et al. 2011). In this study, precipitation samples were collected during 2015–2016 in Nanchang to (1) ascertain the chemical and sulfur isotopic characteristics of precipitation, (2) determine the origin of major chemicals and (3) explore sulfur isotopes as tracers of dissolved sulfates.
Study area
Nanchang, with a population of approximately 7 million residents, is located to the southwest of Lake Poyang (Fig. 1). It is subtropically wet and mild, with a mean annual temperature and precipitation of 18 °C and 1600 mm, respectively, in Nanchang, according to meteorological data (http://www.weather.com.cn/). It usually rains from April to July. The dominant wind is southerly in summer and northerly in winter. The urban land area, local population, fertilizer use, coal consumption, and industrial GDP increased by 2.3 times, 17%, 12%, 200%, and 760%, respectively from 2005 to 2015 in Nanchang according to statistical data (http://tjj.jiangxi.gov.cn).
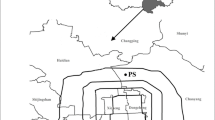
Map showing a the pH distribution during 2016 in China, b the area of Jiangxi Province, and c the Nanchang region and the sampling point. The pH data were from China National Environmental Monitoring Center (http://www.cnemc.cn/)
Samples and methods
Sampling and pretreatment
Precipitation samples were collected on the roof of a teaching building at the Nanchang Institute of Technology during Nov. 2015 and Oct. 2016. Prior to use, the sampler was cleaned with 2 N HCl solution and 18.2 MΩ·cm Milli-Q water. Between rain events, the sampler was closed to avoid dry deposition. In total, 95 samples were taken for chemical analysis. Of these, 71 samples were enough for sulfur isotope analysis. All samples were filtered through a 0.45-μm Millipore membrane. The filtrate was separated into three aliquots for each sample: one was directly used for anion measurement, one was acidified to pH < 2 with 2 N HCl solution for cation measurement, and the remaining aliquot was acidified to pH < 2 for sulfur isotopic analysis after it was poisoned with HgCl2 solution to inhibit microbial activities. The third aliquot was further treated to obtain purified BaSO4 powder samples for δ34S determination according to the method of Xiao et al. (2011).
Chemical and sulfur isotopic analyses
The pH and Ec (electrical conductivity) values were measured in situ by a digital pH meter (PHJS-4A, Jisi, China) with an error of 0.01 and a digital conductivity meter (DDS-IIA, Xiangtian, China) with an error of 1 μS/cm, respectively. Major cations (K+, Na+, Ca2+, Mg2+) were measured by inductively coupled plasma atomic emission spectroscopy (ICP 6300, Thermo, USA). Major anions (F−, C1−, NO3−, SO42−) were measured by using ion chromatography (ICS-2100, Dionex, USA). Reagent and procedural blanks were analyzed in parallel with sample treatments using identical procedures. The analytical precision was better than ± 3%. The NH4+ concentration was determined by using the Nessler colorimetric-spectrophotometric method with an analytical precision of better than ± 5%. The H+ concentration was calculated using the equation, H+ = 10pH (μeq/L).
Sulfate δ34S analysis was performed on an EA-IRMS (EA3000, IsoPrime, Germany) mass spectrometer at the Institute of Geochemistry, Chinese Academy of Sciences. The analytical procedure was described in detail by Grassineau et al. (2001). The δ34S data were reported on the VCDT scale after normalization using three international standards (IAEA-SO5, IAEA-SO6 and NBS127). The standard deviation for δ34S determination was below 0.2‰ (n = 10, one sigma). The sulfur isotopic composition was calculated as follows:
where x and VCDT stand for samples and Vienna Canyon Diablo Troilite (standard material), respectively.
Statistical treatment and backward trajectory analysis
The volume-weighted mean (VWM) values of the species (pH, ions and δ34S) were calculated by the formula: XVWM = (X1 P1 + X2 P2 + … + Xi Pi)/(P1+ P2 + …Pi), where Pi is the precipitation amount corresponding to the ith sample and Xi is the concentration of the species in the ith sample.
