Opinion statement
Inflammatory breast cancer (IBC) remains the most aggressive type of breast cancer. During the past decade, enormous progress has been made to refine diagnostic criteria and establish multimodality treatment strategies as keys for the improvement of survival outcomes. Multiple genomic studies enabled a better understanding of underlying tumor biology, which is responsible for the complex and aggressive nature of IBC. Despite these important achievements, outcomes for this subgroup of patients remain unsatisfactory compared to locally advanced non-IBC counterparts. Global efforts are now focused on identifying novel strategies that will improve treatment response, prolong survival for metastatic patients, achieve superior local control, and possibly increase the cure rate for locally advanced disease. Genomic technologies constitute the most important tool that will support future clinical progress. Gene-expressing profiling of the tumor tissue and liquid biopsy are important parts of the everyday clinical practice aiming to guide treatment decisions by providing information on tumor molecular drivers or primary and acquired resistance to treatment. The International IBC expert panel and IBC International Consortium made a tremendous effort to define IBC as a distinct entity of BC, and they will continue to lead and support the research for this rare and very aggressive disease. Finally, a uniform platform is now required to develop and lead large, multi-arm, proof-of-concept clinical trials that perform rapid, focused, and cost-effective evaluations of potential novel therapeutics in IBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory breast cancer (IBC) is a very distinct entity among breast cancer subtypes. It accounts for only 5% of all breast tumors but has the highest rate of recurrence and tumor-related morbidity and mortality compared to other subtypes [1].
The diagnosis of IBC is determined by clinical and histopathologic characteristics. The clinical presentation is so unique that it usually is considered sufficient to confirm the diagnosis. Fulminant development of skin erythema and texture changes, often described as “peau d’orange”, along with breast engorgement with or without an underlying discrete breast mass are pathognomonic signs of IBC [2] (Fig. 1).
Symptoms progress rapidly, usually in a few weeks but less than 6 months, and affect more than one-third of the breast [3]. The absence of a palpable breast mass in some cases can lead to a misdiagnosis of IBC as mastitis or breast abscess, which will waste valuable time and increase the risk of metastatic spread [4]. It is very important to differentiate skin changes typical of IBC from those observed in locally advanced breast cancer (LABC) in which a neglected tumor invades the skin causing erythema, edema, and ulceration of the breast. In LABC, the skin changes never extend uniformly in the entire skin, and typically, this involvement is not associated with diffuse edema. The absence of estrogen receptor expression, higher tumor grade, and younger age at presentation may be more indicative of IBC [5].
Multiple small retrospective studies have shown that IBC has a higher recurrence rate and worse survival compared to non-inflammatory LABC. For example, in a retrospective study from MD Anderson, 240 patients diagnosed with IBC and 831 patients with LABC enrolled in prior clinical studies were evaluated for recurrence-free survival (RFS) and overall survival (OS) during a 69-month median follow-up period. Treatment regimens were very similar in both cohorts. Data analyses showed that patients with IBC had higher incidences of locoregional recurrence, including skin and lymph nodes and distant soft tissue and bone disease. Patients with IBC had a 35% 5-year RFS compared to 56% for patients who had LABC; 5-year OS was 40.5% and 63.2%, respectively. Interestingly, in both cohorts, patients who achieved pathological complete response (pCR) after induction chemotherapy had no difference in RFS and OS [6].
Little is known about predisposing factors for the development of IBC. Classic risk factors for BC such as early menarche and late menopause, nulliparity, lack of breast feeding, obesity, hormone-replacement therapy, family or personal history of BC, and alcohol and tobacco consumption have also been shown to play a role in IBC. African American women have a higher prevalence of IBC compared to other BC subtypes, while white race is associated with a higher risk of the disease [7].
To define the most relevant risk factors, a retrospective study from MD Anderson evaluated 246 patients with IBC compared to 397 women who were cancer-free. Obesity was found to be a relevant risk factor for IBC regardless of the histologic subtype. Having an increased number of children was identified as a risk factor for triple-negative breast cancer (TNBC) and triple-negative IBC, but also for HER2-expressing IBC. No breastfeeding history was associated with a higher risk of triple-negative and estrogen receptor (ER)–positive IBC [8].
In a more recent case-control study from Schairer and colleagues, 617 patients with IBC, 1151 with LABC, and 7600 who had invasive BC with no chest wall or skin involvement were selected from the Breast Cancer Surveillance Consortium database. According to this study, there was an increased risk of IBC in obese and overweight women regardless of their menopausal status. A family history of BC and high mammographic breast density were associated with an increased risk of IBC, while women with a higher level of education had a lower risk [9].
