Opinion statement
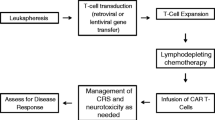
Chimeric receptor antigen (CAR) T cells are an innovative cellular immunotherapeutic approach that involves genetic modification of T cells to express CAR targeting tumor antigen. Prior to the development of CAR-T, the only potential cure for patients with relapsed or refractory (RR) acute lymphoblastic leukemia (ALL) was allogeneic hematopoietic stem cell transplantation (HSCT). Several CAR-T cell products have been studied in prospective clinical trials which ultimately have resulted in the approval of one anti-CD19 CAR-T cell product in pediatric RR ALL: tisagenlecleucel (CD3ζ and 41BB). While some patients achieve durable responses with CAR-T, lack of response and relapse remains clinical challenges. Reasons for sub-optimal response include lack of CAR-T cell persistence and target antigen down-regulation. Future CARs are under development to improve long-term persistence and to be able to overcome resistance mechanisms associated with the disease and the hostile tumor microenvironment. With evolving understanding about CARs and new constructs under investigation, there is optimism that future products will improve the safety and efficacy from the current standard of care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B-cell acute lymphoblastic leukemia (ALL) is an aggressive hematological malignancy that is fatal without immediate treatment. This malignancy is more common in children but can present at any age from infancy to adulthood. The standard management involves induction chemotherapy followed by consolidation and maintenance therapies in pediatric and young adult patients, while allogeneic hematopoietic stem cell transplantation (allo-HCT) is often considered as a consolidation therapy in adult patients (age > 40) [1]. While the clinical outcomes of ALL have improved over the last two decades as a result of novel drugs and targeted therapies, for those with relapsed and refractory disease (RR), outcomes remains dismal [2,3,4,5]. Gokbuget et al. conducted a retrospective analysis to evaluate outcome of RR ALL patients and demonstrated that response rates to conventional chemotherapy decreases with successive salvage regimens and only 20–25% can achieve long-term remission with allo-HCT [2]. Outcome of pediatric ALL is significantly better than adult ALL, and around 80–85% of patients can be cured in the frontline [6]. However, for patients who have chemotherapy refractory disease, outcomes remain poor, and ALL remains one of the leading causes of death in children [1]. In order to improve outcome of chemo-resistant patients, novel therapies such as chimeric antigen receptor (CAR) modified T cells have been developed [7].
CAR-T cells are an innovative cellular immunotherapeutic approach that involves genetic modification of T cells to express CAR specific to tumor antigen [8]. Structurally, a CAR is a recombinant receptor redirected against a tumor antigen that consist of an antibody-derived extracellular single-chain variable fragment (scFv) linked to intracellular T cell signaling domains of the T cell receptor (TCR) and is HLA-independent [9, 10]. Several iterations of CARs have been investigated in clinical trials. First-generation CARs composed of target specific scFv fused to the CD3ζ endodomain of the TCR/CD3 complex had limited perseverance, expansion, and efficacy. Second-generation CARs have cytoplasmic signaling domains of T cell co-stimulatory receptors (i.e., CD28 and 4-1BB), which not only increases T cells cytotoxicity but also enhances T cells functions and longevity [9, 11]. The initial target for CAR-T cells was the CD19 receptor on B cells. CD19 is a 95 kDa glycoprotein present on the B cell surface from early development until differentiation into plasma cells. Its expression is restricted to B lineage cells and is not expressed by pluripotent blood stem cells or on most other normal tissues, making it an ideal target for CAR-T cell therapy [12]. Currently, one form of a second-generation anti-CD19 CAR-T cell is approved for RR B cell ALL, tisagenlecleucel [13••]. Although, we have seen remarkable responses and long-term remission in subset of RR B cell ALL patients, treatment complications (cytokine release syndrome [CRS], neurotoxicity, B cell aplasia), lack of CAR-T cells persistence, and downregulation of tumor antigen limit its effectiveness [14].
Apart from composition of CAR-T cells, several other aspects influence CARs efficacy including CAR-T cells dose, disease burden, and lymphodepletion strategy before CAR-T cells administration [11]. In this review, we will highlight clinical results of FDA-approved CAR-T cells and CAR-T cells under current investigation in patients with B cell ALL. We will also discuss differences in outcomes of different CAR-T cells studies in the light of CAR design, production, and administration.