The HYSPLIT (Hybrid Single Particle Lagrangian Integrated Trajectory) model devised by the NOAA Air Resources Laboratory (https://ready.arl.noaa.gov/HYSPLIT.php) was used to calculate each backward trajectory of air masses during precipitation events for a 72-h duration with a final level of 1000 m above ground level. The percent frequency of wind was calculated by \( \left(\sum \limits_{i=1}^n{W}_i\right)/{W}_t \), where Wi means the ith occurrence of wind in a given area and Wt is the total occurrences of wind in all areas during a given season. The amount (percentage) of precipitation was determined by \( \left(\sum \limits_{i=1}^n{P}_i\right)/{P}_t \), where Pi means the ith precipitation amount in a given area and Pt is the total precipitation in all areas during a given season. The SO42− concentration or δ34S was described by \( \left(\sum \limits_{i=1}^n{X}_i\times {P}_i\right)/{P}_t \), where Xi and Pi are the SO42− concentration (δ34S) and amount of the ith precipitation measurement, respectively, in a given area.
Results and discussion
pH, Ec, and ionic composition of precipitation
The pH values of precipitation samples collected during 2015–2016 in Nanchang ranged from 3.6 to 7.0 with a VWM value of 5.1 (Table 1), and the values were lower and higher than 5.6 for 72.6% and 27.4% of the samples, respectively (Table S1). Samples with pH values below 5.6 reflect the impact of anthropogenic pollution, whereas samples with pH values above 5.6 indicate the addition of alkaline species (Charlson and Rodhe 1982). Overall, precipitation pH values were lower in the rainy months than in the other months (Fig. 2). The VWM pH value of precipitation in Nanchang approximated those reported for Chengdu and Nanjing, and was higher than those in other large cities of South China but lower than those in cities of North China (Table S2). Compared with overseas monitoring data, the precipitation pH value in Nanchang was higher than those in New York and Tokyo, close to that in Belgium but lower than those in other cities (Table S2). The Ec values of precipitation samples ranged from 3 to 86 μS cm−1 with a VWM of 16 μS cm−1, and only approximately 16% of the values were greater than 50 μS cm−1 during 2015–2016 in Nanchang (Table 1). The Ec value of precipitation in Nanchang was roughly similar to those in cities of South China but lower than those in cities of North China (Han et al. 2010; Wu et al. 2016; Niu et al. 2017).
The total cation concentrations (TZ+) varied from 35 to 1448 μeq l−1, and the total anion concentrations (TZ−) varied from 32.7 to 1201 μeq l−1 (Table 1). The mean equivalent ratio of TZ−/TZ+ was 0.95. The small imbalance between cations and anions was attributed to unmeasured anionic species such as bicarbonate, acetate, and formate. On average, the concentrations of measured ionic species in precipitation decreased in the order of SO42− > Ca2+ > NH4+ > NO3− > H+ > Cl− > Na+ > Mg2+ > K+ > F− (Table 1). The former four ions were the dominant constituents, accounting for 77% of the total ions in precipitation (Fig. S1). NO3− and SO42− explained 84.9% of the total anions and were thus the main acidifying factors while Ca2+ and NH4+ comprised 69.43% of the total cations and were thus the main neutralizing factors (Fig. S1). The VWM values of chemical species in precipitation were lower than the arithmetic mean values, implying links between low ion concentrations and high precipitation. In addition to pH, the concentrations of ions such as SO42−, NO3−, Ca2+, and NH4+ were lower in the rainy months than in the other months (Fig. 2). Among cities in China, the precipitation of Nanchang was moderate in terms of ion concentrations (Table S2). The SO42−, NO3−, Ca2+, and NH4+ concentrations of precipitation in Nanchang were higher than those in Maolan, which is located in the acid rain zone of China (Table S2). The ion concentrations of Nanchang precipitation, especially SO42− and NO3−, were also significantly higher than those of some megacities in Europe and America (Table S2). These results show that the precipitation chemistry of Nanchang was affected to a large degree by anthropogenic pollution.
Origin of major ions in precipitation
Based on factor analyses with varimax rotation of standardized component loadings, three major factors with eigenvalues > 0.9 were extracted for the precipitation chemistry of Nanchang and accounted for 77.7% of the total variance (Table 2).
