Opinion statement
Leiomyosarcoma arises from smooth muscle and represents one of the most common soft tissue sarcomas. Despite aggressive multimodality care, over half of the patients will ultimately develop metastatic and incurable disease with a median survival of 12–18 months. At present, there is no standard system to classify leiomyosarcoma, which itself is a heterogeneous disease. Classification by tumor location is the most simplistic approach and is most frequently utilized in clinical practice. Tumor location impacts diagnosis (recognition pre-operatively versus at the time of surgery) as well as treatment (ability to completely resect with clear margins with minimal morbidity). While tumor location can impact prognosis, for example, extremity tumors would generally be considered as lower risk than inferior vena cava tumors, leiomyosarcoma can exhibit a heterogeneous behavior irrespective of tumor location. Specifically, some patients have rapidly progressing disease despite aggressive chemotherapy, while others display a more indolent course even in the metastatic setting. The pathogenic drivers of the heterogeneity observed in tumor behavior are not well understood. As we learn more about the molecular composition of leiomyosarcoma, various classification groups have been proposed as discussed here. Ultimately, it is unlikely that one variable will be adequate for tumor classification, and a combination of location and molecular composition will be necessary to develop appropriate risk stratification nomograms and treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoma is a heterogeneous group of rare solid tumors which originate from connective tissue, blood vessels, and lymphatic tissues (mesenchymal cells). Sarcomas comprise less than 1% of solid tumors in adults and approximately 10% of pediatric tumors diagnosed annually [1]. Sarcomas are broadly divided as bone or soft tissue tumors and can be further characterized by the specific tissue of origin with over 75 different subtypes including osteosarcoma, Ewing’s sarcoma, gastrointestinal stromal tumor, liposarcoma, undifferentiated pleomorphic sarcoma, and leiomyosarcoma (LMS) to name a few. LMS is one of the three most common soft tissue sarcomas (STS), comprising 10–20% of STS diagnoses [2]. LMS is derived from smooth muscle cells and can occur anywhere in the body including extremities, large vessels of the retroperitoneum such as the inferior vena cava, and the uterus [3]. The median age of diagnosis for uterine LMS is 54 years old, and non-uterine LMS is 61 years old [3].
Risk factors for LMS include prior radiation therapy exposure and genetic syndromes such as hereditary retinoblastoma and Li-Fraumeni syndrome; though the vast majority of tumors are sporadic and without hereditary association [4•]. Clinical presentation varies depending on tumor location. Patients may be asymptomatic with a tumor found incidentally on imaging or may present with a painful lump, abnormal uterine bleeding, gastrointestinal bleeding, or general constitutional symptoms based on tumor location. Staging evaluation for tumors located in the extremity, trunk, or pelvic girdle should include magnetic resonance imaging (MRI) scans and for tumors located within the abdomen and pelvis should include computed tomography (CT) scans of the primary site. All patients should undergo a CT chest to assess for lung metastases. The utility of additional imaging such as positron emission tomography CT (PET CT) is not clear for all patients, although it is often utilized in the staging of primary bone sarcomas.
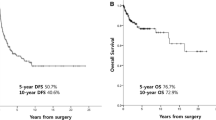
Despite aggressive multimodal treatment which may include some combination of surgery, radiation, and chemotherapy, outcomes in LMS remain poor. Nearly 50% of tumors will recur after definitive therapy of localized disease, with an average overall survival in stage IV disease of approximately 11–15 months [5]. The standard of care for the management of patients with localized LMS includes complete surgical resection, often with pre-operative radiation. The role of adjuvant and neoadjuvant chemotherapy is not well defined and has failed to show an overall survival advantage in prospective studies [6, 7]. STS spreads hematogenously, with the lung being the most common site of metastases in all STS including LMS, although liver, bone, and soft tissue can be involved [2, 3]. For advanced unresectable or metastatic disease, chemotherapy is the mainstay of treatment with the intent of disease control; however, currently available regimens show limited efficacy and are often associated with significant toxicity [8].
LMS can exhibit unpredictable behaviors, with some showing very aggressive growth despite multiple therapeutic approaches while others have a more indolent disease course. The drivers of disease variability are not well understood. Although there are several systems for classifying LMS, including anatomical location and molecular composition, these classifications are not routinely used in clinical practice for risk stratification or prognostication. Molecular biology has radically transformed the field of medical oncology, and the introduction of targeted and immunotherapies has significantly impacted the outcomes of many tumor types. Over the past 20 years, research within LMS has focused on classifications by molecular markers rather than solely by anatomical location [9••]. Molecular features have been shown to have a clinical impact and predict tumor behavior. LMS literature in the past year has used molecular data to test targeted therapies and is showing promise in improving overall survival. We will review two proposed classification systems for LMS with updates from recent literature.