First- vs second- vs third-generation CAR constructs
Early clinical studies employing first-generation CAR-T cells showed modest clinical efficacy and persistence of CAR-T cells [15, 16]. Investigators at Baylor College of Medicine found striking difference in CAR-T cell expansion and persistence with the addition of CD28 endodomain in the first-generation CD19-targeted CAR-T cells for RR B cell lymphoma [17]. Later, in vivo studies were conducted to evaluate persistence of CARs with different signaling domains (TCR-ζ, 41BB, and CD28) in mice engrafted with hematological malignancies [18]. In contrast to CD28 CARs, CD137 (41BB) CARs were suggested to have better antileukemic activity and CAR-T cell persistence. However, the superiority between different second-generation CARs (CD28 vs. 41BB) is yet to be explored in clinical setting in a randomized fashion. These second-generation CARs are the dominant form of CAR-T utilized in clinical trials. Third-generation CARs with > 1 co-stimulatory (e.g., 41BB and CD28) signaling domains have been evaluated in mouse models [19] for better efficiency, but clinical data is very limited [20].
Lymphodepletion strategy
Lymphodepletion is pivotal before administration of CAR-T cells in order to eradicate regulatory T cells (Tregs) and other competing cytokines to enhance the efficacy of CAR-T cells [21]. The most common chemotherapy regimen used for lymphodepletion is a combination of cyclophosphamide ± fludarabine with variable doses and schedules dependent on clinical trial and CAR-T cells product (Table 1). Apart from intensity of lymphodepletion chemotherapy and duration of cytopenia, higher intensity lymphodepletion also correlated with degree of CRS [27]. The optimal lymphodepletion strategy is still unknown and needs to be explored in future studies, prospectively.
Toxicity
Almost all CD19-targeted CAR-T cells therapies have a similar range of toxicities including cytokine release syndrome (CRS), neurotoxicity, infection risk, and prolonged B cell aplasia. CRS is a unique complication of CAR-T cell therapy, characterized by fever, hypotension, respiratory distress, and capillary leak syndrome. Severity of symptoms may vary depending upon disease burden, lymphodepletion chemotherapy, and CAR construct [10, 13••, 22, 27]. The incidence of CRS and neurotoxicity in different CAR-T cells trials and its management are often trial specific (Table 1). Early clinical trials for CAR-T cell therapy led to individualized grading systems for CRS [13••, 22, 28] making comparison of toxicities and standardizing management challenging. Due to these issues, the American Society of Transplant and Cellular Therapy (ASTCT) developed a consensus grading system for CRS as summarized in Table 2 [29••]. While CRS can be life-threatening, most cases are reversible with administration of tocilizumab, an anti-IL6r antibody.
The other common toxicity observed post-CAR-T cell treatment is neurotoxicity. It is characterized by a wide range of symptoms including generalized encephalopathy, obtundation, aphasia, and focal neurological deficits. Unlike CRS, neurotoxicity does not generally respond to tocilizumab, as it does not generate significant drug levels in the cerebrospinal fluid [30]. Supportive care and corticosteroids are the mainstay of treatment, and most patients recover without residual deficits. ASTCT similarly developed a consensus grading of neurotoxicity, which is now labeled as Immune Effector Cells Associated Neurotoxicity Syndrome (ICANS) [29••]. Lastly, B cell aplasia is an on-target, off-tumor expected toxicity commonly observed with CD19-targeted CAR-T cells. The duration of B cell aplasia is dependent on the CAR-T construct and persistence of the modified cells but may be lifelong [13••, 22]. This toxicity is usually managed with intravenous replacement of immunoglobulins.
CD19 CAR-T cell in B cell ALL
Pediatric B cell ALL
Pediatric ALL was one of the earlier tested indications for initial CD19 CAR-T cells clinical trials. One of the first reported CAR-T cells trials in pediatric B cell ALL utilized an anti-CD19 CAR with CD3ζ and CD28 co-stimulatory domain (19-28z). In a Phase 1 study, 21 children and young adults (aged 1–30 years) with RR B cell ALL or non-hodgkin lymphoma (NHL) were evaluated for safety and efficacy [22]. Dose-limiting toxicity was observed at a dose of 3 × 106 cells per kg. Maximum tolerated dose was established at 1 × 106 cells per kg of CAR-T cells. Cytokine release syndrome was observed in 16 patients, occurring at a median of 4 days (range, 1–7) of infusion. Grade (G) 3–4 CRS was observed in four patients. Treatment with steroids and tocilizumab was able to reverse the syndrome. Among the 20 patients with B cell ALL, 14 (70%) achieve complete response (CR). Of note, 12/14 patients with CR were also negative for minimal residual disease (MRD-ve). Ten out of 12 patients with CR MRD-ve procced with a consolidative allo-HCT.
Separately, at the University of Pennsylvania, tisagenlecleucel, an anti-CD19 CAR with 41BB co-stimulatory domain (19-41BB) was under development [31]. In their first reported trial, a total of 30 children and adults received anti-CD19 CAR-T cells at a dose range of 0.76 × 106 to 20.6 × 106 per kg. Twenty-seven (90%) achieved CR with a 6-month event-free survival (EFS) and OS of 67% and 78%, respectively. All patients had some degree of CRS, but G3–4 toxicities were only observed in 27% of patients. Patients with high disease burden were more susceptible to develop higher-grade CRS.
This data supported the development of a Phase 2, global, multicentered study of tisagenlecleucel in pediatric patients up to the age of 26 with RR B cell ALL [13••]. In this pivotal trial, among 75 evaluable patients, 61 (81%) responded. Forty-five (60%) patients achieved CR, and 16 (21%) patients had CR with incomplete count recovery (CRi). Of note, 46 (61%) patients had previous allo-HCT. A 6-month relapse-free survival (RFS) was 80%. The median EFS was not reached (NR), and 73% and 50% of patients were free of event at 6 and 12 months, respectively. Eight (10%) patients who achieved CR proceeded with allo-HCT and were alive at the time of report (four without relapse and four with unknown disease status). Twenty patients relapsed, including three with occurrence of MRD and two patients did not sustain remission for at least 28 days. Among them, 15 patients had CD19-, and 1 patient had CD19+ recurrence. CD19 status was not known in six patients. The median OS was NR, and 90% and 76% of patients were alive at 6 and 12 months, respectively. According to the authors, clinical response was correlated with persistence of tisagenlecleucel in peripheral blood and persistent B cell aplasia. Cytokine release syndrome was observed in 58 (77%) patients, and 35 (47%) patients had G3–4 CRS requiring management in the intensive care unit. Median time to development of CRS was 3 days (range, 1–22), and median duration was 8 days (range, 1–36). Neurologic adverse events were reported in 30 (40%) patients, occurred within 8 weeks of infusion. Grade 3 neurologic adverse events were reported in ten (13%) patients. None of the patients had G4 neurological adverse events. Most of the toxicities were observed in the first 8 weeks of CAR-T cells infusion. In majority of patients, adverse events resolved with supportive care and cytokine blockade by tocilizumab. These data led to the approval of tisagenlecleucel for the management of RR B cell ALL in patients up to 25 years of age.
Adult B cell ALL
Similar to the aforementioned pediatric study, 19-28z CAR-T cells were evaluated for safety and efficacy in adult relapsed B cell ALL in a Phase 1 study [7]. All patients received lymphodepletion chemotherapy followed by CAR-T cell dose of 1–3 × 106 cells per kg [32]. Cytokine release syndrome was observed in 14/53 (26%) patients, and 1 patient died due to severe CRS. Complete response was observed in 44 (83%) patients. Minimal residual disease assessment was done in 48 patients, of them 32 (67%) patients achieved CR with MRD-ve. On long-term follow-up of 29 months (range, 1–65), the median RFS was 6.1 months, and median OS was 12.9 months. The responses were correlated with disease burden at the time of CAR-T cells infusion. Patients with low disease burden had an EFS of 10.6 months and median OS of 20.1 months [7]. Of the 44 patients who achieved CR, 17 (39%) patients underwent allo-HCT: 5 patients were alive, 6 had relapse, and 6 died from allo-HCT related toxicities. Nine patients who achieved CR with MRD had relapse with CD19+ blasts. Among 32 patients who achieved CR with MRD -ve, 16 (50%) patients had relapse, including 6 patients who had allo-HCT. Four of these patients had relapse with loss of CD19 antigen.
Researchers from Fred Hutchinson Cancer Research Center conducted a Phase 1 study of CD19-specific CAR-T cells in 29 adult RR ALL patients [33]. The key difference from earlier study was incorporation of 4-1BB co-stimulatory domain instead of CD28. Twenty-four out of 26 evaluable patients achieved CR. Severe CRS, requiring management in intensive care unit, was observed in 26% of patients. Severe CRS was significantly associated with higher leukemia marrow infiltration and CAR-T cells dose (Table 1).
Turtle et al. [24] conducted a study in adult B cell ALL patients by using selected CD4+ and CD8+ T cells subset that were lentivirally transduced to express the CD19 and truncated epidermal growth factor receptor (EGFRt). The intention was to provide a reproducible potency and facilitate correlation between cell dose and efficacy. Among 30/32 patients who received CAR-T cells, CR rate was 97% (90% MRD-ve). Cytokine release syndrome was observed in 83% of patients, and 28% had G3–4 syndrome. Fifty percent had G3 neurotoxicity. The study suggests that using well-defined CD4+ and CD8+ T cells subset in uniform ratio to express CD19 was feasible and effective. Moreover, cell dose was correlated with expansion of clonally diverse T cells.
In another Phase I/II study (ZUMA-3), 19-28z CAR with lymphocytes enrichment (KTE-X19) was evaluated for safety and efficacy in adult RR B cell ALL [23]. Forty-five patients were treated with 2, 1, or 0.5 × 106 KTE-X19 cells/kg after lymphodepleting chemotherapy. Grade ≥ 3 CRS and neurotoxicity was observed in 13 (29%) and 17 (38%) patients, respectively. Two patients died from CRS. Among 41 patients with more than 2-months follow-up, CR/CRi rate was 68% (73% with MRD-ve). In 19 patients who were treated with a dose of 1 × 106 KTE-X19 cells/Kg, 16 (84%) patients achieved CR/CRi with median EFS of 15 months.
Novel CAR-T cell in ALL
Although we have seen excellent short-term responses with CD19 CAR-T cells in patients with B cell ALL, durability of response is variable, and relapse or failure to respond remains a clinical challenge. One cause of treatment failure with CD19 CAR-T cells therapy is downregulation or alteration in the CD19 receptor making CD19 CAR-T cells ineffective [25, 34]. This has led to further research to develop CARs targeting other tumor antigens apart from CD19. CD22 is B cell lymphocyte antigen that is highly expressed in ALL and is generally maintained following loss of CD19 antigen. A Phase 1 study was conducted to evaluate safety and efficacy of anti-CD22 CAR in 21 children and adult B cell ALL patients [25]. Of note, 17 of these patients were previously treated with CD19 antigen-directed therapies. Completed remission was observed in 11/15 (73%) patients who received ≥ 1 × 106 CD22 CAR-T cells per kg body weight. Median remission duration was around 6 months. Relapses were seen in association with decrease expression of CD22 antigen.
Other novel approaches to CAR-T cell therapy is dual targeting to reduce the risk of antigen downregulation [35]. A Phase I study was conducted to evaluate safety and feasibility of CD19/CD22 CAR in pediatric B cell ALL patients [26•]. Among the four patients treated, three had low disease burden detected by MRD. All four patients responded. Three patients had G1–2 CRS, and two had G1 neurotoxicity. Longer follow-up and more accrual are warranted to establish safety and efficacy of this CAR construct.
There is some evidence to suggest that CAR-binding affinity may adversely affect T cell responses. Consequently, investigators have developed a novel CAR with a lower affinity to CD19 (CAT) compared to previously tested CD19 CARs [36]. Initial data is promising, and among 14 pediatric patients with RR B cell ALL treated, 12 (86%) patients achieved complete molecular remission. Toxicities were low, and none of the patients developed severe CRS. EFS and OS at 1 year was 46% and 63%, respectively. Eleven out of 14 patients had persistence of CAR-T cells at last follow-up.
Role of allo-HCT in CAR-T cell-treated patients
Allogeneic hematopoietic stem cell transplantation may be ineffective in RR B cell ALL patients who are resistant to salvage chemotherapies. In this context, CAR-T cells therapy can be efficiently used to bridge RR B cell ALL patients to transplant. Lee et al. [37] reported long-term outcome of 51 ALL patients who received 19-28z CAR-T cells. Complete remission was observed in 31 (61%) patients of which 21 underwent subsequent allo-HCT. Relapses were more common among patients who did not receive allo-HCT (6/7 [86%]) compared to those who had allo-HCT (2/21 [9.5%]). The median leukemia free survival was NR in patients who received allo-HCT with leukemia free survival rate of 62% at 18 months. Similarly, in another study, clinical outcome post allo-HCT was analyzed in ALL patients who received anti-CD19 or anti-CD22 CAR-T cells on clinical trials [38•]. A total of 85 patients were treated, 51 (60%) achieved CR, of whom 43 achieved MRD -ve. Twenty-five patients who attained CR with MRD-ve received allo-HCT. Cumulative incidence of relapse post allo-HCT was 11.3% at 24 months. Six patients died from transplant related toxicities. Data suggest that by attaining deep remission, CAR-T cells therapy may have a synergistic activity with allo-HCT in improving long-term outcome. The role of allo-HCT is evolving but remains a consideration in patients after CAR-T cells therapy given it is an established procedure with known curative ability.
Cost effectiveness of CAR-T cell therapy
While CAR-T cell therapy has changed the landscape of RR B cell ALL, the widespread accessibility to this product is limited by the cost and complexity of this treatment. At present, CAR-T cells therapy is the most expensive oncological therapy with one-time infusion of tisagenlecleucel costing about $475,000 [39••]. Lin et al. conducted a study to evaluate cost-effectiveness of tisagenlecleucel in RR pediatric ALL compared to blinatumomab, clofarabine-based combination therapy, or clofarabine monotherapy [39••]. Markov modeling was used, and main outcomes looked were life years gained, costs, quality-adjusted life years gained, and incremental cost-effectiveness ratio (incremental cost per quality adjusted life years gained). With an assumption that tisagenlecleucel will attain 5-years RFS rate of 40%, life expectancies can be significantly increased to 12.1 years with reasonable cost of $61,000/quality adjusted life years gained. While with an assumption of 5-years RFS benefit of 20%, the gain was small with increase in life expectancies of 3.8 years and expensive at a cost of $151,000/quality adjusted life years gained. Moreover, at current price, if tisagenlecleucel is used to bridge patients to allo-HCT, the therapy would not be cost-effective with increased life expectancies of 5.7 years at a cost of $184,000/quality adjusted life years gained.
Future directions and conclusion
While CAR-T cell therapy has transformed outcomes for patients with RR B cell ALL, a significant number of patients will fail treatment, and it remains unclear which patients will be cured by CAR-T cell therapy alone and which ones would benefit from a consolidative allogeneic transplant. Cost, manufacturing challenges, relapse, and toxicity remain clinical challenges to widespread application of this therapy. To date, one anti-CD19 CAR-T cell product has been approved in pediatric B cell ALL with optimism for future approvals in adult B cell ALL. Different methods of T cells transduction, CAR-T cells dosing, novel targeting, and modification of the construct may impact its effectiveness and safety profile in the future. In this rapidly evolving field, there is an optimism that novel constructs and combination approaches will improve clinical outcomes.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukemia. Lancet Oncol. 2013;14(6):e205–17.
Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–41.
Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O’Brien S, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86(7):1216–30.
Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–9.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73.
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127(26):3312–20.
Park JH, Brentjens RJ. Adoptive immunotherapy for B cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discov Med. 2010;9(47):277–88.
Park JH, Brentjens RJ. Are all chimeric antigen receptors created equal? J Clin Oncol. 2015;33(6):651–3.
Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5–6):385–97.
•• Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48 This is a pivotal, global study which led to approval of Tisagenlecleucel (anti-CD19 CAR-T cell) in relapsed refractory B cell acute lymphoblastic leukemia patients up to 25 years of age.
Erratum: Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. Blood. 2016;128(11):1533.
Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–15.
Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–70.
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–6.
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther: J Am Soc Gene Ther. 2009;17(8):1453–64.
Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–5.
Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–50.
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–12.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28.
Shah BD, Bishop MR, Oluwole OO, Logan A, Baer MR, Donnellan WB, et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). 2019;37(15_suppl):7006.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8.
• Schultz LM, Davis KL, Baggott C, Chaudry C, Marcy AC, Mavroukakis S, et al. Phase 1 study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with B cell acute lymphoblastic leukemia (ALL). Blood. 2018;132(Suppl 1):898 One of the reasons for treatment failure with CD19 CAR-T cell is down-regulation of CD19 receptor making CD19 CAR-T cells ineffective. This papers highlights novel multi-targeted (CD19/CD22) CAR-T cell to improve effectiveness of CAR-T cell in B cell malignancies.
Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):567–72.
Schultz LM, Davis KL, Baggott C, Chaudry C, Marcy AC, Mavroukakis S, et al. Phase 1 Study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with B Cell Acute lymphoblastic leukemia (ALL). 2018;132(Suppl 1):898-.
•• Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release Syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38 Initial clinical trials of CAR-T cell therapy use different grading system for CRS. Later, the American Society of Transplant and Cellular Therapy (ASTCT) developed a consensus grading system for CRS, outlined in this paper.
Nellan A, McCully CML, Cruz Garcia R, Jayaprakash N, Widemann BC, Lee DW, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132(6):662–6.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
Park JH, Riviere I, Wang X, Bernal Y, Purdon T, Halton E, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol. 2015;33(15_suppl):7010.
Turtle CJ, Hanafi L-A, Berger C, Sommermeyer D, Pender B, Robinson EM, et al. Addition of fludarabine to cyclophosphamide lymphodepletion improves in vivo expansion of CD19 chimeric antigen receptor-modified T cells and clinical outcome in adults with B cell acute lymphoblastic leukemia. Blood. 2015;126(23):3773.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–95.
Shah NN, Maatman T, Hari P, Johnson B. Multi-targeted CAR-T Cell therapies for B cell malignancies. Front Oncol. 2019;9:146.
Ghorashian S, Kramer AM, Onuoha S, Wright G, Bartram J, Richardson R, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25(9):1408–14.
Lee DW III, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C, Yates B, et al. Long-term outcomes following CD19 CAR T cell therapy for B-ALL are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Blood. 2016;128(22):218.
• Shalabi H, Delbrook C, Stetler-Stevenson M, Yuan C, Steinberg SM, Yates B, et al. Chimeric antigen receptor T cell (CAR-T) therapy can render patients with ALL into PCR-negative remission and can be an effective bridge to transplant (HCT). Biol Blood Marrow Transplant. 2018;24(3):S25–S6 This retrospective analysis evaluated clinical outcome of patients who had allogeniec hematopoietic stem cell transplantation after CAR-T cell therapy. Data suggest that CAR-T cell therapy can be effectively used to bridge patient to transplant to improve long term outcome.
•• Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, et al. Cost-effectiveness of chimeric antigen receptor T cell therapy in relapsed or refractory pediatric B cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192–202 This is an important study that focuses on cost implications of CAR-T cell therapy. This study emphasizes the need for reducing cost of CAR-T cell therapy to make it universally accessible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Talha Badar declares that he has no conflict of interest.
Nirav N. Shah has received honoraria and/or travel support from Incyte, Celgene, and Miltenyi Biotec; has served on scientific advisory boards for Kite, Celgene, and Cellectar Biosciences; and has received institutional research support for clinical trials from Bristol-Myers Squibb and Miltenyi Biotec.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Badar, T., Shah, N.N. Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Curr. Treat. Options in Oncol. 21, 16 (2020). https://doi.org/10.1007/s11864-020-0706-6
Published:
DOI: https://doi.org/10.1007/s11864-020-0706-6