Factor 1 was characterized by high positive loadings for major anions (SO42− and NO3−) and major cations (Ca2+, Mg2+, K+, and NH4+) and a negative loading for precipitation amount (Table 3). There were positive correlations between SO42−, NO3−, and NH4+ (Table 3). Furthermore, SO42− and NO3−, which are usually considered to be two sensitive indicators of anthropogenic pollution (Ciężka et al. 2016), were remarkably enriched in precipitation relative to marine and crustal sources (Table S3). In addition, NH4+ in precipitation has been ascribed to biomass burning, fertilizer usage, and human excrement (Ciężka et al. 2016). Therefore, the high loadings for SO42−, NO3−, and NH4+ implied the predominance of acid and alkaline pollutants from anthropogenic sources. Ca2+, Mg2+, and K+ were positively correlated with one another and were concentrated in precipitation compared with seawater values (Table 3 and Table S3). Ca2+ and Mg2+ result from silicate weathering and are also enriched in carbonate dust (Han et al. 2019). K+ could be sourced partly from biological burning because it was correlated with NH4+ (Table 3). However, K+ in precipitation was mainly related to the weathering of silicates in soil dust since sylvites are scarce in Nanchang. Therefore, the high loadings of K+, Ca2+, and Mg2+ were associated with crustal sources. Factor 1 revealed two aspects: (1) crust-sourced dust and anthropogenic particulates were jointly carried into the atmosphere by wind, which was supported by positive correlations of major anions with major cations (Table 3), and (2) there was a dilution effect of precipitation on ion concentrations because almost all ions were negatively correlated with precipitation amount to some degree (Table 3).
Factor 2 showed positive loadings on Cl−, Na+, SO42−, and NH4+ (Table 2). Cl− was positively correlated with SO42+ and NH4+ and had a certain correlation with Na+ (Table 3). Moreover, Cl− was slightly and significantly enriched in precipitation relative to the seawater and crust values, respectively (Table S3). Therefore, Cl− in precipitation originated partly from anthropogenic pollution but was predominantly of marine origin. Na+ is the best reference ion for seawater; however, its EFcrust value is 0.371 (Table S3). Thus, a small amount of Na+ in precipitation could be from crustal sources. As a whole, the positive loadings for Cl−, Na+, SO42−, and NH4+ reflected a combined effect of sea salt and anthropogenic sources.
Factor 3 had positive loadings for Ca2+ and pH (Table 2). Ca2+ increased as the pH value increased with a correlation coefficient of r = 0.51 (Table 3). Ca2+ is a typical alkaline cation of crustal origin, and pH is an indicator of precipitation acidity (Al-Khashman 2005). Therefore, factor 3 emphasized the effect of acid rain on Ca-bearing materials, including construction materials, surface soils, and rocks.
Sulfur isotopes as tracers of SO42− in precipitation
Sulfur isotopic characteristics
The δ34S value of SO42− in Nanchang precipitation during 2015–2016 ranged from − 1.0‰ to + 4.5‰ (Table 1), similar to the values reported by Xiao et al. (2011). On average, precipitation δ34S values in Nanchang approximated the values in the Three Gorges Reservoir and were higher than those in Guiyang but lower than those in most other domestic cities (Mukai et al. 2001; Xiao et al. 2014; Wu and Han 2015). Compared with foreign cities (Panettiere et al. 2000; Tichomirowa et al. 2007), precipitation δ34S values were lower in Nanchang. In addition, the precipitation δ34S values in Nanchang were roughly higher in the dry months than in the rainy months (Fig. 2), which was similar to the sulfur isotopic pattern of precipitation in Guiyang and Japan (Ohizumi et al. 1997; Xiao et al. 2014).