BMI and obesity seem to be important risk factors and play a prognostic role in IBC. Excessive production of estrogen from adipose tissue steroids through aromatase inhibitor enzymes has been linked with an increased risk of BC in postmenopausal women [10]. In addition to direct stimulation of breast epithelial cell proliferation from excessive estrogens, more complex mechanisms of oncogenesis have been proposed. Insulin resistance that occurs in overweight or obese individuals activates insulin-like growth factor 1 (IGF-1) receptor pathway, which in turn activates cell-signaling pathways such as PI3K/AKT, leading to cell proliferation and decreased apoptosis [11].
For decades, researchers investigated the possible implication of viral infection in the pathogenesis of BC and IBC in particular. An underlying infection from viruses such as herpesviruses, papillomaviruses, or polyomaviruses was hypothesized to be responsible for the inflammatory manifestation of IBC.
Fina and colleagues analyzed 509 BC samples from geographical areas with high, intermediate, or low risk of nasopharyngeal cancer for the presence of Epstein-Barr virus (EBV). The virus was present in 31.8% of samples localized to the tumor epithelial cells. There was no significant difference based on geographical distribution except for higher viral loads in the endemic EBV areas [12].
In an Algerian study, 155 paraffin-embedded breast cancer specimens, including approximately two-thirds non-IBC and one-third IBC, were screened for the presence of viral DNA. They found viral DNA in 22 patients, with the most prevalent being Epstein-Barr 1 virus and human papillomavirus 16. Triple-negative tumors and IBC were more likely to be positive for viral DNA compared to other subtypes [13].
The mouse mammary tumor virus is another retrovirus that has been studied as a possible link to BC. However, the results from multiple studies in a variety of BC subtypes and geographic areas are contradictory, and no clear association has been proven to date [14,15,16].
Diagnostic workup
Diagnostic imaging is necessary to detect the primary breast lesion and to determine the extension of disease, including nodal status and distant metastases. Mammography is the first diagnostic modality performed to evaluate the clinical findings. An underlying breast lesion, skin thickening, and enlarged axillary lymph nodes are the most common findings shown on mammography [17]. Particularly for IBC, mammography can fail to detect the primary tumor and/or pathologic axillary lymph nodes in more than 40% of cases [18]. Ultrasonography has demonstrated high sensitivity in identifying lymph node involvement and can be used in combination with mammography to increase the test sensitivity [19].
Breast MRI is a very important diagnostic tool for IBC and has demonstrated higher sensitivity in detecting primary breast lesions in these patients compared to mammogram [20]. Extensive skin thickening, edema, and enhancement along with diffuse non-mass enhancement in the breast are typical radiographic features of IBC that clearly differentiate it from LABC [21] (Fig. 2).
For the assessment of nodal status, MRI and US can perform equally well. However, MRI has higher sensitivity in detecting N2-3 disease, including subpectoral and internal mammary lymph nodes and infraclavicular disease [22]. Monitoring treatment response and assessment of residual disease prior to surgery is required to optimize the surgical plan. MRI should be considered for this purpose because as outlined above, it has the higher sensitivity to detect the primary breast lesions and lymph node involvement.
Considering the very fulminant presentation of IBC, a detailed staging is recommended at the time of diagnosis [23]. Standard imaging techniques such as CT scans and nuclear bone scans are widely used for the initial staging of IBC, often with very low sensitivity.
The majority of patients diagnosed with IBC have axillary lymph node involvement at the time of presentation [23]. The use of advanced imaging techniques such as PET/CT scans has demonstrated that it is not uncommon to find distant metastases very early on in the disease course. PET/CT has demonstrated exceptionally high sensitivity on identifying nodal and distant metastases [24] (Fig. 3).
Several studies, most of them performed retrospectively, have investigated the role of PET/CT scans for IBC staging. According to this studies, PET/CT scans were able to detect distant metastatic lesions that were not identified by classic imaging [20, 25, 26].
Pathological aspects of IBC
While clinical and radiographic features of IBC are very specific, indicating a distinct entity of BC, the pathological diagnosis follows the classical principles of breast tumors in general. A core biopsy of the underlying mass and/or skin changes is required to confirm the malignancy.
IBCs are predominantly ductal carcinomas, but they can also be lobular or other histologic subtypes of BC. The assessment of immunohistochemical tumor markers ER/PR and HER-2 is necessary to decide the adequate systemic treatment regimen. IBCs usually are high-grade tumors and lack expression of endocrine receptors [27]. During the past years, an increase in the incidence of the HER2 overexpressing IBC has been reported [28].
Invasion of breast or dermal lymphatics by tumor emboli represents the pathologic hallmark of IBC [29]. This leads to drainage obstruction of the lymphatic vessels, which is responsible for the breast and skin clinical inflammatory manifestations and also the aggressive nature of IBC [30•].
Skin punch biopsy from the site of the most intense erythema should be considered to confirm the presence of tumor emboli in the dermal lymphatics. However, the absence of this finding does not exclude the diagnosis of IBC.
A very interesting and exclusive finding of IBC tumors is the presence of macrophages and mammary stem cells in the normal breast tissue surrounding the primary tumor. According to research data, CD44/CD49/CD133-positive mammary cells and CD68-positive macrophages are remarkably higher in IBC-surrounding normal breast tissue compared to those in non-IBC [31•].