Classification of LMS
Anatomical location
LMS classification by anatomical location is the most simplistic way to categorize disease and is commonly used in clinical practice, with a major distinction between uterine and non-uterine LMS. While the anatomical location does have some predictive value on outcomes, this may be more closely related to the stage at presentation rather than tumor biology. For example, a mass in the extremity may become symptomatic and present at an earlier stage than a retroperitoneal mass. The most consistent predictor of outcome is complete surgical resection at the time of primary treatment, and certainly, the ability to achieve complete resection with clear margins is impacted by tumor location [4•]. LMS uses two staging systems depending on the site of origin. Uterine LMS are staged based on the Federation of Gynecology and Obstetrics (FIGO) system, and all other sites of origin are staged based on the American Joint Committee of Cancer staging system. There are also several grading systems used for LMS proposed by the United States National Cancer Institute (NCI) and the French Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC); however, grading has been found to be inconsistent in predicting prognosis in LMS [10].
For the localized disease of any anatomical location, surgery is the primary treatment modality. In tumors that are determined to have a high recurrence rate (tumor > 5 cm, nodal positive, or mitotic rate > 4) or difficult surgical location, neoadjuvant radiation therapy or chemotherapy may be considered. When peri-operative chemotherapy is deemed appropriate, recommended regimens are primarily anthracycline-based including doxorubicin, ifosfamide, and mesna (AIM); or ifosfamide, epirubicin, and mesna [6]. These prospective studies have failed to demonstrate an overall survival advantage with adjuvant chemotherapy. In our practice, high-risk individuals such as those with high-grade, large (> 5 cm), and deep-seated tumors should be considered for peri-operative treatment if no comorbid contraindications. Data for the utility of palliative chemotherapy in the metastatic setting is well established. In a phase 3 trial with 228 patients comparing doxorubicin monotherapy to doxorubicin with ifosfamide/mesna in advanced or metastatic LMS, progression-free survival (PS) was improved in the AIM group (7.4 months vs 4.6 in doxorubicin monotherapy group); however, there was not a statistically significant difference in overall survival (OS) (14.3 months in AIM group vs 12.8 months in doxorubicin group) [6]. Another phase 3 trial with 257 patients evaluated doxorubicin monotherapy to docetaxel plus gemcitabine in STS and found no difference in progression-free or overall survival (PS 23.3 vs 23.7 weeks respectively) [7].
Neoadjuvant radiation therapy is often utilized in addition to surgical resection, with the National Comprehensive Cancer Network (NCCN) recommending treatment to 50 Gy. While radiation therapy is commonly utilized in extremity and head and neck tumors, the use in retroperitoneal disease is less convincing as shown in the recent STRASS study (abdominal recurrence-free survival 4.5 years vs 5 years in the surgery along vs surgery + radiation group) [11]. Ongoing research with the STRASS2 trial is evaluating the effect of neoadjuvant chemotherapy (ADM 75 mg/m2 + DTIC (dacarbazine) 1 g/m2 Q3 weeks) on PS and OS (NCT04031677) and may further inform on the utility of neoadjuvant chemotherapy in LMS of retroperitoneal origin. While doxorubicin with ifosfamide has long been the standard for neoadjuvant or adjuvant chemotherapy, a recent study from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) utilized propensity score matching analysis to assess doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as first-line treatment for advanced LMS [12]. Combination doxorubicin with dacarbazine was noted to have a higher ORR compared to AIM and doxorubicin monotherapy (30.9%, 19.5%, and 25.6%, respectively). Furthermore, PFS and OS were significantly longer with doxorubicin plus dacarbazine (HR 0.72 and HR 0.66, respectively). While a retrospective analysis, these data suggest the utility of dacarbazine rather than ifosfamide with anthracycline as first-line therapy for leiomyosarcoma. Results from STRASS2 will further inform on the utility of this treatment approach.
While uterine LMS (uLMS) is the most common anatomical subtype, it remains a rare cause of uterine masses. The lifetime incidence of uterine leiomyoma is approximately 70–80% and of uLMS is only 0.05–0.28% [13]. It is often not possible to distinguish uLMS from uterine leiomyoma from imaging. Most women are diagnosed with uterine LMS in the post-operative setting and therefore miss the opportunity to be considered for neoadjuvant therapy. This is in clear contrast to LMS of other primary locations, which are often amenable to pre-operative biopsy for diagnostic confirmation. Żak et al. discussed potential tools (clinical features, serum tumor markers, multimodal imaging findings, and artificial intelligence) to distinguish benign vs malignant intrauterine masses noninvasively; however, no validated method was identified [14]. This is an active area of research which could impact the treatment approach to allow for neoadjuvant therapy if able to definitively diagnose uterine LMS pre-operatively.
Retroperitoneal LMS often arises from the inferior vena cava (IVC). Similar to uterine tumors, patients can present with large lesions due to the absence of symptoms. Historically, outcomes for IVC tumors have been poor, owing to challenging surgical resection and reconstruction. One of the largest historical series of 141 patients reported 5-year survival at < 30% [15]. Not surprisingly, as surgical techniques have evolved, outcomes for IVC LMS have also improved, with more contemporary long-term survival rates ranging from 31 to 66% [16, 17]. While showing improvement, outcomes remain starkly inferior to those for extremity soft tissue sarcomas, with long-term disease-free survival often exceeding 75–80% based on tumor location and histology.
While anatomical classifications have been used in clinical practice to guide treatment decision and inform on prognosis, it has been noted that this variable alone does not consistently predict behavior or outcomes [2]. Multiple recent studies have explained our knowledge on the molecular composition of LMS, suggesting its role in tumor classification.
Molecular composition
Molecular subtyping has been revolutionary within medical oncology, providing a wide array of targetable mutations which have significantly improved overall survival in many solid tumor types. Due to the success of these treatment strategies, biological subtyping is becoming heavily researched within all tumor types, including sarcoma.
STS consists of over 75 histologic subtypes. Classically, LMS will stain positive for alpha-smooth muscle actin, desmin, and h-caldesmon [4•]. Cells are spindle shaped containing eosinophils and elongated/hyperchromic nuclei [4•]. Histologic diagnosis has been standardized by the Stanford criteria which includes diffuse moderate-to-severe atypia, a mitotic count of at least 10 mitotic figures/10 HPF, and tumor cell necrosis criteria; with absence of necrosis and atypia, ≤ 4 mitosis indicating benign leiomyoma. Whole-genome sequencing has shown significant heterogeneity within LMS and identified four frequent gene alterations in TP53, RB, ATRX, and MED12 [4•].
Molecular subtyping for LMS was first proposed in 2009 by Beck et al., and since then, there have been many different systems proposed [9••, 18•]. In the landmark publication, Beck et al. identified three reproducible groups of LMS based on gene expression microarray profiling. In their analysis, group I, which contained enriched muscle-related genes, phosphoproteins, and kinases with lower genomic stability, showed improved overall survival compared to other groups (Table 1). Subsequently, Guo et al. defined 3 subtypes of LMS by biologic makeup [19]. Type 1 showed smooth muscle differentiation; type 2 showed poor differentiation, and type 3 encompassed tumors which did not classify as type 1 or 2 (Table 1). Type 3 tumors were primarily of uterine origin, supporting the clinical practice that uterine LMS is biologically distinct from other types.
Barlin et al. identified two distinct molecular populations of uterine LMS based on 73 genes, which differed in clinical behavior and outcomes [20]. Clade 1 showed gene upregulation in immature B-cell immunophenotype, while clade 2 showed enrichment in histidine metabolism genes [20]. These two subtypes showed clinical differences in behavior with clade 2 showing superior survival to clade 1 (PS 4 vs 26 months; OS of 18 vs 77 months respectively) [20]. Using these methods, it may be possible to define clinically relevant molecular subtypes of LMS which could provide prognostic information along with informing treatment decision.
In 2017, the Cancer Genome Atlas (TCGA) consortium proposed molecular definitions for uterine LMS and 2 groups of LMS [21]. The uterine group was found to have a high DNA damage response rate along with hypomethylation of ESR1 and altered AKT pathway [21]. The two soft tissue groups both had high HIF1α and hypermethylation as compared to the uterine type; however, type C1 had an alteration in the AKT pathway and MYOCD amplification, whereas, C2 had high inflammatory signatures with NK and mast cells [21]. Type C1 was found to have reduced recurrence-free survival as compared to type C2 [21]. Chudasama et al. proposed a subclassification for uterine LMS including SG1, enriched in platelet degranulation with complement activation and metabolic genes, SG2, enriched in muscle-related genes, and SG3, with an intermediate expression of muscle-related genes [22]. The most recent classifications came from Hemming et al. in 2020 and Anderson et al. in 2021 [23, 24]. Hemming et al. defined 3 subtypes by enriched genes: conventional LMS, inflammatory LMS, and uterogenic LMS [25]. Conventional LMS is defined as enrichment in SYNM and ADIRF, inflammatory LMS with enrichment in PDGFRA and DCN, and uterogenic LMS as enrichment in ESR1 and CHRDL2 [24]. Anderson et al. evaluated primary and metastatic LMS by underlying mutational processes defining subtype 1 with DMD deletions, type 2 with MYOCD amplifications, and type 3 by a combination of DMD deletions and MYOCD amplifications [23] (Table 1).