Sulfur isotopic constraint on the provenance of SO42−
Precipitation SO42− usually has multiple potential sources with different sulfur isotopic compositions, as shown in Fig. 3. Active volcanoes do not occur in Nanchang and surrounding areas. Sea spray and marine biogenic sulfur are isotopically different from Nanchang precipitation (Fig. 3). Therefore, these three potential sources contributed little to Nanchang precipitation. Southwesterly, southerly, and easterly winds were dominant, and the frequency of wind directions agreed with the precipitation amount (Fig. 4a, b). However, the frequency of wind directions were not associated with δ34S and the concentration of SO42− (Fig. 4c, d), suggesting a local source of SO42− in Nanchang precipitation. Continental biogenic S, originating from waters, wetlands, and soils (Novák et al. 2001; Wu and Han 2015), has a lower δ34S value than the SO42− in Nanchang precipitation (Fig. 3). Furthermore, anthropogenic SO2 emissions were significantly correlated with atmospheric SO2 concentrations during 2012–2015 (Fig. 5a). It was thus concluded that anthropogenic SO2 emissions contributed much to precipitation SO42−, but continental biogenic S emissions did not.
The plot of δ34S vs. SO42− for precipitation events. Curve A: mixing between pollutant sulfate and seawater sulfate (Ohizumi et al. 1997). Curve B: mixing between pollutant sulfate and continental biogenic sulfur (Panettiere et al. 2000). The data of local coals were from Hong et al. (1992). ① Southern Chinese coals (Motoyama et al. 2011). ② Northern Chinese coals (Motoyama et al. 2011). ③ Australian coals (Smith and Batts 1974). ④ Indonesian coals (Motoyama et al. 2011). ⑤ Chinese oil and natural gas (Maruyama et al. 2000). ⑥ Marine biogenic source (Amrani et al. 2013). ⑦ Continental biogenic source (Panettiere et al. 2000; Norman et al. 2004). ⑧ Volcanogenic source (Nielsen et al. 1991). ⑨ Miyakejima volcano (Imai et al. 2007). ⑩ Sea spray (Amrani et al. 2013). ⑪ Copper ore, Jiangxi of China (Liu et al. 2013). ⑫ Iron ore, Jiangxi of China (Xu et al. 2016)
Diagrams showing a atmospheric SO2 concentrations versus anthropogenic SO2 emissions in Nanchang, b the percentages of coal and oil in the total energy consumption in Jiangxi Province, c industrial and anthropogenic SO2 emissions in Nanchang, and d the proportion of different energy consumption sources in Jiangxi Province. The data of atmospheric SO2 concentrations in Nanchang were from Department of Ecology and Environment of Jiangxi Province (http://sthjt.jiangxi.gov.cn/) and other data were from Statistic Bureau of Jiangxi (http://tjj.jiangxi.gov.cn/)
Anthropogenic SO2 is emitted from fossil fuel combustion, ore smelting, vehicle emission, and so on (Górka et al. 2017). The usages of coal and oil comprised approximately 69% and 17% of the annual total energy consumption, respectively, during 2012–2015 in Nanchang (Fig. 5b). Moreover, industrial SO2 emissions comprised > 98% of anthropogenic SO2 emissions (Fig. 5c). Smelters are rare in Nanchang. Therefore, coal combustion was a dominant contributor to precipitation SO42− in Nanchang, which was supported by the δ34S value of precipitation falling in the range of coal values (Fig. 3). The proportion of local:northern Chinese:imported coals used in Jiangxi Province was generally maintained at 1:2:0.4 from 2012 to 2015, showing stable sources of coal consumption (Fig. 5d). On average, the three types of coals were different in terms of δ34S values and sulfur concentrations: − 3.1‰ and 0.9% for local coal (Hong et al. 1992); + 6.6‰ and 0.77% for northern Chinese coal (Motoyama et al. 2011); and + 6.7‰ and 0.19% for imported coal from Australia and Indonesia (Smith and Batts 1974; Motoyama et al. 2011). Through calculation, the relative contributions of sulfur emissions from the burning of local, northern Chinese, and foreign coals occupied 36%, 61%, and 3%, respectively. Therefore, the burning of northern Chinese coal was dominant affecting precipitation SO42− in Nanchang.
The causes for sulfur isotopic variation between dry and wet months
Precipitation δ34S seasonality is commonly caused by several mechanisms: (1) the removal of aerosol particles and soluble gases by raindrops below clouds (Xiao et al. 2014), (2) seasonal variation in sulfate sources (Mukai et al. 2001), (3) isotope fractionation during the oxidation of SO2 to SO42− (Harris et al. 2013), and (4) fluctuations in anthropogenic S from coal burning (Górka et al. 2017).