Current treatment strategies - international expert panel consensus guidelines
The treatment of IBC remains a challenge despite the progress made in defining underlying tumor biology and breast cancer treatment in general. The rarity of the disease makes conduction of specific, large prospective clinical trials more difficult. Furthermore, the lack of uniformity in the diagnostic criteria and in treatment sequence and regimens creates barriers in analyzing IBC data retrospectively. Current expert panel guidelines recommend a multimodality approach as the standard treatment strategy for IBC. Application of preoperative systemic therapy followed by surgery and radiation has significantly improved survival outcomes [32].
Upfront chemotherapy with anthracycline and taxane regimens and the addition of HER2-targeted agents for HER2-expressing tumors is currently the standard approach for IBC and LABC. The prognostic significance of a pathological complete response (pCR) to the neoadjuvant chemotherapy (NAC) in BC patients including IBC is well-established. Multiple retrospective clinical studies have suggested that pCR is associated with excellent survival outcomes.
In a retrospective study of 240 IBC patients, 178 were treated with fluorouracil, doxorubicin, and cyclophosphamide combination (FAC), and 62 patients were treated with FAC and paclitaxel sequentially. Patients who received the FAC/paclitaxel regimen had a significantly higher pCR rate compared to patients who received FAC alone (25% vs 10%). Progression-free survival was 27 vs 18 months, and overall survival was 54 vs 32 months in favor of the paclitaxel combination [33].
Liu and colleagues analyzed data from the National Cancer Database of 593 patients with IBC that compared pCR rate and OS between HR and HER2-positive or HER2-negative cohorts. Interestingly, the HER2+/HR− cohort achieved the highest pCR rate, and HER2+ patients, regardless of HR status, had better survival compared to HER2−/HR+ and TN-IBC. According to this study, HR+ status is not a favorable prognostic marker for IBC as it is established for non-IBC patients. TN-IBC, advanced stage, and uncomplete surgical resection were associated with worse prognosis [34•].
Another study from Van Uden and colleagues evaluated 679 IBC patients with stage III tumors who received NAC between 2006 and 2015. Patients with HER2+/HR− tumors had a higher possibility of achieving a pCR (43%, p < 0.001) compared to other subtypes. Notably, patients from all subtypes who achieved a pCR after NAC had improved survival outcomes, but the benefit was higher for those who had HER2+ tumors [35•]. The above results are in concordance with large, prospective studies in which the addition of dual HER2-targeted therapy, trastuzumab, and pertuzumab, to backbone chemotherapy, has drastically improved clinical response rates and survival for HER2-overexpressing BC [36, 37].
After completing NAC, a radical mastectomy with axillary lymph node dissection is the recommended surgical approach for patients with IBC. Breast-conserving or skin-sparing surgery is not an appropriate treatment choice, especially for patients who have residual disease after the NAC. During the past few years, efforts have been made to consider potential breast-conserving treatment for IBC. Specifically, for patients who have a very good response to NAC, it has been reported that a breast-conserving approach is adequate and has similar long-term outcomes compared with standard mastectomy [38, 39]. However, these results are in contradiction to those previously reported in large, retrospective study, which suggested that the breast-conserving approach was associated with a higher rate of recurrence [40]. Longitudinal prospective studies are warranted to investigate the adequacy of breast-conserving surgery for IBC patients.
Postsurgical radiation to the chest wall and axillary, internal mammary, and infraclavicular and supraclavicular lymph nodes should be offered to all IBC patients. In specific situations, when tumors respond poorly to chemotherapy, radiation can be given preoperatively. Dose escalation up to 60–66 Gy and hypofractionation have demonstrated superiority in locoregional control [41].
Finally, adjuvant endocrine therapy and/or HER2-targeted treatment are recommended for patients who have HR+ and HER2+ disease. For patients who have significant residual disease escalation, strategies applied for BC subtypes in general can also be used for IBC. Data reported from large, randomized trials suggested that patients who did not achieve a pCR from NAC can receive additional adjuvant treatment to improve survival outcomes. CDK4/6 inhibitors in combination with endocrine therapy are currently being evaluated as an adjuvant treatment for patients with HR+ disease who have high-risk tumors and/or residual disease after NA chemotherapy. In HER2-positive patients, adjuvant T-DM1 or neratinib can be considered for patients with residual invasive disease who have an increased risk of recurrence [42, 43]. For TN-IBC, chemotherapeutic drugs and novel agents such as capecitabine or immune checkpoint inhibitors are being used to improve survival [44,45,46].