Through investigations to better understand the molecular makeup of LMS, uterine LMS has been shown to have a higher incidence of mutations in DNA repair mechanisms [26]. This is being leveraged as a potential treatment strategy using PARP inhibitors and temozolomide as targeted therapy in LMS. There is an ongoing phase 2 clinical trial which was recently presented at ASCO 2022 showing that in patients with locally advanced or metastatic uterine LMS which has progressed through first-line therapy, combination with a PARP inhibitor and temozolomide extended progression-free survival by 6.9 months (NCT03880019) [27].
The evolution of molecular alterations in tumors over time is well described. Recently, Shaefer et al. reported on tumor suppressor alterations in primary and non-primary (local recurrences and distant metastases) LMS [28•]. Results demonstrated dysregulation of TP53, p16/RB, and PTEN in 90%, 95%, and 41% of non-primary LMS, respectively. PTEN activation was more common in soft tissue than uterine non-primary LMS (55% vs 31%, p = 0.0005), while ER expression was more common in uterine non-primary LMS (50% vs 7%, p < 0.0001). Co-activation of TP53 and RB1 was present in 81% of non-primary LMS including soft tissue and uterine. These data highlight the role for evaluating therapies targeting DNA damage repair mechanisms in advanced LMS.
Literature updates from 2022
While anthracyclines have remained the standard of care for first-line treatment in metastatic disease, novel drugs and drug-drug combinations continue to be explored. LMS-04, a phase 3 study of doxorubicin alone versus doxorubicin with trabectedin in patients with metastatic or unresectable LMS showed a significantly improved PFS (12.2 months vs. 6.2 months, HR 0.41, p < 0.0001) [29]. A recent pre-clinical study showing the use of patient-derived xenografts to identify novel therapy targets for LMS found that the study population was susceptible to a cyclin-dependent kinase (CDK) inhibition, suggesting a potential role for targeted therapy [30]. ATR and IGF1R inhibitors were also studied due to their frequent mutation within LMS without significant change in tumor size [30]. An article in August 2022 by Kim et al. sought to determine the efficacy and safety of using a combination of Eribulin and gemcitabine [31]. Gemcitabine is an accepted monotherapy in LMS, with eribulin single agent showing some promise. Median progression-free survival was 5.6 months with median overall survival of 31.9 months [31]. Ultimately, a combination of eribulin-gemcitabine may provide an additional systemic treatment option for metastatic LMS pending further studies. Another study looking at the efficacy of eribulin in metastatic liposarcoma and LMS was presented as an oral abstract at the ASCO 2022 meeting and showed progression-free survival and overall survival (8.5 months and 26 months) which are comparable to standard therapy [32].
Conclusion
LMS remain a rare and aggressive tumor type with a high mortality rate despite multimodality therapy. While various classification systems (anatomical location vs molecular makeup) have been proposed with the goal of improving treatment and outcomes, these classification systems have not significantly changed therapeutic approaches at this time. Despite the challenges of studying rare disease, there is ongoing research into the molecular composition of LMS, its impact on outcomes, and the potential to inform on therapeutic targets.
Over the past 20 years, the landscape of LMS is shifting towards molecular subtyping as a predictor of tumor behavior. Although anatomical location remains the primary factor influencing treatment decision in current clinical practice, molecular subtyping is showing promise as a more versatile system of classification. Recent literature has focused not only on defining the biologic makeup of LMS and its impact on prognosis but also in finding targeted therapies which may enhance the effectiveness of LMS systemic treatments. We are seeing biologic markers which explain indolent vs aggressive LMS behavior. There are three distinct molecular subtypes which have already been defined and two specific uterine LMS subtypes. These have demonstrated different yet consistent clinical behaviors. Research over the past year has primarily focused on incorporating targeted therapies into LMS treatment. The preliminary trials show promise with further Phase III trials needed.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. 2021;28(1):44–58.
Brennan MF, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416–21 (discussion 421-2).
Lamm W, et al. Distinctive outcome in patients with non-uterine and uterine leiomyosarcoma. BMC Cancer. 2014;14(1):981.
• George S, et al. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018;36(2):144–50. Contemporary comprehensive review of leiomyosarcoma.
Wang Z, et al. Survival of patients with metastatic leiomyosarcoma: the MD Anderson Clinical Center for targeted therapy experience. Cancer Med. 2016;5(12):3437–44.
Judson I, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–23.
Seddon B, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397–410.
Oosten AW, et al. Outcomes of first-line chemotherapy in patients with advanced or metastatic leiomyosarcoma of uterine and non-uterine origin. Sarcoma. 2009;2009:348910.
•• Burns J, Jones RL, Huang PH. Molecular subtypes of leiomyosarcoma: moving toward a consensus. Clin Transl Discov. 2022;2(4):e149. A review of the literature for molecular subtyping and clinical imact.
Neuville A, Chibon F, Coindre J-M. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology. 2014;46(2):113–20.
Bonvalot S, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1366–77.
D’Ambrosio L, et al. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: a propensity score matching analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer. 2020;126(11):2637–47.
Pritts EA, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–77.
Zak K, Zaremba B, Rajkak Ak, Kotarski J, Amant F, Bobinski M. Perioperative differentiation of uterine leiomyoma and leiomyosarcomas: current possibilities and future directions. Cancers. 2022;14(8):1966–91.
Mingoli A, et al. Leiomyosarcoma of the inferior vena cava: analysis and search of world literature on 141 patients and report of three new cases. J Vasc Surg. 1991;14(5):688–99.
Wachtel H, et al. Outcomes after resection of leiomyosarcomas of the inferior vena cava: a pooled data analysis of 377 cases. Surg Oncol. 2015;24(1):21–7.
Hollenbeck ST, et al. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. J Am Coll Surg. 2003;197(4):575–9.
• Beck AH, et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene. 2010;29(6):845–54. First discovery of variable molecular subtypes in leiomyosarcoma.
Guo X, et al. Clinically relevant molecular subtypes in leiomyosarcoma. Clin Cancer Res. 2015;21(15):3501–11.
Barlin JN, et al. Molecular subtypes of uterine leiomyosarcoma and correlation with clinical outcome. Neoplasia. 2015;17(2):183–9.
Cancer Genome Atlas Research Network. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4):950–65.
Chudasama P, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. 2018;9(1):144.
Anderson ND, et al. Lineage-defined leiomyosarcoma subtypes emerge years before diagnosis and determine patient survival. Nat Commun. 2021;12(1):4496.
Hemming ML, et al. Oncogenic gene-expression programs in leiomyosarcoma and characterization of conventional, inflammatory, and uterogenic subtypes. Mol Cancer Res. 2020;18(9):1302–14.
Guo X, Forgó E, van de Rijn M. Molecular subtyping of leiomyosarcoma with 3’ end RNA sequencing. Genomics data. 2015;5:366–7.
Oza J, et al. Homologous recombination repair deficiency as a therapeutic target in sarcoma. Semin Oncol. 2020;47(6):380–9.
Yang J, et al. Therapeutic perspectives for adult soft tissue sarcoma-updates from the 2022 ASCO annual meeting. Cancer Biol Med. 2022;19(10):1496–502.
• Schaefer IM, et al. Relationships between highly recurrent tumor suppressor alterations in 489 leiomyosarcomas. Cancer. 2021;127(15):2666–73. Molecular analysis of larges leiomyosarcoma cohort to date.
Pautier P, et al. Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2022;23(8):1044–54.
Hemming ML, et al. Preclinical modeling of leiomyosarcoma identifies susceptibility to transcriptional CDK inhibitors through antagonism of E2F-driven oncogenic gene expression. Clin Cancer Res. 2022;28(11):2397–408.
Kim CG, et al. Phase II clinical trial of eribulin-gemcitabine combination therapy in previously treated patients with advanced liposarcoma or leiomyosarcoma. Clin Cancer Res. 2022;28(15):3225–34.
Chen TW, et al. A single-arm phase Ib/II study of lenvatinib plus eribulin in advanced liposarcoma and leiomyosarcoma. Clin Cancer Res. 2022;28(23):5058–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any potential conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sarcoma
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hickman, A., Siontis, B.L. Not All Leiomyosarcomas Are the Same: How to Best Classify LMS. Curr. Treat. Options in Oncol. 24, 327–337 (2023). https://doi.org/10.1007/s11864-023-01067-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01067-2