As shown in Table 3, no notable correlation existed between the δ34S value and amount of precipitation in Nanchang. In addition, coal combustion was the dominant contributor to precipitation SO42− and had constant sources in Jiangxi Province, as elucidated in the former paragraph. Therefore, the former two mechanisms had a minimal effect on δ34S fluctuations in precipitation in Nanchang.
Oxidation of SO2 to SO42− is responsible for precipitation δ34S seasonality and has two pathways: homogeneous and heterogeneous (Harris et al. 2013). Górka et al. (2017) thought that sulfates in precipitation originated mainly from secondary emissions in Wroclaw, ranging from ~ 80% during the nonheating period to ~ 60% during the heating period. As in Wroclaw, power plants are not equipped with desulfurization units in Nanchang. Therefore, secondary emissions should be a major contributor to the total sulfate content of precipitation in Nanchang. Furthermore, sulfate δ34S in precipitation should have a corresponding response if the δ34S of secondary sulfate changes significantly with ambient temperature, reflecting heterogeneous oxidation. Otherwise, sulfate δ34S in precipitation should have no response to ambient temperature, indicating homogeneous oxidation. Atmospheric temperature was not significantly correlated with precipitation δ34S in Nanchang (Table 3), showing the control of homogeneous oxidation on the δ34S value of Nanchang precipitation during the whole year.
Homogeneous oxidation of SO2 leads to isotopically lighter S accumulation in the resulting sulfate, whereas heterogeneous oxidation of SO2 causes isotopically heavier S enrichment in droplets (Novák et al. 2001). In summer (Jun. to Aug.), with a mean air temperature of 28.2 °C, the photochemical production of gaseous oxidants was high and SO2 solubility was low (Saltzman et al. 1983); more SO2 was thus converted to SO42− by homogeneous oxidation, causing lower δ34S values in precipitation except in August (Fig. 6). In winter (Dec. to Feb.), with a mean air temperature of 6.6 °C, homogeneous oxidation became weaker, accompanied by heterogeneous oxidation, resulting in higher δ34S values in precipitation (Fig. 6).
Variations of air temperature, coals for electricity, and precipitation δ34S values with month during 2015–2016. Air temperatures of 16–26 °C are the suitable temperatures to human beings, at which heating and air conditioning were not needed. The data for air temperature and coals for electricity in Jiangxi Province were from the China Weather website (http://www.weather.com.cn/) and the Jiangxi Energy Bureau website (http://www.cma.gov.cn/), respectively
The summers were hot, with the highest temperature approaching 40 °C in Jiangxi (Fig. 6). Thus, a large amount of electricity was needed for air conditioning and agricultural irrigation, which was inferred from the coal consumption of power plants during summer (particularly August). Coal consumption for electricity increased from June to August and then decreased from August to September, and the δ34S of precipitation SO42− also changed similarly (Fig. 6). Moreover, the precipitation δ34S value was positively correlated with coal consumption in summer (coal consumption = 209.5 × δ34S − 267.9, R2 = 0.99). Therefore, anthropogenic S emissions from coal-burning power plants affected the δ34S seasonality of precipitation in Nanchang. However, the precipitation δ34S value was inversely correlated with coal consumption in winter (coal consumption = − 84.9 × δ34S + 527.6, R2 = 0.95) (Fig. 6), probably due to the oxidation of SO2 to SO42−.
