Opinion statement
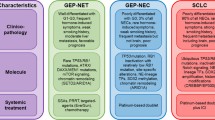
Ongoing advances in our understanding of neuroendocrine tumor (NET) biology, genetics, and immunology, will continue to expand the availability of targeted therapies, thus improving the outcomes of patients. Well-differentiated neuroendocrine tumors (NETs) are grouped into pancreatic and non-pancreatic NETs (includes GI and thoracic NETs) for treatment considerations (Fig. 1). For panNETs, initial therapy is driven by the need of radiographic response, and targeted agents are typically reserved for second and third line based on the toxicity profile. Treatment options for non-pancreatic NETs are also expanding and while SSAs are the typical first-line option, everolimus and PRRT both remain approved therapies for future lines, and VEGF TKIs are showing promising results in research settings. Sequencing these agents and best time to incorporate peptide receptor radio therapy into the management algorithm remains an unmet need.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine neoplasms (NENs) are rare diverse cancers; they have recently gained significant interest as a result of their increased incidence: the age-adjusted incidence has increased over six times in the last four decades (1.09 per 100,000 in 1973 to 6.98 per 100,000 by 2012), per a recent SEER data analysis [1•]. The majority originate in the gastrointestinal tract (55%) and bronchopulmonary system (30%) [1•]. Because of their indolent course, they are often diagnosed at an advanced stage, particularly if they are localized in the small intestine or pancreas [2].
While most NENs are relatively slow growing; their histologic grade and differentiation are closely correlated with their clinical behavior. NEN classification systems are based on proliferative indices and continue to evolve [3, 4] (Table 1). For instance, the recent WHO Digestive NEN classification system subdivides G3 tumors into poorly differentiated neuroendocrine carcinomas and well-differentiated, high-grade neuroendocrine tumors (NETs); their differences are highlighted in Table 1 [5, 6••]. At the same time, evidence-based medicine has changed the treatment landscape in the last decade. This review summarizes the established treatment options for well-differentiated, advanced gastrointestinal, pancreatic, and lung NETs, with a focus on targeted therapies, recent efforts to improve outcomes, and novel approaches.
Biological features of NETs and novel therapeutic targets
Although NETs that arise in primary sites outside of the pancreas have limited mutations in common oncogenic pathways, several such pathways nevertheless demonstrate increased activity; these pathways have guided the development of targeted therapy (Fig. 1) [7].
Several growth-promoting targets are expressed in NET cells, such as platelet-derived growth factors (PDGFR α and β), vascular endothelial growth factor (VEGF), insulin-like growth factor 1, and tumor growth factor β [8,9,10,11,12]. Many different targeted agents against these and other targets have been explored; however, few drugs have advanced to randomized controlled phase III trials. Anti-VEGF (sunitinib) and anti-mTOR (everolimus) drugs represent the only current FDA-approved targeted options for NETs.
VEGF pathway
NETs have a key role in angiogenesis, as suggested by clinical observations that they are vascular tumors. VEGF overexpression has been demonstrated in carcinoid and pancreatic NETs (panNETs) [13, 14] and carcinoid cell lines [15, 16]. In addition, panNETs showed widespread expression of VEGF receptor (VEGFR)-2 and -3, in addition to PDGFR α and β and stem cell factor receptor (c-kit) [17,18,19]. Preclinical data also suggest that activation of mesenchymal-epithelial transition factor (MET) leads to the growth of NETs and thus provides a potential therapeutic target [20,21,22]; MET overexpression in panNETs is associated with poor overall survival (OS) [23]. Furthermore, treatment with an anti-VEGF antibody or sunitinib has been shown to be associated with activation of MET and metastatic spread in a transgenic mouse model of spontaneous panNETs [22].
PI3K/mTOR pathway
Mammalian target of rapamycin (mTOR) is a conserved serine/threonine protein kinase and is a member of the family of phosphoinositide-3 (PI3)-related kinases [24]. Aberrant signaling through the mTOR pathway has also been implicated in neuroendocrine tumorigenesis [25]. Some NETs are part of familial cancer syndromes that are associated with aberrations of the mTOR pathway (neurofibromatosis type 1, tuberous sclerosis). In addition, a whole exome sequencing analysis identified somatic mutations in the mTOR pathway genes in 15% of panNET cases [26]. Studies have also demonstrated that the expression of mTOR and its downstream targets are associated with clinical outcome, especially in small intestinal NETs, whereas overexpression of mTOR or its activated downstream products has been associated with higher grade and shorter survival duration [27].
Table 2 provides a summary of the major randomized studies that used targeted therapies in well-differentiated NETs.
VEGF inhibitors
Sunitinib is a small orally active multi-targeted TKI that blocks the VEGF receptor, as well as PDGFR β, KIT, and RET [28]. In a phase II study, 107 patients with advanced NET received 50 mg of sunitinib for 4 weeks, followed by a 2-week break [29]. The objective response rate (ORR) was 16.7% in patients with panNETs compared with 2.4% in those with carcinoids, which suggests that there are biological differences between the two cohorts. Unfortunately, grade 3 fatigue was noted in 24.3% of patients.
Based on these results, a phase III double-blind, placebo-controlled trial was conducted in patients with low-to-intermediate-grade panNETs. Patients were randomly assigned to receive either 37.5 mg of sunitinib daily (lower dose than phase II because of reported grade 3 fatigue with a higher dose) or a placebo. This study was designed to enroll 340 patients but was stopped prematurely after an early, unplanned analysis of 171 patients demonstrated worse outcomes (death and serious adverse events) in the placebo arm. The PFS duration favored the experimental arm, with a median of 11.4 months compared with 5.5 months in the placebo group (hazard ratio [HR] = 0.42; 95% confidence interval [CI] = 0.26–0.66; P < 0.001). Based on these results, sunitinib was approved for the treatment of patients with unresectable or metastatic progressive, well-differentiated panNETs. The most common adverse events were diarrhea, nausea, asthenia, vomiting, and fatigue (all incidences > 30%), palmar-plantar erythrodysesthesia (23%), and hypertension (26%). The most common grade 3 or 4 adverse events were neutropenia (12%) and hypertension (10%). The premature closure of the study prompted the FDA’s request for a post-approval study.
In the recently reported phase IV clinical trial, 61 treatment-naïve and 45 previously treated patients with progressive panNETs were treated with sunitinib [30]. The median PFS duration was 13.2 months (95% CI = 10.9–16.7), and the ORR was 24.5%. The median OS duration, although not yet mature, was 37.8 months at the time of the final report. The treatment-related toxicities reported by ≥ 20% of patients in this trial were neutropenia, diarrhea, leukopenia, fatigue, hand-foot syndrome, hypertension, abdominal pain, dysgeusia, and nausea. The most common grade 3 and 4 toxicities were neutropenia (22%) and diarrhea (9%) [30].
mTOR inhibitors
Everolimus binds to FKBP-12, resulting in the formation of an inhibitory complex with mTOR complex 1 (mTORC1); it is FDA approved for the treatment of all well-differentiated panNETs as well as non-functioning non-panNETs [31]. In a phase II study of everolimus plus octreotide in 30 patients with advanced gastrointestinal NETs, the ORR was 17% [32]. Guided by early data, the RADIANT-2 trial randomly assigned 429 patients with progressive, functional, and advanced gastrointestinal NETs to octreotide LAR, with or without everolimus (10 mg daily) [33]. Combined therapy was associated with a prolongation in median PFS duration, but it missed the bar for statistical significance (16.4 versus 11.3 months; HR = 0.77; 95% CI = 0.59–1.0). It was postulated that the primary outcome results were affected by imbalances between the study groups, with important prognostic variables (e.g., disease site and performance status) and informed censoring that favored the control group. A later analysis found that everolimus had a significant PFS benefit after adjusting for randomization imbalances (HR for progression = 0.62; 95% CI = 0.51–0.87; P = 0.003) [34]. In the final analysis, there was no significant difference in OS between the two groups (HR for death = 1.17; 95% CI = 0.92–1.49) [35]; however, crossover to the active drug was allowed, potentially obscuring any meaningful survival benefit.
A further demonstration of the benefits of everolimus in non-panNETs comes from the RADIANT-4 trial, a phase III study in which 302 patients with advanced, non-functional lung cancer or gastrointestinal NETs (lung = 30%, ileum = 24%, and rectum = 13%) were randomly assigned to everolimus or placebo [36••]. Everolimus was associated with a significant improvement in the median PFS duration, the primary endpoint (11 versus 3.9 months; HR = 0.48; 95% CI = 0.35–0.67). The ORR was low in both groups, but the disease control rate was 81% for everolimus compared with 64% for placebo. The adverse events included stomatitis, diarrhea, peripheral edema, fatigue, and rash. The most frequent grade 3 or 4 toxicities were diarrhea (7%), stomatitis (9%), and anemia (5%). In the most recent update, at a median follow-up of 33 months, everolimus reduced the risk of death, but the difference was not statistically significant (2-year survival rate, 77% versus 62%; HR = 0.73; 95% CI = 0.48–1.11) [37]. HRQOL was maintained, with no relevant differences noted between the everolimus and placebo groups [38].
Everolimus was independently investigated in panNETs in the RADIANT-3 trial. This was a randomized, double-blind, placebo-controlled phase III study that evaluated the efficacy of everolimus in 410 patients with low- to intermediate-grade panNETs [39]. Median PFS durations of 11 versus 4.6 months (HR = 0.35; 95% CI = 0.27–0.45; P < 0.001) were observed in the everolimus and placebo arms, respectively. The OS durations were comparable between the two groups (44.0 and 37.7 months; HR = 0.94; 95% CI = 0.73–1.20; P = 0.30) because of crossover to the active agent at disease progression. An exploratory analysis to correct for crossover suggested survival rates of 82% and 75% at 12 months and 67% and 55.6% at 24 months for the everolimus and placebo arms, respectively (HR = 0.60; 95% CI = 0.09–3.95) [40].
Based on data from the abovementioned studies, everolimus has been approved for the treatment of advanced panNETs and non-functional carcinoids [41••]. It is however listed under NCCN guidelines for all NETs, with a caveat suggesting lack of efficacy data in functional tumors [41••]. No predictive biomarkers are currently available for everolimus. The side effects of everolimus include stomatitis, diarrhea, fatigue, infection, rash, cytopenias, and atypical infections. The incidence and severity of the most common adverse event, stomatitis, can be markedly reduced by the prophylactic use of dexamethasone oral solution (0.5 mg/5 ml, swished for 2 min and spit out; 4 times daily for 8 weeks). This was demonstrated in the SWISH trial that enrolled breast cancer patients being treated with everolimus (≥ grade II = 2% versus 33%; no stomatitis = 79% versus 39% compared to historical controls) [42].
Combination and other approaches
Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, and has been explored in the management of NETs. Its activity was studied in a randomized phase II trial in which 44 patients with advanced carcinoids were assigned to 18 weeks of bevacizumab or pegylated IFNα-2b [43], followed by combination therapy with bevacizumab plus interferon. The ORR was 18% in the bevacizumab arm and no responses were noted in the interferon arm; the 18-week PFS rates were 95% versus 68% for the bevacizumab vs the interferon arms. These results led to a large randomized study of octreotide plus bevacizumab versus octreotide plus interferon in 427 patients with progressive, advanced carcinoids [44•]. Although the bevacizumab arm had a higher ORR (12% versus 4%), there was no improvement in the primary endpoint of median PFS (16.6 months in the bevacizumab arm versus 15.4 months in the interferon arm [HR = 0.93; 95% CI = 0.73–1.18; P = 0.55]) [44•].
These and other studies have shown that interferon is an active drug in patients with NETs. Immune stimulation, angiogenesis inhibition, and the induction of cell cycle arrest are postulated mechanisms for its anti-secretory and anti-proliferative effects in GEP-NETs. However, its use is limited by its adverse effects: influenza-like symptoms, depression, and myelosuppression [45, 46].
There was initial interest in combination therapies using mTOR inhibitors, somatostatin analogs, and various VEGF-targeted therapies in NETs. However, their limited benefits and/or cumulative side effect profiles as discussed below have halted their further development.
The phase II COOPERATE-2 study in which 160 patients with advanced panNETs were randomly assigned to receive everolimus plus pasireotide (a somatostatin analogue with greater affinity to the somatostatin receptors 1, 3, and 5) versus everolimus [47•]. Despite an improvement in the ORR (20% versus 6%), the combination did not confer a PFS benefit compared to everolimus alone (HR = 0.991; 95% CI = 0.64–1.54), thus limiting its clinical utility. The glycemic complication rates were also significantly higher with the combination (grade 3 or 4 fasting hyperglycemia: 37% versus 11%; diabetes mellitus: 26% versus 7%).
The LUNA trial was a prospective randomized phase II study with three arms (everolimus versus pasireotide versus everolimus plus pasireotide) in 124 patients with advanced, well-differentiated carcinoids of lung and thymus origin [48•]. The adverse event profile was in line with the reported toxicities from the individual drugs. However, there was one death in the everolimus group and two deaths in the combination group; both suspected to be related to everolimus treatment. The primary endpoint (disease control rate at 9 weeks) was met in all three arms. No one approach was found to be significantly more effective (39.0% in the long-acting pasireotide arm, 33.3% in the everolimus arm, and 58.5% in the combination arm).
CALGB 80701 was a randomized phase II study that compared the combination of everolimus and bevacizumab to everolimus alone in 150 patients with advanced, progressive panNETs [49]. The primary endpoint, PFS, was marginally improved in the combination arm, per the pre-specified statistical cut-off of P < 0.15 (16.7 months versus 14 months; HR = 0.80; P = 0.12). The ORR was also improved significantly with the addition of everolimus (31% versus 12%; P = 0.005). However, the toxicities were notable, with 81% of patients on the combination arm reporting grade 3 and 4 adverse events (compared to 49% in the everolimus arm). Grade 3 and 4 hypertension (38% versus 8%), proteinuria (16% versus 1%), diarrhea (11% versus 1%), and hypophosphatemia (10% versus 1%) were more common in the combination arm. Even though the study met its primary endpoint, the higher type 1 error rate and the significantly higher toxicity rate made it a less promising approach.
Along similar lines, the toxicity of combining mTOR inhibitors with VEGF TKIs has hampered their development in clinical trials. A phase I study of sorafenib with everolimus in patients with advanced NETs resulted in a significant toxicity burden (thrombocytopenia, hand-foot skin reaction, and rash or allergic reaction), which precluded its further development [50]. Similarly, the combination of sorafenib and bevacizumab was accompanied by an unfavorable safety profile, despite some activity in a phase II study [51].
Emerging targeted agents
Multiple other novel TKIs have been evaluated in advanced NETs in phase II and III trials with promising results but not approved by regulatory bodies.
Surufatinib
Surufatinib is a small molecule inhibitor that targets VEGF receptors, FGFR 1, and colony-stimulating factor 1 receptor. In a phase I study, surufatinib demonstrated antitumor activity in hepatocellular carcinoma and NETs, which are both highly vascularized tumors [52]. The results of a phase Ib/II multicenter study of surufatinib in advanced, well-differentiated NETs (n = 81; 42 panNETs and 39 GINETs and lung NETs) were encouraging [53••]. In the panNET and extra-panNET cohorts, the ORRs were 19% in and 15%, the disease control rates were 91% and 92%, and the median PFS durations were 21.2 months (95% CI = 15.9–24.8 months) and 13.4 months (95% CI = 7.6–19.3 months), respectively. The most common grade ≥ 3 toxicities were in line with those of other VEGF-targeted therapies. Biomarker analyses suggested that higher serum VEGFR-2 levels and lower basic FGF levels at the baseline were associated with a longer median PFS duration, although this analysis was retrospective.
Based on these encouraging results, two phase III randomized studies were conducted in panNET and non-panNET patients in China. SANET-ep was a double-blind, multi-center phase III trial that randomly assigned patients with progressive, advanced extra-panNETs in a 2:1 fashion to receive surufatinib versus placebo [54]. The primary endpoint of the study was PFS duration. The study was stopped at the interim analysis as the primary endpoint had been met after 198 patients had been enrolled. The primary sites were the gastrointestinal tract (47.0%) and lungs (11.6%). The majority of patients (84%) had pathological grade 2 disease. The investigator-assessed median PFS duration was longer in the surufatinib arm (9.2 versus 3.8 months; HR = 0.33; 95% CI = 0.22–0.5; P < 0.0001). The most common (≥ 5%) grade 3 or higher adverse events were hypertension (36.4% in the surufatinib arm versus 13.2% in the placebo arm), proteinuria (19.4% versus 0%), and anemia (7.0% versus 2.9%).
A recent press release from the Chi-Med group reported encouraging results from the SANET-p study [55]. In this phase III study, 195 Chinese patients with low- or intermediate-grade advanced panNETs were randomly assigned, in a 2:1 fashion, to receive either 300 mg of oral surufatinib daily or placebo on a 28-day cycle. Prior VEFF therapy was not permitted on this study. The primary endpoint of PFS was met at the time of the interim analysis, and the study was closed to further accrual [56]. More details of the study and results are eagerly awaited (NCT02589821). Based on the results of these two studies, surufatinib is under review for approval in China. To establish the safety and efficacy of this novel targeted agent in the American population, a dose escalation /expansion study was conducted, where the maximum tolerated dose as well the recommended phase II dose was 300 mg, similar to previous data [57]. Thirty-two patients with gastroentero-pancreatic NETs (GEPNETs), who had previously received at least one targeted agent, were enrolled and ORR of 9.4% was observed (only in panNETs). Toxicity profile was consistent with available phase III data from China where most common adverse events reported were hypertension, fatigue, diarrhea, proteinuria and nausea. Additionally, pharmacokinetic and dose exposure data was consistent with results from large randomized phase 3 trials.
Pazopanib
Pazopanib exerts anti-tumor and anti-angiogenic effects by targeting VEGFR-1, -2, and -3; PDGFR α and β; FGFGR-1, -2, and -3; and c-Kit [58]. It was approved by the FDA as a treatment for metastatic renal cell carcinoma and advanced soft tissue sarcoma. Data from clinical trials suggest that pazopanib has anti-tumor activity in advanced NENs.
A multicenter, single-arm, phase II study evaluated patients with advanced, well-differentiated pancreatic or extra-panNENs who received 800 mg of pazopanib orally once a day plus octreotide LAR [59]. Fifty-two patients were enrolled, with an ORR of 21.9% (7 of 32) in patients with panNETs. The median PFS durations were 14.4 and 12.2 months for panNET and extra-panNET patients, respectively. Treatment was generally well tolerated. The most frequently observed toxic effects were fatigue (75%), nausea (63%), diarrhea (63%), and hypertension (54%).
Another phase II study of pazopanib from Spain enrolled 44 patients with advanced NETs (18 pancreatic, 15 gastrointestinal, five pulmonary, and six other or unknown) who had experienced disease progression on prior therapies [60]. The disease control rate at 6 months was 59.5% (four partial responses and 21 stable disease), with a median PFS duration of 9.5 months. Pazopanib had higher activity in patients with panNETs, and its benefit was similar in patients who had been previously treated with TKIs and those who had been treated with mTOR inhibitors (73% and 60%, respectively).
Alliance A021202 was a multicenter, randomized, double-blind, phase II study of pazopanib (800 mg/day) versus placebo in progressive non-pancreatic GEPNETs [61]. Of 171 accrued patients, most (66%) had small bowel primary tumors, and the majority (87%) were on concurrent somatostatin analogs. The median PFS duration in patients receiving pazopanib (n = 97) was 11.6 months compared with 8.5 months in those receiving placebo (n = 74; HR = 0.53; P = 0.0005). There was no improvement in OS duration; however, crossover at disease progression (n = 49) most likely confounded the OS endpoint.
Cabozantinib
Cabozantinib is a TKI that is known to influence tumor growth, metastasis, and angiogenesis through inhibition of multiple tyrosine kinases including MET, VEGFR-2, AXL, and RET [62]. The clinical activity of cabozantinib in patients with NETs was evaluated in a phase II study that included patients with advanced panNETs and carcinoid tumors, many of whom had experienced tumor progression on prior therapy [63]. Radiographic responses were observed in 3 of 20 (15%) patients with panNETs and 6 of 41 (15%) patients with carcinoid tumors. The PFS was 21.8 months in patients with panNETs and 31.4 months in patients with carcinoid tumors. Treatment-related adverse events that were associated with cabozantinib in NET patients were similar to those that had been reported in other diseases. Grade 3 and 4 toxicities included hypertension (13%), hypophosphatemia (11%), diarrhea (10%), lymphopenia (7%), thrombocytopenia (5%), fatigue (5%), and increased lipase or amylase (8%). Unexpected toxicities included heart failure and autoimmune hemolytic anemia, each in one patient. Based on these encouraging results, the Alliance cooperative group (A021602) is evaluating the efficacy of cabozantinib compared with placebo in patients with advanced NETs in the ongoing randomized phase III CABINET trial (NCT03375320).
Axitinib
Axitinib is a selective inhibitor of VEGFR-1, -2, and -3 that is clinically indicated in the management of renal cell carcinoma. In pre-clinical studies, axitinib induces growth inhibition in pancreatic endocrine cell lines and causes a reduction in tumor vasculature, with widespread hypoxia and significant mTOR pathway activation, leading to resistance [64, 65].
A phase II trial of 5 mg of axitinib twice daily in patients with progressive advanced low-to-intermediate grade carcinoid tumors enrolled 30 patients [66]. The study reported a median PFS duration of 26.7 months (95% CI = 11.4–35.1 months), with a 12-month PFS rate of 74.5% (± 10.2). The median OS duration was 45.3 months (95% CI = 24.4–45.3 months). The ORR was low, with a partial response in 1 of 30 patients (3%) and stable disease in 21 (70%). Hypertension was the most common toxicity (27 patients [90%]), with grade 3 or 4 hypertension reported in 19 patients (63%); the condition led to treatment discontinuation in six patients (20%). The authors concluded that axitinib has an inhibitory effect on tumor growth in patients with advanced, progressive carcinoid tumors, but the high rate of grade 3 or 4 hypertension may represent an impediment to its use in unselected patients. The AXINET trial is a phase II/III study of Octreotide LAR with axitinib or placebo in patients with extra-panNETs (n = 255) without prior exposure to anti-VEGF therapies. The primary endpoint is PFS and the trial has completed accrual, and the results are awaited (NCT01744249).
Nintedanib
Nintedanib is an oral TKI that targets VEGFR, PDGFR, and FGFR [67, 68]. It has been approved for the treatment of advanced non-small-cell lung cancer and idiopathic pulmonary fibrosis. In pre-clinical mouse models of NETs, prolonged exposure to nintedanib led to strong suppression of angiogenesis, accompanied by a reduced tumor burden, which translated to significant prolongation of survival [69]. A phase II study of nintedanib in advanced non-pancreatic and lung NETs enrolled 30 patients [70]. The median PFS and OS durations were 11 and 27.6 months, respectively, but the ORR was only 4% (1 of 30), with stable disease in 83% (20 of 30). The incidence of grade 3 toxicities was 27% (hypertension and decreased appetite). In addition, the results of recent studies suggest that high-grade diarrhea and dose-dependent elevated transaminases are possible side effects of therapy [71].
Lenvatinib
Lenvatinib is an oral TKI that is directed against VEGFR 1-3, FGFR 1-4, PDGFR α, and RET. It has been approved for the treatment of radioiodine-refractory differentiated thyroid cancer and hepatocellular cancer.
The TALENT trial was a prospective multicohort phase II study of lenvatinib in patients with well-differentiated, advanced digestive NETs; it recruited 111 patients (55 with panNETs and 56 with GEPNETs) [72]. Thirty percent of patients had received sunitinib previously. The ORR was 29% (42.3% for panNETs and 16.3% for GEPNETs). With a median follow-up duration of 19 months, the PFS and OS durations were 15.5 months (95% CI = 11.3 months-not reached) and 29.2 months (95% CI = 23.2 months-not reached) for panNETs and 15.4 months (95% CI = 11.5–19.4 months) and not reached for GEPNETs, respectively. The most frequent grade 3 or higher adverse events were hypertension (22%), fatigue (11%), and diarrhea (11%). Dose reductions and interruptions were required in 91.8%, with a median dose of 20 mg daily.
Conclusions
The increasing number of options that are available for the treatment of patients with advanced NETs are exciting but have also made it challenging to devise optimal treatment algorithms. We typically place well-differentiated NETs into two big buckets for treatment considerations, panNETs and non-panNETs (which includes gastrointestinal and thoracic NETs). In panNETs, initial therapy is typically driven by the need for radiographic response and if so, cytotoxic therapy is initiated first. For others, somatostatin analogues form the first line of therapy; targeted agents are typically reserved for second- and third-line therapies. The sequencing of these agents and the optimal time to incorporate peptide receptor radiotherapy (PRRT) into the management algorithm remains an unmet need. Studies such as SEQTOR and COMPETE (Table 2) are actively enrolling patients to help answer these questions. Treatment options for non-panNETs are also expanding, and while somatostatin analogues are the typical first-line option, everolimus and PRRT are both approved therapies for subsequent lines; VEGF TKIs have also shown promising results in research settings. Further research is also needed to identify reliable biomarkers to help prioritize effective therapies and avoid overtreatment in patients with very slow-growing, asymptomatic tumors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42 The study highlights the burden of NETs in United States and essential to understand epidemiology of this rare cancer.
Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin N Am. 2011;40(1):1–18.
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–12.
Kim JY, Hong S-M, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11–6.
Inzani F, Petrone G, Rindi G. The New World Health Organization classification for pancreatic neuroendocrine neoplasia. Endocrinol Metab Clin N Am. 2018;47(3):463–70.
•• Klimstra DS KG, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. WHO Classification of Tumours Digestive System Tumours. Edited by Board The WHO Classification of Tumours Editorial. 5th ed International Agency for Research on Cancer, Lyon. 2019:16–21. This publication highlights the new classification of NETs and recognized Grade 3 well differentiated NETs as a category.
Vijayvergia N, Boland PM, Handorf E, et al. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a fox Chase Cancer Center pilot study. Br J Cancer. 2016;115(5):564.
Welin S, Fjällskog ML, Saras J, Eriksson B, Janson ET. Expression of tyrosine kinase receptors in malignant midgut carcinoid tumors. Neuroendocrinology. 2006;84(1):42–8.
Chaudhry A, Funa K, Oberg K. Expression of growth factor peptides and their receptors in neuroendocrine tumors of the digestive system. Acta Oncol (Stockholm, Sweden). 1993;32(2):107–14.
Wulbrand U, Wied M, Zofel P, Goke B, Arnold R, Fehmann H. Growth factor receptor expression in human gastroenteropancreatic neuroendocrine tumours. Eur J Clin Investig. 1998;28(12):1038–49.
von Wichert G, Jehle PM, Hoeflich A, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60(16):4573–81.
Nilsson O, Wangberg B, Theodorsson E, Skottner A, Ahlman H. Presence of IGF-I in human midgut carcinoid tumours--an autocrine regulator of carcinoid tumour growth? Int J Cancer. 1992;51(2):195–203.
Zhang J, Jia Z, Li Q, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer. 2007;109(8):1478–86.
Terris B, Scoazec JY, Rubbia L, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32(2):133–8.
Silva SR, Bowen KA, Rychahou PG, et al. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int J Cancer. 2011;128(5):1045–56.
Bowen KA, Silva SR, Johnson JN, et al. An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J Gastrointest Surg : official journal of the Society for Surgery of the Alimentary Tract. 2009;13(10):1773–80.
Hansel DE, Rahman A, Hermans J, et al. Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Modern Pathol: an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16(7):652–9.
Fjallskog ML, Lejonklou MH, Oberg KE, Eriksson BK, Janson ET. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2003;9(4):1469–73.
Fjallskog ML, Hessman O, Eriksson B, Janson ET. Upregulated expression of PDGF receptor beta in endocrine pancreatic tumors and metastases compared to normal endocrine pancreas. Acta Oncologica (Stockholm, Sweden). 2007;46(6):741–6.
Reuther C, Heinzle V, Spampatti M, et al. Cabozantinib and tivantinib, but not INC280, induce antiproliferative and antimigratory effects in human neuroendocrine tumor cells in vitro: evidence for ‘Off-Target’ effects not mediated by c-met inhibition. Neuroendocrinology. 2016;103(3–4):383–401.
Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer discovery. 2012;2(3):270–87.
Sennino B, Ishiguro-Oonuma T, Schriver BJ, Christensen JG, McDonald DM. Inhibition of c-Met reduces lymphatic metastasis in RIP-Tag2 transgenic mice. Cancer Res. 2013;73(12):3692–703.
Krampitz GW, George BM, Willingham SB, et al. Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A. 2016;113(16):4464–9.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–84.
Chan J, Kulke M. Targeting the mTOR signaling pathway in neuroendocrine tumors. Curr Treat Options in Oncol. 2014;15(3):365–79.
Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science (New York, NY). 2011;331(6021):1199–203.
Qian ZR, Ter-Minassian M, Chan JA, et al. Prognostic significance of MTOR pathway component expression in neuroendocrine tumors. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2013;31(27):3418–25.
Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6(9):734–45.
Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(20):3403–10.
Raymond E, Kulke MH, Qin S, et al. The efficacy and safety of sunitinib in patients with advanced well-differentiated pancreatic neuroendocrine tumors. J Clin Oncol. 2017;35(4_suppl):380.
Chan DL, Segelov E, Singh S. Everolimus in the management of metastatic neuroendocrine tumours. Ther Adv Gastroenterol. 2017;10(1):132–41.
Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2008;26(26):4311–8.
Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet (London, England). 2011;378(9808):2005–12.
Yao JC, Hainsworth JD, Wolin EM, et al. Multivariate analysis including biomarkers in the phase III RADIANT-2 study of octreotide LAR plus everolimus (E+O) or placebo (P+O) among patients with advanced neuroendocrine tumors (NET). J Clin Oncol. 2012;30(15_suppl):4014.
Pavel ME, Baudin E, Oberg KE, et al. Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann Oncol : official journal of the European Society for Medical Oncology. 2017;28(7):1569–75.
•• Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet (London, England). 2016;387(10022):968–77 Randomized study that led to approval of Everolimus for this disease population.
Yao JC, Fazio N, Singh S, et al. Everolimus (EVE) in advanced, nonfunctional, well-differentiated neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin: second interim overall survival (OS) results from the RADIANT-4 study. J Clin Oncol. 2016;34(15_suppl):4090.
Pavel ME, Singh S, Strosberg JR, et al. Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 2017;18(10):1411–22.
Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23.
Yao JC, Pavel M, Lombard-Bohas C, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 study. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2016;34(32):3906–13.
•• Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, Version 2.2018. J Natl Compr Cancer Netw : JNCCN. 2018;16(6):693–702 NCCN guidelines provide the latest concise description of treatment options available for NETs.
Rugo HS, Seneviratne L, Beck JT, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18(5):654–62.
Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(8):1316–23.
• Yao JC, Guthrie KA, