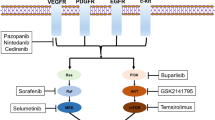
Opinion statement
Patients with advanced and recurrent ovarian, uterine, and cervical cancers have limited efficacious treatment options and poor outcomes. The development of agents that target DNA repair mechanisms, angiogenesis, immune checkpoints, and hormone receptor expression provides additional options for these patients. Many available targeted therapies have limited efficacy as single agents, so clinical trials investigating combination therapies as well as continued identification and validation of predictive biomarkers are critical. Many novel small molecule therapies, antibody drug conjugates, and therapeutic vaccines are also in development. This review will focus on recent evidence supporting the use of clinically available targeted therapies for gynecologic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the most deadly gynecologic cancer. There will be a projected 21,410 new cases of ovarian cancer and 13,770 deaths in the USA in 2021 [1]. Standard treatment for newly diagnosed ovarian cancer consists of cytoreductive surgery and platinum-based chemotherapy. Though the majority of patients are left without evidence of disease after first-line treatment, about 70% will recur within the first 5 years [2]. The prognosis for recurrent ovarian cancer is poor, and patients often receive multiple additional lines of therapy before succumbing to their disease. Recent advances in ovarian cancer research have introduced additional therapeutic options for these patients. Most recently, poly(adenosine-diphosphate-ribose) polymerase (PARP) inhibitors have shown substantial benefit for patients with BRCA mutations in the upfront setting and in the maintenance setting regardless of BRCA status [3].
In the USA, endometrial cancer is the most common gynecologic cancer with a predicted 66,570 new cases in 2021 and incidence continues to rise [4]. Prognosis for advanced endometrial cancer is poor with a 5-year overall survival of 40–65% for stage III and 15–17% for stage IV disease [5]. The Cancer Genome Atlas (TCGA) project identified four molecular subgroups: polymerase epsilon (POLE) ultra-mutated, microsatellite instability hypermutated, copy-number low, and copy-number high (p53 abnormal) [6, 7]. In a molecular analysis of the PORTEC 3 patient cohort, Leon-Castillo et al found that molecular classification by TCGA subgroup has strong prognostic value in high-risk endometrial cancer. Specifically, they found that p53 abnormal patients had a significantly improved recurrence free survival (RFS) with chemotherapy and radiation therapy as compared to radiation alone [6]. Furthermore, the POLE ultra-mutated subtype demonstrated excellent RFS in both adjuvant therapy arms. The work of the TCGA has enhanced our understanding of this heterogeneous disease and has opened the door for targeted therapies in the treatment of endometrial cancer.
Cervical cancer is the fourth most common cause of cancer-related death in women worldwide. There is a large variation in the incidence and mortality of cervical cancer with developing nations more severely affected. Incidence in developed nations has been decreasing due to screening, and further decline is expected with widespread HPV vaccination. Regardless, in the USA in 2021, about 14,480 new cases will be diagnosed with 4,290 deaths [8]. Patients with FIGO stage IB to IIA disease have a recurrence risk of 10 to 20%, while those with stage IIB to IVA disease have a 30–70% chance of recurrence [9]. Efficacious treatment options in the advanced and recurrent settings are limited, but multiple targeted therapies are currently in phase II/III trials that may offer options for this patient population.
The exciting evolution of cancer treatment over the last decade is due to a deep understanding of the cancer genome, discovery of actionable molecular biomarkers, and an understanding of the immune landscape. These advances have led to development of targeted agents including those that target DNA repair mechanisms, immune checkpoint inhibition, hormonal therapies, and anti-angiogenics. Many novel small molecule therapies, antibody drug conjugates, and therapeutic vaccines are also in development.
This review will discuss the current evidence supporting the use of clinically available targeted therapies for gynecologic cancer. Select agents in development will also be discussed.
Anti-angiogenic Agents
The vascular endothelial growth factor (VEGF) signaling pathway is a major driver of angiogenesis in solid tumors. VEGF is a growth and proliferation factor for endothelial cells that binds to VEGFR-1 and VEGFR-2. VEGFR-2 is expressed mainly on endothelial cells, while VEGFR-1 is expressed on macrophages, tumor cells, and fibroblasts [10]. Multiple targeted anti-angiogenic therapies are used in gynecologic cancers, including the monoclonal antibody, bevacizumab, and small-molecule tyrosine kinase inhibitors (e.g., cediranib and pazopanib). Bevacizumab is a recombinant humanized monoclonal antibody against circulating VEGF. It also functions to normalize tumor vasculature which helps to improve sensitivity to chemotherapy [10].
Anti-angiogenic therapy has been studied for two decades in ovarian cancer. In 2018, the United States Food and Drug Administration (FDA) approved front-line use of bevacizumab in combination with standard chemotherapy in patients with stage III/IV ovarian cancer based on the results of GOG 218 [11]. Patients received either carboplatin and paclitaxel (control), carboplatin and paclitaxel with concurrent bevacizumab and placebo maintenance, or carboplatin-paclitaxel with bevacizumab and bevacizumab maintenance. Initial results showed a 3.8-month improvement in progression-free survival (PFS) with concurrent and maintenance bevacizumab (p < 0.001) [11]. However, in 2019, updated results from 9 years of follow-up found no survival benefit compared to standard chemotherapy [12]. An exploratory analysis of a subset of patients from GOG 218 with stage IV disease revealed a 10-month improvement in overall survival (OS) in the concurrent and maintenance bevacizumab arm compared to standard chemotherapy [13]. Additionally, ICON7 showed survival benefit of chemotherapy plus bevacizumab for patients at highest risk for recurrence: stage IV, sub-optimally debulked stage III, or inoperable stage III (39.3 vs. 34.5 months (p = 0.030) [14, 15]. In light of this data, many providers choose to reserve upfront bevacizumab for patients with stage IV disease, sub-optimally debulked stage III, or those with platinum refractory disease. There was no statistical difference in quality of life in either study population with the addition of bevacizumab.
Cediranib is a tyrosine kinase inhibitor of VEGFR 1/2/3 and c-KIT. Phase III trial, ICON6, investigated the combination of cediranib with chemotherapy and as maintenance in patients with recurrent platinum sensitive ovarian cancer [16]. Final overall survival (OS) analysis was performed at a median 25.6 months and showed an improvement in median OS by 7.4 months in favor of the cediranib arm (HR 0.85; p = 0.210) [17]. Cediranib is not yet FDA approved for use in ovarian cancer.
Anti-angiogenic therapies also have an important role in the treatment of advanced or recurrent cervical cancer. GOG 240 compared cisplatin/paclitaxel and topotecan/paclitaxel with or without bevacizumab in patients with recurrent or persistent disease. The study found a median OS of 16.8 months in the chemotherapy plus bevacizumab arms versus 13.3 months in the chemotherapy-alone arms (HR 0.77; p = 0.007) [18, 19]. The results of GOG 240 resulted in US FDA approval of bevacizumab in first-line management of advanced cervical cancer in 2014.
PARP Inhibitors
The introduction of PARP inhibitors (PARPi) has led to major changes in the management of epithelial ovarian cancer (EOC). PARPi function by inhibiting the PARP enzyme, which prevents the repair of single-strand breaks in DNA via base excision repair. Inability to repair single-strand breaks leads to double-strand breaks [5]. In homologous recombination (HR) proficient cells, double-strand breaks are able to be repaired by BRCA-mediated homologous recombination repair. However, BRCA-mutated or HR-deficient (HRD) cells are unable to utilize this repair mechanism which leads to accumulation of double-strand breaks and cell death [5].
There have been 17 phase II/III trials published from 2009 through 2020 that investigate the use of PARP inhibitors compared to standard of care or placebo in EOC [3]. Three PARP inhibitors, olaparib, rucaparib, and niraparib, are commercially available and approved by the FDA for the treatment of patients with EOC—each with different clinical indications and toxicity profiles. Veliparib and talazoparib have also been evaluated in EOC and are still under clinical investigation.
Multiple phase II/III trials have established the clinical benefit of treatment with olaparib, rucaparib, and niraparib in the recurrent setting in patients with BRCA mutated or HRD disease. These are listed in Table 1. Based on the results of these studies, olaparib and rucaparib are approved for patients with BRCA mutations who have received ≥3 lines or ≥ 2 prior lines of chemotherapy, respectively [3]. Niraparib is approved for patients with HRD-positive tumors who have received ≥3 prior lines of chemotherapy [3].
STUDY 19, SOLO2, NOVA, and ARIEL 3 investigated the efficacy of PARP inhibitor maintenance monotherapy for recurrent disease [29,30,31,32,33]. Based on the results of these studies, the American Society of Clinical Oncology (ASCO) recommends that PARP inhibitor monotherapy maintenance (second line or more) may be offered to patients with EOC who have responded to platinum-based therapy regardless of BRCA status [3]. Olaparib, rucaparib, and niraparib are available for this indication.
SOLO1, PAOLA1, PRIMA, and VELIA are four clinical trials published in 2018–2019 which address the use of PARP inhibitors for newly diagnosed EOC [2, 34,35,36,37] SOLO1 investigated olaparib as first-line maintenance in BRCA1/2 mutants with FIGO stage III–IV disease after complete or partial response to platinum-based chemotherapy. Patients were randomized to olaparib alone or placebo. The trial showed that in this population, maintenance with olaparib improved PFS at 3 years with 60% of patients without disease in the olaparib group vs. 27% in the placebo group (HR 0.30; p < 0.001) [2]. Though final OS data is not yet published, after a median of 4.8 and 5.0 years of f/u, median PFS was 56 vs. 14 months for olaparib and placebo, respectively [34].
The PAOLA1 trial was the first phase III trial to examine the efficacy of a PARPi with bevacizumab as first-line maintenance therapy in patients with advanced EOC. Patients must have had a complete or partial response to standard platinum-based chemotherapy with bevacizumab. Patients were randomized to olaparib + bevacizumab or placebo + bevacizumab. A statistically significant improvement in PFS was demonstrated in the intention to treat population compared with placebo with median PFS of 22.1 months vs. 16.6 months [35]. Pre-specified subgroup analyses showed that patients with somatic BRCA mutations and patients with positive HRD status had the greatest PFS benefits. However, no significant benefit was observed in HR-proficient patients.
The PRIMA trial investigated the efficacy of niraparib maintenance after response to platinum-based chemo in patients with newly diagnosed, advanced EOC at high risk for recurrence. In the overall population, a significant benefit in median PFS was seen with niraparib over placebo, 13.8 months vs. 8.2 months. PFS was also significantly improved in the niraparib group in those with HR-proficient tumors (8.1 months vs. 5.4 months; HR 0.68, CI 0.49–0.94) [36]. The PRIMA trial was the first to demonstrate clinical benefit of first-line treatment with niraparib in all patients with advanced EOC regardless of HRD status.
The VELIA trial assessed the efficacy of veliparib added to first-line chemotherapy with carboplatin and paclitaxel and then continued as maintenance monotherapy in patients with previously untreated, advanced EOC. Patients were randomized to placebo with carboplatin/paclitaxel followed by placebo which served as the control group, veliparib with carboplatin/paclitaxel followed by placebo, or veliparib with carboplatin/paclitaxel followed by veliparib maintenance. Clinical benefit was observed in the HRD cohort (median PFS 31.9 months in veliparib throughout vs. 20.5 months in control group, p < 0.001), while no benefit was seen in HRD BRCA-wildtype or HR-proficient subgroups [37]. Veliparib is not currently commercially available.
Based on the results of these studies, olaparib is approved for upfront maintenance in patients with BRCA 1/2 mutations, while niraparib is approved for all women regardless of BRCA status.
PARPi, though generally well tolerated by patients, have class-specific adverse events (AEs). The most common include fatigue, anemia, neutropenia, thrombocytopenia, persistent cytopenias, and nausea. Less common AEs include vomiting, diarrhea, headache, elevation of liver enzymes, increased creatinine, pneumonitis, and increased risk of leukemia [3]. Because PARP inhibitors are daily, continuously administered agents, it is important to pay attention to low-grade AEs. Any grade 2 AE that requires a dose hold should be followed by a dose reduction.
PARP inhibitor therapy remains under investigation for endometrial and cervical cancers.
Hormonal Therapy
Estrogen and Progesterone Receptor-Directed Therapy
Many endometrial cancers express estrogen (ER) and progesterone (PR) receptors. It is known that estrogen-based hormonal therapy increases the incidence of endometrial hyperplasia and cancer, while progesterone is an important inducer of endometrial differentiation and an inhibitor of carcinogenesis mediated through estrogen [5]. For appropriately selected patients with grade 1 disease confined to the endometrium who desire a fertility sparing treatment approach, a continuous progestin-based therapy such as megestrol, medroxyprogesterone, or the levonorgestrel IUD can be considered [38,39,40,41,42]. A durable complete response occurs in about 50% of patients [38].
For non-surgical candidates with uterine-confined disease of endometrioid histology, external beam radiation therapy and brachytherapy are the preferred treatment [42]. In these patients, hormonal therapy can be considered an initial systemic therapy. Progesterone-based therapy, tamoxifen (selective estrogen receptor modulator) with alternating progestational agents, and aromatase inhibitors have been used [43,44,45].
The role of hormonal therapy in recurrent or metastatic disease is also typically limited to patients with endometrioid histology. Predictors of treatment response include well-differentiated tumors, expression of ER/PR receptors, a long disease-free interval, and location and extent of extra-pelvic metastasis. Hormonal options for these patients include progestational agents alone, tamoxifen alone, megestrol or medroxyprogesterone acetate with alternating tamoxifen, aromatase inhibitors, or fulvestrant (selective estrogen receptor downregulator) [43,44,45,46,47,48].
HER2- and EGFR-Targeted Therapy
The human epidermal growth factor receptor (HER) family includes EGFR (HER1), HER2, HER3, and HER4. HER2/neu provides critical signaling for cancer cell growth, survival, and proliferation. Overexpression of the HER2 receptor occurs in approximately 30% of uterine serous carcinomas (USC; TP53-mutated carcinomas) [49]. A randomized phase II study by Fader et al investigated the addition of trastuzumab, a humanized monoclonal antibody against HER2/neu, to carboplatin and paclitaxel compared to carboplatin/paclitaxel alone in patients with stages III or IV or recurrent USC [49]. The recently published final OS analysis reported an updated median PFS favoring the trastuzumab arm (8.0 months in control arm versus 12.9 months in the trastuzumab arm, HR = 0.46; 90% CI, p = 0.005) [50]. OS was also greater in the trastuzumab compared with the control arm, with medians of 29.6 months versus 24.4 months (HR = 0.58; p = 0.046) [50]. The benefit was most significant in the stages III to IV disease group as the survival median was not reached in the trastuzumab arm vs. 24.4 months in the control arm (HR = 0.49; p = 0.041) [50]. Additionally, Fader et al established that patients with 3+ HER2/neu overexpression by immunohistochemistry or 2+ by confirmatory FISH can likely achieve clinical benefit with this therapeutic combination [49]. A multi-institutional cohort study by Erickson et al found that HER2/neu positivity was associated with a 3-fold greater risk of recurrence in early stage USC [51].
Multiple HER2 inhibitors have been investigated for use in ovarian and cervical cancer. However, clinical efficacy has been modest, and none are FDA approved for use [52, 53]. Additionally, multiple studies of EGFR inhibitors including gefitinib, erlotinib, and cetuximab alone and in combination with chemotherapy have shown minimal activity in cervical cancer [54,55,56,57].
The combination of hormonal therapy with targeted therapies is a novel strategy in the treatment of certain gynecologic cancers. These combinations are actively being assessed in ongoing clinical trials.
Immune Checkpoint Inhibition
Immunotherapy has revolutionized the field of oncology as it has become a standard treatment option for many solid tumors. Immune checkpoint inhibitors (ICI) in the form of monoclonal antibodies against programmed death protein 1 (PD-1) (pembrolizumab, nivolumab, cemiplimab), programmed death protein ligand (PD-L1) (atezolizumab, avelumab, and durvalumab), and CTLA-4 (cytotoxic T lymphocyte-associated protein) (ipilimumab) are generally well tolerated and have an ever expanding role in the treatment of gynecologic cancers. The PD-1/PD-L1 pathway represents an adaptive immune resistance mechanism exerted by tumor cells in response to endogenous immune anti-tumor activity. PD-L1 is overexpressed on tumor cells in the tumor microenvironment. PD-L1 expressed on the tumor cells binds to PD-1 receptors on the activated T cells, which leads to the inhibition of cytotoxic T cells [58].
Pembrolizumab gained tumor agnostic FDA approval based on its clinical utility in mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) tumors in 2017. In KEYNOTE 028, patients with PD-L1-positive solid tumors and failure of previous therapy received pembrolizumab monotherapy. A 17% ORR was observed in the cervical cancer cohort, 13% in the endometrial cancer cohort, and 11.5% in the ovarian cancer cohort [59,60,61].
The prevalence of MSI high tumors may be as high as 40% in endometrial cancer [62]. Preliminary results from a phase II study evaluating the efficacy of pembrolizumab in nine patients with recurrent or persistent endometrial cancer with dMMR suggested an ORR of 56% and 12-month OS rate of 89% [63]. Additionally, the endometrial cancer cohort of the phase II, KEYNOTE 158 trial (single-agent pembrolizumab in patients with histologically confirmed MSI-H/dMMR disease), had an ORR of 57.1%, and median OS was not reached [64]. For endometrial cancer patients without microsatellite stable (MSS) disease, KEYNOTE 146 assessed the activity and safety of lenvatinib, an oral multikinase inhibitor, plus pembrolizumab in patients with biomarker-unselected advanced endometrial cancer. The objective response rate (ORR) was 39.6%. Most patients had MSS disease. These results led to the accelerated FDA approval of this combination for advanced or recurrent endometrial cancer with MSS disease in September 2019 [65]. An ongoing phase III trial, KEYNOTE 775, is investigating lenvatinib + pembrolizumab vs. physician’s choice chemotherapy in patients with advanced endometrial cancer with progression after one prior platinum-based therapy (NCT03517449). Additional ongoing clinical trials are aimed at the combination of immunotherapies with other anti-angiogenics and chemotherapies.
Despite the fact that EOC has a high prevalence of tumor-infiltrating lymphocytes (TILS) (approximately 50%) at diagnosis and a known resistance mechanism using the PD-L1 pathway, response rates to monotherapy ICI are poor, ranging from 6 to 22% [61, 66,67,68,69,70]. Strategies to overcome the immunosuppressive microenvironment of the tumor include combination therapy. A select list of clinical trials that investigate the efficacy immunotherapy with PARPi, anti-angiogenic agents, or both is listed in Table 2.
The results from GOG 3015, a phase II trial of carboplatin/paclitaxel + bevacizumab with or without atezolizumab for newly diagnosed stage III/IV ovarian cancer, found that the addition of atezolizumab did not significantly improve PFS in the intention to treat (18.4 months with placebo vs. 19.5 months with atezolizumab) or PD-L1-positive population (18.5 vs. 20.8 months) [72].
Immunotherapy is of interest for cervical cancer due to the immunogenic nature of human papilloma virus (HPV) integration and persistence. Unfortunately, responses to ICI monotherapy have been modest. In KEYNOTE 158, single-agent pembrolizumab in recurrent or metastatic cervical cancer resulted in an ORR of 14.6% in PD-L1-positive tumors [73]. The median duration of response was not reached. Due to the limited number of efficacious treatment options for this patient population, durable responses seen in responders from KEYNOTE 158 led to FDA approval of pembrolizumab for patients with recurrent or metastatic cervical cancer with PD-L1-positive tumors in June 2018.
AE’s associated with ICIs are known as immune-related AEs (irAEs). Most are mild to moderate in severity, but vigilance is necessary to recognize subtle signs of toxicity. The most commonly reported AEs are fatigue, pruritus/rash, diarrhea/colitis, autoimmune hepatitis, and endocrinopathies including thyroid dysfunction and hypophysitis [64, 74]. Pneumonitis is an irAE unique to PD-1/PD-L1 inhibition that is not often seen with CTLA-4 inhibition [74].
The future of immunotherapy in the treatment of gynecologic cancer is identifying synergistic combinations and predictive biomarkers. The biomarkers with the most evidence are PD-L1 expression, tumor mutational burden, and immune gene expression profile signatures [74]. The role of tumor-infiltrating lymphocytes as a predictive biomarker in gynecologic cancer is also under investigation.
Looking Towards the Future
Tisotumab Vedotin
Tisotumab vedotin is an investigational antibody drug conjugate (ADC) directed to tissue factor, which is a cell-surface protein associated with tumor growth, angiogenesis, metastasis, and poor prognosis. Results from the multicenter innovaTV 204/GOG-3023/ENGOT-cx6 phase II, single-arm trial of Tisotumab vedotin in patients with previously treated advanced or recurrent cervical cancer, showed a 24% ORR including 7 patients (7%) with a complete response and 17 patients (17%) with a partial response [75]. After a median follow-up of 10 months, the median duration of response was 8.3 months [75]. Based on the results of this study, an application for accelerated FDA approval of Tisotumab vedotin was submitted in February 2021.
Tumor-Infiltrating Lymphocytes (TILs)
The presence of T cells in the tumor microenvironment is associated with better response to chemotherapy and improved survival in EOC [76]. Adoptive cell therapy (ACT) with tumor-infiltrating lymphocytes (TIL) is the infusion of autologous T cells obtained from the tumor microenvironment of the individual patient. The T cells are then expanded and activated in vitro before reinfusion. This treatment has been successful in malignant melanoma with response rates ranging from 40 to 70% and durable responses in up to 20% [77]. Multiple early studies have demonstrated the potential of ACT in ovarian cancer [78, 79]. More recently, a study of six patients with recurrent high-grade serous ovarian cancer received immune therapy consisting of ipilimumab followed by surgery to obtain TILs and infusion of expanded TILs, low-dose IL-2, and nivolumab [76]. Regression of target tumor lesions was observed in all patients, and two patients achieved a partial response.
TIL treatment of HPV-positive cervical cancer has also shown promising clinical efficacy. Stevanovic et al reported objective tumor responses in 5 of 18 (28%) patients with recurrent cervical cancer with two complete responses ongoing at 67 and 53 months after treatment [80].
Therapeutic Vaccines
Multiple therapeutic vaccines have been the subject of clinical trials and continue to be actively explored in combination with other targeted therapies. Cervical cancer therapeutic vaccines aim to eradicate HPV-infected cells by stimulating cytotoxic T cells against the tumor/viral antigens. The HPV E6 and E7 oncoproteins are expressed in HPV-associated cancers and are ideal targets for a therapeutic vaccine. Axalimogene filolisbac (ADXS11-001) is a live, attenuated, listeria monocytogenes bacterial vector secreting HPV-16 E7 which is under active investigation for treatment of HPV-associated malignancies [9]. ADXS11-001 was explored as a monotherapy in the phase II GOG/NRG0265 trial in patients with persistent/recurrent cervical cancer. Results from 26 patients showed a 12-month OS rate of 38.5%, indicating possible therapeutic activity in this population [81].
Therapeutic vaccines are also being investigated for patients with EOC. These trials have found that systemic specific T cell and antibody responses can be induced. One study by Tanyi et al used a personalized vaccine generated by autologous dendritic cells pulsed with oxidized autologous whole-tumor cell lysate. The vaccine was then injected into selected lymph nodes of patients with recurrent ovarian cancer either alone or in combination with bevacizumab with or without low-dose cyclophosphamide. This study demonstrated that this safe and tolerable combination elicited antitumor immunity, which was associated with improved survival [82]. Another phase Ib study evaluated DPX-Survivac, a vaccine containing a mix of HLA class I peptides designed to evoke a T cell response against survivin which is expressed in gynecologic malignancies. The vaccine was given in combination with metronomic cyclophosphamide and epacadostat [83]. Partial responses were observed in 30%, and stable disease was observed in 50% of the 32 patients. A phase II study investigated the use of dendritic cell vaccine (DCVAC) with platinum-based chemotherapy vs. chemotherapy alone in patients with recurrent epithelial ovarian. DCVAC + chemotherapy decreased the risk of death by 62% which corresponds to 73% survival at 2 years, compared to 41% survival with chemotherapy alone [84].
Countless other targeted therapies are being investigated in early-phase trials for use in gynecologic cancer. These include, but are not limited to, PI3K/AKT/mTOR pathway inhibitors, MEK inhibitors, JAK/STAT inhibitors, Wee1 inhibitors, and ADCs. Discussion of these agents in detail is beyond the scope of this review.
Conclusion
In the era of precision medicine, there is a focus on understanding the molecular and immunologic features of tumorigenesis. This in turn has led to the development of targeted therapies that have revolutionized cancer care. Though there has been recent success in treatment of gynecologic cancer with agents such as PARPi, numerous challenges remain. Many of the treatments discussed have limited efficacy as single agents, so clinical trials investigating combination therapies are necessary. In addition, continued identification and validation of predictive biomarkers is critical to ensure careful patient selection. Financial toxicity is also an important consideration as these agents become widely available for our patients. Despite these challenges, precision care has been a long-sought-after goal which is starting to become reality for patients with gynecologic cancer.
Data Availability
All cited material is available from the corresponding journal listed in the reference.
References and Recommended Reading
Society, A.C. Key statistics for ovarian cancer. 2021 [cited 2021 February 3]; Available from: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.
Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–505.
Tew WP, et al. PARP inhibitors in the management of ovarian cancer: ASCO Guideline. J Clin Oncol. 2020;38(30):3468–93.
Society, A.C. Key statistics for endometrial cancer. 2021 [cited 2021 February 3]; Available from: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html#:~:text=The%20American%20Cancer%20Society%20estimates,or%20corpus)%20will%20be%20diagnosed.
Wang Q, et al. Targeted therapies in gynecological cancers: a comprehensive review of clinical evidence. Signal Transduct Target Ther. 2020;5(1):137.
Leon-Castillo A, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38(29):3388–97.
Cancer Genome Atlas Research, N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
Society, A.C. Key statistics for cervical cancer. 2021 [cited 2021 February 3]; Available from: https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html.
Vora C, Gupta S. Targeted therapy in cervical cancer. ESMO Open. 2018;3(Suppl 1):e000462.
Crusz SM, Miller RE. Targeted therapies in gynaecological cancers. Histopathology. 2020;76(1):157–70.
Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83.
Tewari KS, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37(26):2317–28.
Coleman R. Analysis of survivorship in high-risk patients on treated on GOG-218. Gynecol Oncol. 2013;130(1):e112–3.
Perren TJ, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96.
Oza AM, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–36.
Ledermann JA, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387(10023):1066–74.
Ledermann JA. Overall survival results of ICON6: a trial of chemotherapy and cediranib in relapsed ovarian cancer. J Clin Oncol. 2017;35(15):5506.
Tewari KS, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–43.
Tewari KS, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654–63.
Domchek SM, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203.
Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61.
Vanderstichele A. Randomized phase II CLIO study on olaparib monotherapy versus chemotherapy in platinum-resistant ovarian cancer. J Clin Oncol. 2019;37:5507.
Liu JF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–14.
Kaye SB, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–9.
Penson RT, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38(11):1164–74.
Kristeleit R, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095–106.
Swisher EM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87.
Moore KN, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636–48.
Ledermann J, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92.
Ledermann JA, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17(11):1579–89.
Pujade-Lauraine E, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84.
Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64.
Coleman RL, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–61.
Moore K. Maintenance olaparib for patients with newly diagnosed, advanced ovarian cancer and a BRCA mutation: 5-year follow-up from SOLO1. Ann Oncol. 2020;31(4):S1334.
Ray-Coquard I, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–28.
Gonzalez-Martin A, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–402.
Coleman RL, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403–15.
Gunderson CC, et al. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125(2):477–82.
Baker J, et al. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125(1):263–70.
Ushijima K, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25(19):2798–803.
Hubbs JL, et al. Systemic and local hormone therapy for endometrial hyperplasia and early adenocarcinoma. Obstet Gynecol. 2013;121(6):1172–80.
Abu-Rustum, N. NCCN clinical practice guidelines in oncology - uterine neoplasms. 2021 October 20, 2020 [cited 2021 February 5]; Version 1.2021:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
Rose PG, et al. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–6.
Altman AD, et al. Use of aromatase inhibitors as first- and second-line medical therapy in patients with endometrial adenocarcinoma: a retrospective study. J Obstet Gynaecol Can. 2012;34(7):664–72.
Fiorica JV, et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(1):10–4.
Whitney CW, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(1):4–9.
Herzog TJ. What is the clinical value of adding tamoxifen to progestins in the treatment [correction for treatment] of advanced or recurrent endometrial cancer? Gynecol Oncol. 2004;92(1):1–3.
Singh M, et al. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106(2):325–33.
Fader AN, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol. 2018;36(20):2044–51.
Fader AN, et al. Randomized phase II trial of carboplatin-paclitaxel compared with carboplatin-paclitaxel-trastuzumab in advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin Cancer Res. 2020;26(15):3928–35.
Erickson BK, et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: a multi-institutional cohort study. Gynecol Oncol. 2020;159(1):17–22.
Bookman MA, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21(2):283–90.
Monk BJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28(22):3562–9.
Schilder RJ, et al. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009;19(5):929–33.
Goncalves A, et al. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol Oncol. 2008;108(1):42–6.
Farley J, et al. Phase II study of cisplatin plus cetuximab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immunohistochemical expression: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;121(2):303–8.
Santin AD, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122(3):495–500.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Frenel JS, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the Phase Ib KEYNOTE-028 Trial. J Clin Oncol. 2017;35(36):4035–41.
Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 Study. J Clin Oncol. 2017;35(22):2535–41.
Varga A, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol. 2019;152(2):243–50.
Cancer Genome Atlas Research, N, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378–84.
Fader AN. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Gynecol Oncol. 2016;141:206–7.
Marabelle A, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38(1):1–10.
Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981–92.
Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Hamanishi J, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–22.
Disis ML, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019;5(3):393–401.
Matulonis UA, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–7.
Infante JR. Safety, clinical activity and biomarkers of atezolizumab (atezo) in advanced ovarian cancer. Ann Oncol. 2016;27(871P):vi300.
Moore KN, Pignata S. Trials in progress: IMagyn050/GOG 3015/ENGOT-OV39. A Phase III, multicenter, randomized study of atezolizumab versus placebo administered in combination with paclitaxel, carboplatin, and bevacizumab to patients with newly-diagnosed stage III or stage IV ovarian, fallopian tube, or primary peritoneal cancer. Int J Gynecol Cancer. 2019;29:430–3.
Moore K. Primary results from IMagyn050/GOG 3015/ENGOT-OV39, a double-blind placebo-controlled randomised phase III trial of bevacizumab-containing therapy +/- atezolizumab for newly diagnosed stage III/IV ovarian cancer. Ann Oncol. 2020;31:S1161–2.
Chung HC, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the Phase II KEYNOTE-158 Study. J Clin Oncol. 2019;37(17):1470–8.
Levinson K, et al. Immunotherapy in gynecologic cancers: what we know now and where we are headed. Am Soc Clin Oncol Educ Book. 2019;39:e126–40.
Coleman R. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer: results from the phase II innovaTV 204/GOG-3023/ENGOT-cx6 study. Ann Oncol. 2020;31:S1162–3.
Kverneland AH, et al. Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer. Oncotarget. 2020;11(22):2092–105.
Pedersen M, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Oncoimmunology. 2018;7(12):e1502905.
Fujita K, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1(5):501–7.
Aoki Y, et al. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res. 1991;51(7):1934–9.
Stevanovic S, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–93.
K., H.W. ADXS11-001 immunotherapy in squamous or non-squamous persistent/recurrent metastatic cervical cancer: results from stage I of the phase II GOG/NRG0265 study. J Clin Oncol. 2016;34:5516.
Tanyi JL, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018;10(436):eaao5931.
Dorigo O. Clinical data from the DeCidE1 trial: assessing the first combination of DPX-Survivac, low dose cyclophosphamide (CPA), and epacadostat (INCB024360) in subjects with stage IIc-IV recurrent epithelial ovarian cancer. J Clin Oncol. 2018;36:5510.
Cibula D. Dendritic cell-based immunotherapy (DCVAC/OvCa) with chemotherapy in patients with platinum-sensitive, relapsed, epithelial ovarian carcinoma: survival analysis of a phase II, open-label, randomized, multicenter trial (study SOV02). Gynecol Oncol. 2019;154:18.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Dr. Bruce performed the literature search and drafted the review. The work was critically revised by Dr. Powell. All authors read and approved the final submission.
Corresponding author
Ethics declarations
Shaina F. Bruce declares that she has no conflict of interest. Matthew A. Powell has received compensation for service as a consultant from Tesaro, Merck, Roche/Genentech, Clovis Oncology, AstraZeneca, Johnson & Johnson, and Eisai.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Bruce, S.F., Powell, M.A. The Use of Targeted Agents in the Treatment of Gynecologic Cancers. Curr. Treat. Options in Oncol. 23, 15–28 (2022). https://doi.org/10.1007/s11864-021-00918-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-021-00918-0