Conclusions
The chemical and sulfur isotope compositions of precipitation were investigated in Nanchang, South China, during 2015–2016 in this study. The pH values of precipitation in Nanchang ranged from 3.6 to 7.0, and 72.6% of the values were < 5.6, indicating the severity of acid rain. The Ec values in Nanchang varied from 3 to 86 μS cm−1, similar to those in cities of South China. Precipitation was characterized by SO42−, NO3−, Ca2+, and NH4+ in Nanchang. SO42− and NO3− were the two main acidifying factors, whereas Ca2+ and NH4+ were the major neutralizing species. As observed for the pH value, the chemical species of precipitation all showed lower values in the rainy months than in other months. Compared with foreign cities, the concentrations of ions in precipitation were higher in this region, reflecting the effect of anthropogenic pollution. SO42−, NO3−, and NH4+ in precipitation were controlled by anthropogenic pollutants. K+, Mg2+, and Ca2+ could mainly originate from the weathering of silicates and/or carbonates. Cl− in precipitation originated partly from anthropogenic pollution but was predominantly of marine origin. The δ34S value of SO42− fluctuated from − 1.0‰ to + 4.5‰, and its variation trend with time was opposite to pH in Nanchang precipitation. The use of northern Chinese coal was considered to be the most dominant contributor to precipitation SO42− in Nanchang. The oxidation of SO2 to SO42−, particularly homogeneous oxidation, was the most dominant mechanism affecting precipitation δ34S fluctuations with time. Our findings give deep insights into the origin of atmospheric pollutants in South China and are helpful for local governments to improve air quality. Our work may also provide a significant reference for acid rain studies in other regions. Our current work only focused on 1 year of precipitation events. In the future, a long-term precipitation monitoring program should be conducted to systematically explore the multiyear variations in precipitation chemistry and air pollutants.
References
Al-Khashman OA (2005) Ionic composition of wet precipitation in the Petra Region, Jordan. Atmos Res 78:1–12. https://doi.org/10.1016/j.atmosres.2005.02.003
Amrani A, Said-Ahmad W, Shaked Y, Kiene RP (2013) Sulfur isotope homogeneity of oceanic DMSP and DMS. Proc Natl Acad Sci 110:18413–18418. https://doi.org/10.1073/pnas.1312956110
Charlson RJ, Rodhe H (1982) Factors controlling the acidity of natural rainwater. Nature 295:683–685. https://doi.org/10.1038/295683a0
Ciężka M, Modelska M, Górka M, Trojanowska-Olichwer A, Widory D (2016) Chemical and isotopic interpretation of major ion composition of precipitation: a one-year temporal monitoring study in Wrocław, SW Poland. J Atmos Chem 73:61–80. https://doi.org/10.1007/s10874-015-9316-2
Duan L, Yu Q, Zhang Q, Wang ZF, Pan YP, Larssen T, Tang J, Mulder J (2016) Acid deposition in Asia: emissions, deposition and ecosystem effects. Atmos Environ 146:55–69. https://doi.org/10.1016/j.atmosenv.2016.07.018
Górka M, Skrzypek G, Hałas S, Jędrysek MO, Strąpoć D (2017) Multi-seasonal pattern in 5-year record of stable H, O and S isotope compositions of precipitation (Wrocław, SW Poland). Atmos Environ 158:197–210. https://doi.org/10.1016/j.atmosenv.2017.03.033
Grassineau NV, Mattey DP, Lowry D (2001) Sulfur isotope analysis of sulfide and sulfate minerals by continuous flow-isotope ratio mass spectrometry. Anal Chem 73:220–225. https://doi.org/10.1021/ac000550f
Gu JX, Du SY, Han DW, Hou LJ, Yi J, Xu J, Liu GH, Han B, Yang GW, Bai ZP (2014) Major chemical compositions, possible sources, and mass closure analysis of PM2.5 in Jinan, China. Air Qual Atmos Health 7:251–262. https://doi.org/10.1007/s11869-013-0232-9