Molecular signature of IBC
It is suggested that the aggressive nature of IBC is linked with the explicit molecular signature. Van Laere and colleagues analyzed data collected from 137 IBC and 252 non-IBC patient samples under the World IBC Consortium. PAM50 algorithm was used to classify Affymetrix (HGU133-series) gene expression profiles of IBC patients from three research institutions in the USA and Europe. According to this study, IBC displayed four intrinsic clusters that corresponded to four molecular subtypes, with 75% of the samples classified as aggressive subtypes including Basal-like, ErbB2+, and Luminal B, while 19% were Luminal A. For patients with non-IBC, the distribution was 54% and 42%, respectively. In addition, ErbB2+ subtype accounted for 22% of IBC compared with 9% in non-IBC patients [47]. Of note, this study identified that IBC tumors have characteristically reduced transforming growth factor beta (TGF-β) signaling which has been reported to be involved in tumor-cell motility. Inhibition of TGF-β signaling prevents single-cell motility but permits collective-cell migration that can lead to massive lymphatic invasion, which is the hallmark of IBC [48].
Liang and colleagues analyzed NGS data from 156 IBC and 197 non-IBC stages III and IV untreated tumors. Alterations in several genes such as TP53, ERBB4, BRCA2, BRCA1, NOTCH2, ESR1, FGFR3, and EGFR were found more frequently in IBC, while CDH1 was more typical for non-IBC tumors. In this study, alterations on PI3k/AKT/mTOR pathway were common for IBC especially for HR+ tumors. However, the presence of PIK3CA mutations was associated with worse survival outcomes for HER2+ and TN-IBC, while such a prognostic significance was not observed for HR+ IBC [49].
Consistent with the abovementioned results are also the data from Ross and colleagues who performed comprehensive genomic profiling of 53 IBC tumor specimens. Of note, 96% of sequenced cases had at least one genomic alteration associated with an available clinical trial or FDA-approved drug. The most common alterations were TP53, MYC, PIK3CA, ERBB2, FGFR1, BRCA2, and PTEN [50].
A recent study from Bertucci and colleagues analyzed NGS data from 101 IBC and 2351 non-IBC treatment–naïve primary tumors. According to this study, IBC has a very distinct genomic landscape compared to non-IBC tumors. Specifically, molecular subtype distribution is very different between IBC and non-IBC; HR+/HER2−, HER2+, and TN were 25%, 35%, and 40%, respectively for IBC versus 68%, 15%, and 17% for non-IBC tumors. Moreover, IBC had a distinct pattern of genetic alterations with TP53, ERBB2, MYC, PIK3CA, BRCA, CCND1, GATA3, NOTCH1, FGFR1, JAK2, and ARID1A being the most frequently altered genes. Importantly, genomic alterations of TP53 and genes involved in NOTCH and DNA repair pathways were more frequent for IBC, while PIK3CA was more frequent in non-IBC. When stratified based on metastatic status, metastatic IBC tumors had similar genomic expression with non-IBC except for ERBB2 amplification, which was remarkably higher in IBC [51••].
Prospective endeavors
To date, IBC remains the most aggressive form of BC, with very high recurrence rates and poor prognosis. Future efforts should be focused on understanding the biology of this unique disease and identifying novel treatment strategies. Treatment combinations that will increase the pCR rate are warranted for this aggressive disease. In this regard, understanding the molecular mechanism of tumor drivers and resistance to treatment is fundamental for the implementation of biologically informed treatment approaches. Gene-expressing profiling of the primary and residual disease can provide valuable information for the primary and acquired mechanism of resistance to treatment and identify potential actionable alterations. Current targeted agents such as CDK4/6, PARP, and PIK3CA/AKT inhibitors, along with the under development novel agents, constitute a great potential to improve outcomes for IBC patients. Several trials are underway and will likely augment treatment options for this disease in the future (Table 1).
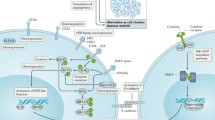
Activated pathways in IBC
EGFR pathway activation was found to be associated with an increased recurrence rate and worse survival for patients who have IBC [52]. It regulates cancer stem cells through COX2 activation [53]. Suppression of this pathway has been shown to control tumor proliferation and increase apoptosis. An open, single-arm study from Matsuda and colleagues evaluated the safety and efficacy of the anti-EGFR antibody panitumumab in HER2− IBC. Panitumumab was administered in combination with NA chemotherapy, four cycles of weekly nab-paclitaxel and carboplatin, followed by four cycles of FEC. According to study results, the addition of panitumumab to NA chemotherapy was associated with a higher pCR rate. Particularly for TN-IBC, a pCR was achieved in 40% of patients [54••]. A larger, randomized phase II trial is ongoing and will define the role of panitumumab in IBC.
Hotspot mutations in PIK3CA were found in 21% of IBC tumors in a study performed by Hamm and colleagues. Furthermore, HER3 point mutations were very frequent in this IBC cohort, which may suggest a role of anti-HER small molecule such as lapatinib, neratinib, or afatinib for the treatment of IBC [55].
The role of angiogenesis and lymphangiogenesis in IBC has been studied extensively. According to research data, IBC displays increased angiogenic activity compared to non-IBC tumors. However, the role of antiangiogenic agents in this disease has not yet been established. A large number of clinical trials investigated the efficacy of bevacizumab, a monoclonal antibody that targets VEGF resulting in inhibition of angiogenesis, in BC patients. The exploratory analyses for the IBC subpopulation did not show any significant clinical benefit from the addition of bevacizumab to standard-of-care chemotherapy. A potential explanation for the poor results may be the fact that IBC tumors are very enriched in cancer stem cells (CSCs) that use the hypoxic environment generated by antiangiogenic agents to develop resistant clones [56]. Furthermore, bevacizumab as a single antiangiogenic agent seems insufficient to abrogate angiogenesis. Based on this observation, the research focus has turned towards identifying novel and more potent antiangiogenic agents [57].
Rebastinib is an oral novel agent targeting the Tie-2 tyrosine kinase receptor, which is often expressed on the endothelial cells and tumor-associated macrophages. It is currently being investigated in a phase 1 clinical trial in combination with paclitaxel for advanced solid tumors, including TN-IBC (NCT03601897).
In a preclinical study, the histone deacetylase (HDAC) inhibitor romidepsine potently inhibited the VEGF and hypoxia-inducible factor 1 alpha (HIF-1a) proteins, resulting in destruction of lymphatic vascular architecture and IBC tumor emboli. When used in combination with paclitaxel, it eliminated primary tumor and distant sites [58]. Further investigation is needed to elucidate the role of HDAC inhibitors as a therapeutic strategy for IBC.
As described in the previously mentioned molecular studies, DNA repair pathway is one of the most frequently altered pathways in IBC. Several genes such as BRCA2, ATM, ATR, BRCA1, POLE, PALB2, and FANCA are found to be altered, very frequently, resulting in a high homologous repair deficiency (HRD) score. This fact suggests a potential benefit from DNA damage repair–targeted agents such as PARP inhibitors or other underdeveloped drugs.
According to Hamm and colleagues who performed a genomic and immunologic profiling of 19 IBC specimens, genetic alterations in the DNA repair pathway were associated with more genomic instability and elevated tumor-infiltrating lymphocytes (TIL) [55]. It is also suggested that a high level of CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in BC patients [59]. Taken together, these results can support a synergistic effect of DNA repair pathway inhibitors and immune checkpoint agents.
A new approach, currently under investigation, is the administration of a PARP inhibitor concurrently with adjuvant radiation treatment for BC patients to achieve a higher local control. PARP inhibitors are proven to have a synergistic effect by preventing the repair of the DNA damage caused by radiation and acting as radiosensitizers [60, 61]. Radiolabeled PARP inhibitors, that are currently underway, are also a very promising therapeutic approach [62].
The Janus kinase (JAK) family consists in four enzymes that are normally found in mammalian cells and are involved in a variety of functions such as growth, neural development, hematopoiesis, and immune response. They phosphorylate and activate transcriptional factor STAT through cytokine signal [63, 64]. The JAK2/STAT3 pathway is suggested to be responsible for treatment resistance in IBC patients [65]. A phase II study is investigating the efficacy of paclitaxel in combination with ruxolitinib, a JAK2/STAT3 pathway inhibitor, as part of NAC for patents with TN-IBC [66] (NCT02876302). Ruxolitinib is currently used for the treatment of myelofibrosis, polycythemia vera, and acute graft-versus-host disease (GVHD) in patients 12 years of age and older. Other JAK inhibitors such as tofacitinib and baricitinib are being used to treat rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.
Genes related to the NOTCH pathway are found to be explicitly altered in IBC. TN tumors are more likely to have NOTCH mutations compared to HR+ disease. Novel agents targeting these genes are potential treatment options for IBC and need to be investigated in the future [67].
Cyclooxygenase-2 (Cox-2) is found to be overexpressed in IBC, and several studies have investigated the antitumorigenic effect of Cox-2 inhibitors. Cox-2 is involved in the prostaglandin E2 (PGE2) production; therefore, inhibition of PGE2 through the blockade of prostanoid receptors such as EP1, EP2, EP3, and EP4 is an alternative way to inhibit Cox-2 activity [68]. In addition, overexpression of COX-2 can lead to upregulation of CCR7, EP2, and EP4 in BC specimens, and CCR7 and EP receptor pathways are associated with lymphatic invasion [69]. Selective EP4 antagonist, GW627368X, has shown an antitumor effect especially in aggressive tumors such as TNBC and IBC, which make it a potential treatment strategy that needs to be explored in the future [70].
Immune checkpoint inhibitors
During the past few years, we have witnessed the radical transformation of the cancer treatment paradigm with the introduction of immunotherapy. Immune checkpoint inhibitors have demonstrated unprecedented results for different types of tumors and established the principle of biology-informed treatments as opposed to histology-based. In BC, the use of immunotherapy is currently approved for the metastatic TNBC patients, with high PD-L1 expression on the tumor-associated immune cells [71••].