Han G, Tang Y, Wu Q, Tan Q (2010) Chemical and strontium isotope characterization of rainwater in karst virgin forest, Southwest China. Atmos Environ 44:174–181. https://doi.org/10.1016/j.atmosenv.2009.10.019
Han GL, Song ZL, Tang Y, Wu QX, Wang ZG (2019) Ca and Sr isotope compositions of rainwater from Guiyang City, South China: implication for the sources of atmospheric aerosols and their seasonal variations. Atmos Environ 214:116854. https://doi.org/10.1016/j.atmosenv.2019.116854
Harris E, Sinha B, Hoppe P, Ono S (2013) High-precision measurements of 33S and 34S fractionation during SO2 oxidation reveal causes of seasonality in SO2 and sulfate isotopic composition. Environ Sci Technol 47:12174–12183. https://doi.org/10.1021/es402824c
Hong YT, Zhang HB, Zhu YX, Piao HC, Jiang HB, Zeng YQ, Liu GS (1992) Sulfur isotope characteristic of coal in China and isotopic fractionation during coal burning. Sci China B 00B:868–873 (In Chinese)
Imai A, Geshi N, Shimano T, Nakada S (2007) Implication of the temporal sulphur isotopic variation during the 2000 eruption of Miyakejima Volcano, Japan. Island Arc 16:83–92. https://doi.org/10.1111/j.1440-1738.2007.00549.x
Kuribayashi M, Ohara T, Morino Y, Uno I, Kurokawa J, Hara H (2012) Long-term trends of sulfur deposition in East Asia during 1981-2005. Atmos Environ 59:461–475. https://doi.org/10.1016/j.atmosenv.2012.04.060
Kurokawa J, Ohara T, Morikawa T, Hanayama S, Janssens-Maenhout G, Fukui T, Kawashima K, Akimoto H (2013) Emissions of air pollutants and greenhouse gases over Asian regions during 2000-2008: Regional Emission inventory in Asia (REAS) version 2. Atmos Chem Phys 13:11019–11058. https://doi.org/10.5194/acpd-13-10049-2013
Liu T, Liu C, Yan Z, Chen Y, Wu X, Fan X (2013) Study of metallogenetic fluids and metallogenic mechanisms of Xingyuanchong copper deposit, Jiangxi Province, China. Procedia Earth and Planet Sci 7:508–512. https://doi.org/10.1016/j.proeps.2013.03.142
Lynam MM, Dvonch JT, Hall NL, Morishita M, Barres JA (2014) Trace elements and major ions in atmospheric wet and dry deposition across Central Illinois, USA. Air Qual Atmos Health 8:135–147. https://doi.org/10.1007/s11869-014-0274-7
Maruyama T, Ohizumi T, Taneoka Y, Minami N, Fukuzaki N, Mukai H, Murano K, Kusakabe M (2000) Sulphur isotope ratios of coals and oils used in China and Japan. Chem Soc Jpn 1:45–51 (In Japanese). https://doi.org/10.1246/nikkashi.2000.45
Motoyama R, Yanagisawa F, Ueda A, Suzuki Y, Kanai Y, Ohsawa E, Kojima T, Akata N (2011) Spatial distribution of stable sulfur isotope ratio in coal samples in East Asian Region. Radioisotopes 60:27–33. https://doi.org/10.3769/radioisotopes.60.27
Mukai H, Tanaka A, Fujii T, Zeng Y, Hong Y, Tang J, Guo S, Xue H, Sun Z, Zhou J, Xue D, Zhao J, Zhai G, Gu J, Zhai P (2001) Regional characteristics of sulfur and lead isotope ratios in the atmosphere at several Chinese urban sites. Environ Sci Technol 35:1064–1071. https://doi.org/10.1021/es001399u
Nielsen H, Pilot J, Grinenko LN, Grinenko VA, Lein AY, Smith JW, Pankina RG (1991) Lithospheric sources of Sulphur. In: Krouse HR, Grinenko VA (eds) Stable isotopes: natural and anthropogeic sulphur in the environment. John Wiley, New York, pp 65–132
Niu YW, Li XL, Huang Z, Zhu CZ (2017) Chemical characteristics and possible causes of acid rain at a regional atmospheric background site in eastern China. Air Qual Atmos Health 10:1–10. https://doi.org/10.1007/s11869-017-0486-8
Norman AL, Belzer W, Barrie LA (2004) Insights into the biogenic contribution to aerosol total sulphate and precipitation in the Fraser Valley afforded by isotopes of sulphur and oxygen. J Geophys Res 109:D05311. https://doi.org/10.1029/2002JD003072
Novák M, Jačková I, Přechová E (2001) Temporal trends in the isotope signature of air-borne sulfur in Central Europe. Environ Sci Technol 35:255–260. https://doi.org/10.1021/es0000753
Ohizumi T, Fukuzaki N, Kusakabe M (1997) Sulfur isotopic view on the sources of sulfur in atmospheric fallout along the coast of the sea of Japan. Atmos Environ 31:1339–1348. https://doi.org/10.1016/S1352-2310(96)00278-6
Panettiere P, Cortecci G, Dinelli E, Bencini A, Guidi M (2000) Chemistry and sulfur isotopic composition of precipitation at Bologna, Italy. Appl Geochem 15:1455–1467. https://doi.org/10.1016/S0883-2927(00)00012-3
Saltzman ES, Brass GW, Price DA (1983) The mechanism of sulfate aerosol formation: chemical and sulfur isotopic evidence. Geophys Res Lett 10:513–516. https://doi.org/10.1029/GL010i007p00513
Sinha B, Hoppe P, Huth J, Foley S, Andreae M (2008) Sulfur isotope analyses of individual aerosol particles in the urban aerosol at a central European site (Mainz, Germany). Atmos Chem Phys 8:7217–7238. https://doi.org/10.5194/acp-8-7217-2008
Smith JW, Batts BD (1974) The distribution and isotopic composition of sulfur in coal. Geochim Cosmochim Acta 38:121–133. https://doi.org/10.1016/0016-7037(74)90198-7
Tichomirowa M, Haubrich F, Klemm W, Matschullat J (2007) Regional and temporal (1992-2004) evolution of air-borne sulphur isotope composition in Saxony, southeastern Germany, Central Europe. Isot Environ Healt S 43:295–305. https://doi.org/10.1080/10256010701702499
Wu QX, Han GL (2015) Sulfur isotope and chemical composition of the rainwater at the Three Gorges Reservoir. Atmos Res 155:130–140. https://doi.org/10.1016/j.atmosres.2014.11.020
Wu Y, Xu ZF, Liu WJ, Zhao T, Zhang X, Jiang H, Yu C, Zhou L, Zhou XD (2016) Chemical compositions of precipitation at three non-urban sites of Hebei Province, North China: influence of terrestrial sources on ionic composition. Atmos Res 181:115–123. https://doi.org/10.1016/j.atmosres.2016.06.009
Xiao HY, Zhu RG, Lin B, Liu CQ (2011) Sulfur isotopic signatures in rainwater and moss Haplocladium microphyllum indicating atmospheric sulfur sources in Nanchang City (SE China). Sci Total Environ 409:2127–2132. https://doi.org/10.1016/j.scitotenv.2011.02.011
Xiao HW, Xiao HY, Long AM, Wang YL, Liu CQ (2014) Sources and meteorological factors that control seasonal variation of δ34S values in rainwater. Atmos Res 149:154–165. https://doi.org/10.1016/j.atmosres.2014.06.003
Xu B, Jiang SY, Luo L, Zhao KD, Ma L (2016) Origin of the granites and related Sn and Pb-Zn polymetallic ore deposits in the Pengshan District, Jiangxi Province, South China: constraints from geochronology, geochemistry, mineral chemistry and Sr-Nd-Hf-Pb-S isotopes. Mineral Deposita 52:337–360. https://doi.org/10.1007/s00126-016-0659-7
Yu H, He N, Wang Q, Zhu J, Xu L, Zhu Z, Yu G (2016) Wet acid deposition in Chinese natural and agricultural ecosystems: evidence from national-scale monitoring. J Geophys Res - Atmos 121:10995–11005. https://doi.org/10.1002/2015JD024441
Acknowledgments
The authors are grateful to Editor-in-Chief Prof. Dr. Yong S. Chung and the anonymous reviewers whose insightful comments were very useful in improving this paper. Many thanks are also given to Prof. Liangfeng Han for polishing the English language of this paper.
Funding
This study was financially supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20191304), the Fundamental Research Funds for the Central Universities (2019B45414) and the National Natural Science Foundation of China (Grant No. 41877487).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, F., Rao, W., Chu, X. et al. Chemical and sulfur isotopic characteristics of precipitation in a representative urban site, South China: implication for anthropogenic influences. Air Qual Atmos Health 13, 349–359 (2020). https://doi.org/10.1007/s11869-020-00798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-020-00798-7