In IBC, PD-L1 is expressed in approximately 40% of tumors and was found to be associated with a better response to chemotherapy. Tumor-infiltrating immune cells are also suggested to be associated with a better response to treatment, and in addition, they had favorable prognostic significance [72, 73]. Several trials are currently underway to investigate the activity of immune checkpoint inhibitors in metastatic IBC, with very promising results.
Immunotherapy is also being investigated in the early setting of the disease. Herein, data reported from the large phase III randomized study, KEYNOTE-522, indicate an increase in pCR rate in patients who received combination pembrolizumab and chemotherapy compared with those who received placebo-chemotherapy. According to this trial, the benefit from pembrolizumab in early-stage BC seems to be independent from PD-L1 expression [74••].
In addition, the identification of biomarkers that can reliably predict the response to immune checkpoint inhibitors is a priority. Thus far, tumor mutational burden (TMB), microsatellite instability-high or mismatch repair deficiency (MSI-H/dMMR), and PD-L1 expression are FDA-approved biomarkers to predict response to immunotherapy. However, there are limitations in their use that warrant an improvement with more robust predictive biomarkers.
Detection of microscopic disease as prognostic and predictive biomarker
Another very important aspect of cancer treatment is the characterization of circulating microscopic disease [75]. Over the past decade, research data have elucidated the prognostic significance of circulating tumor cells (CTCs) for cancer patients [76].
For IBC in particular, tumor spread is highly suspected in the very early course of the disease, and characterization of CTCs is a very important tool to determine the extent of disease [77]. Furthermore, the presence of CTCs is associated with worse prognosis, which is proportional with the volume of microscopic disease [76]. The future mission is to integrate the microscopic disease extent into disease staging and treatment decisions.
A multicenter, phase II, prospective study evaluated 137 patients with IBC separated into two cohorts based on HER2-positive or HER2-negative status. All of the patients received neoadjuvant chemotherapy appropriate for the subtype and were assessed for CTCs at baseline and after treatment. Patients who had nondetectable CTCs at baseline and pCR after NAC had excellent survival outcomes compared with patients who had positive CTCs and no-pCR. Triple-negative IBC was also associated with poor prognoses [78].
In summary, IBC remains the most aggressive form of BC with the highest morbidity and mortality rate. For locally advanced disease, a multimodality treatment approach including NAC, surgery, and radiation has resulted in superior survival outcomes. Research efforts are now focused on identifying novel treatment strategies that will improve survival for the IBC patients. Genomic assays are very important tools for the detection of primary and acquired resistance to treatment and in selecting treatment strategies. Furthermore, in-depth understanding of molecular biology of IBC will help to identify relevant genomic alterations and oncogenic pathways that drive tumor growth and spread. Forthcoming studies will give valuable information that can be applied in clinics to improve treatment outcomes.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fouad TM, et al. Overall survival differences between patients with inflammatory and noninflammatory breast cancer presenting with distant metastasis at diagnosis. Breast Cancer Res Treat. 2015;152(2):407–16.
Walshe JM, Swain SM. Clinical aspects of inflammatory breast cancer. Breast disease. 2005;22:35–44.
Hance KW, et al. Trends in inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results Program at the National Cancer Institute. JNCI: Journal of the National Cancer Institute. 2005;97(13):966–75.
Iglesias A, et al. Benign breast lesions that simulate malignancy: magnetic resonance imaging with radiologic-pathologic correlation. Curr Probl Diagn Radiol. 2007;36(2):66–82.
Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(12):2254–9.
Cristofanilli M, et al. Inflammatory breast cancer (IBC) and patterns of recurrence. Cancer. 2007;110(7):1436–44.
Levine PH, Veneroso C. The epidemiology of inflammatory breast cancer. Semin Oncol. 2008;35(1):11–6.
Atkinson RL, et al. Abstract P6-12-04: Risk factors for inflammatory breast cancer. Cancer Res. 2013;73(24 Supplement):P6-12-04.
Schairer C, et al. Risk factors for inflammatory breast cancer and other invasive breast cancers. J Natl Cancer Inst. 2013;105(18):1373–84.
Key TJ, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–26.
Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–18.
Fina F, et al. Frequency and genome load of Epstein-Barr virus in 509 breast cancers from different geographical areas. Br J Cancer. 2001;84(6):783–90.
Corbex M, et al. Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from algeria compared with other types of breast cancer tumors. PLoS One. 2014;9(12):e114559-e114559.
Pogo BGT, Holland JF, Levine PH. Human mammary tumor virus in inflammatory breast cancer. Cancer. 2010;116(S11):2741–4.
Witt A, et al. The mouse mammary tumor virus-like env gene sequence is not detectable in breast cancer tissue of Austrian patients. Oncol Rep. 2003;10(4):1025–9.
Mant C, et al. Human murine mammary tumour virus-like agents are genetically distinct from endogenous retroviruses and are not detectable in breast cancer cell lines or biopsies. Virology. 2004;318(1):393–404.
Günhan-Bilgen I, Üstün EE, Memiş A. Inflammatory breast carcinoma: mammographic, ultrasonographic, clinical, and pathologic findings in 142 cases. Radiology. 2002;223(3):829–38.
Chow CK. Imaging in inflammatory breast carcinoma. Breast disease. 2005;22:45–54.
Lee KW, et al. Inflammatory breast cancer: imaging findings. Clin Imaging. 2005;29(1):22–5.
Yang WT, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109(3):417–26.
Girardi V, et al. Inflammatory breast carcinoma and locally advanced breast carcinoma: characterisation with MR imaging. La Radiologia medica. 2011;116(1):71–83.
Uematsu T. MRI findings of inflammatory breast cancer, locally advanced breast cancer, and acute mastitis: T2-weighted images can increase the specificity of inflammatory breast cancer. Breast cancer (Tokyo, Japan). 2012;19(4):289–94.
Matro JM, et al. Inflammatory breast cancer management in the national comprehensive cancer network: the disease, recurrence pattern, and outcome. Clin Breast Cancer. 2015;15(1):1–7.
Groheux D, et al. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54(1):5–11.
Carkaci S, et al. Retrospective study of 18F-FDG PET/CT in the diagnosis of inflammatory breast cancer: preliminary data. Journal of nuclear medicine : official publication. Society of Nuclear Medicine. 2009;50(2):231–8.
Alberini J-L, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer. 2009;115(21):5038–47.
Resetkova E. Pathologic aspects of inflammatory breast carcinoma: part 1. Histomorphology and differential diagnosis. Semin Oncol. 2008;35(1):25–32.
Parton M, et al. High incidence of HER-2 positivity in inflammatory breast cancer. Breast (Edinburgh, Scotland). 2004;13(2):97–103.
Bonnier P, et al. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62(4):382–5.
• Hirko KA, et al. Abstract P6-09-10: Association of dermal lymphatic involvement and survival in inflammatory breast cancer. Cancer Res. 2019;79(4 Supplement):P6-09-10. Represents new data on dinstic biology of IBC tightly related with disease prognosis.
• Reddy JP, et al. Mammary stem cell and macrophage markers are enriched in normal tissue adjacent to inflammatory breast cancer. Breast Cancer Res Treat. 2018;171(2):283–93 Elucidates the unique morphology of IBC.
Perez C, Graham M, Taylor M. Management of locally advanced carcinoma of the breast. I Noninflammatory Cancer. 1994;74(1 Suppl):453–65.
Cristofanilli M, Buzdar A, Sneige N. Paclitaxel in the multimodality treatment for inflammatory breast carcinoma. Cancer. 2001;92:1775–82.
• Liu J, et al. Chemotherapy response and survival of inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes approximation: an analysis from the National Cancer Database. Journal of cancer research and clinical oncology. 2017;143(1):161–8. Large cohort study demonstrating the prognosis and treatment strategy based on tumor subtype.
• van Uden DJP, et al. Pathologic complete response and overall survival in breast cancer subtypes in stage III inflammatory breast cancer. Breast cancer research and treatment. 2019;176(1):217–26 Confirm the importance of pCR for IBC as for all BC in general.
Gianni L, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7.
Schneeweiss A, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Annals of oncology : official journal of the European Society for Medical Oncology. 2013;24(9):2278–84.
Brzezinska M, Dixon JM. Inflammatory breast cancer: no longer an absolute contraindication for breast conservation surgery following good response to neoadjuvant therapy. Gland surgery. 2018;7(6):520–4.
Chen H, et al. A standard mastectomy should not be the only recommended breast surgical treatment for non-metastatic inflammatory breast cancer: a large population-based study in the Surveillance, Epidemiology, and End Results database 18. Breast (Edinburgh, Scotland). 2017;35:48–54.
Rueth NM, et al. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the National Cancer Database. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(19):2018–24.
Woodward W, Buchholz T. The role of locoregional therapy in inflammatory breast cancer. Semin Oncol. 2008;35:78–86.
von Minckwitz G, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Martin M, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017:18.
Masuda N, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
Schmid P, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Wang XI, et al. Phase III trial of metronomic capecitabine maintenance after standard treatment in operable triple-negative breast cancer (SYSUCC-001). J Clin Oncol. 2020;38(15_suppl):507-507.
Van Laere SJ, et al. Uncovering the molecular secrets of inflammatory breast cancer biology: an integrated analysis of three distinct affymetrix gene expression datasets. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(17):4685–96.
Giampieri S, et al. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287–96.
Liang X, et al. Targeted next-generation sequencing identifies clinically relevant somatic mutations in a large cohort of inflammatory breast cancer. Breast Cancer Res. 2018;20(1):88.
Ross JS, et al. Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. Breast Cancer Res Treat. 2015;154(1):155–62.
•• Bertucci F, et al. NOTCH and DNA repair pathways are more frequently targeted by genomic alterations in inflammatory than in non-inflammatory breast cancers. Molecular Oncology. 2020;14(3):504–19. Large retrospective study that gives very important information on tumor biology and potential future treatment targets.
Cabioglu N, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2007;18(6):1021–9.
Wang X, et al. EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer. Oncotarget. 2017;8(40):67904–17.
•• Matsuda N, et al. Safety and efficacy of panitumumab plus neoadjuvant chemotherapy in patients with primary HER2-negative inflammatory breast cancer. JAMA Oncol. 2018;4(9):1207–13. New treatment strategies for IBC.
Hamm CA, et al. Genomic and immunological tumor profiling identifies targetable pathways and extensive CD8+/PDL1+ immune infiltration in inflammatory breast cancer tumors. Mol Cancer Ther. 2016;15(7):1746.
Conley SJ, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A. 2012;109(8):2784–9.
Yamauchi H, et al. Molecular targets for treatment of inflammatory breast cancer. Nat Rev Clin Oncol. 2009;6(7):387–94.
Robertson FM, et al. The class I HDAC inhibitor Romidepsin targets inflammatory breast cancer tumor emboli and synergizes with paclitaxel to inhibit metastasis. J Exp Ther Oncol. 2013;10(3):219–33.
Mahmoud SM, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55.
Jagsi R, et al. Concurrent veliparib with chest wall and nodal radiotherapy in patients with inflammatory or locoregionally recurrent breast cancer: the TBCRC 024 Phase I Multicenter Study. J Clin Oncol. 2018;36(13):1317–22.
Kirova YM, et al. Abstract OT3-04-01: a phase I of olaparib with radiation therapy in patients with inflammatory, loco-regionally advanced or metastatic TNBC (triple negative breast cancer) or patient with operated TNBC with residual disease. Cancer Res. 2018;78(4 Supplement):OT3-04-01.
Pirovano G, et al. Targeted brain tumor radiotherapy using an Auger emitter. bioRxiv. 2019:649764.
Banerjee S, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–46.
Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296(5573):1653–5.
Jhaveri K, et al. Hyperactivated mTOR and JAK2/STAT3 pathways: molecular drivers and potential therapeutic targets of inflammatory and invasive ductal breast cancers after neoadjuvant chemotherapy. Clinical breast cancer. 2016;16(2):113-22.e1.
Overmoyer B, et al. Abstract OT3-05-01: TBCRC 039: phase II study of combination ruxolitinib (INCB018424) with preoperative chemotherapy for triple negative inflammatory breast cancer. Cancer Res. 2018;78(4 Supplement):OT3-05-01.
Bertucci F, et al. NOTCH and DNA repair pathways are more frequently targeted by genomic alterations in inflammatory than in non-inflammatory breast cancers. Mol Oncol. 2020;14(3):504–19.
Robertson FM, et al. Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J Exp Ther Oncol. 2008;7(4):299–312.
Pan MR, et al. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J Biol Chem. 2008;283(17):11155–63.
Majumder M, et al. EP4 as a therapeutic target for aggressive human breast cancer. Int J Mol Sci. 2018;19(4).
•• Schmid P, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 2020;21(1):44–59 Practice changing study.
Bertucci F, et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget. 2015;6(15):13506–19.
Van Berckelaer C, et al. Infiltrating stromal immune cells in inflammatory breast cancer are associated with an improved outcome and increased PD-L1 expression. Breast Cancer Res. 2019;21(1):28.
•• Schmid P, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21 New treatment strategy.
Lucci A, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. The Lancet. Oncology. 2012;13(7):688–95.
Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91.
Mego M, et al. Circulating tumor cells (CTCs) are associated with abnormalities in peripheral blood dendritic cells in patients with inflammatory breast cancer. Oncotarget. 2017;8(22):35656–68.
Pierga J-Y, et al. Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2016;28(1):103–9.
Acknowledgements
The authors are grateful to Prof. Sergio Crispino, Scientific Director ASSO and Scientific Director Anticancer Fund, for his insights and suggestions.
Author information
Authors and Affiliations
Contributions
This study received funding from Associazione per lo Sviluppo della Scienza Oncologica (ASSO), Siena, Italy.
Corresponding author
Ethics declarations
Conflict of Interest
Elena Vagia declares that she has no conflict of interest. Massimo Cristofanilli has received research funding from Eli Lilly, Pfizer, and G1 Therapeutics, and has received compensation for service as a consultant from CytoDyn, Eli Lilly, Pfizer, Sermonix, G1 Therapeutics, and Foundation Medicine.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Vagia, E., Cristofanilli, M. New Treatment Strategies for the Inflammatory Breast Cancer. Curr. Treat. Options in Oncol. 22, 50 (2021). https://doi.org/10.1007/s11864-021-00843-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00843-2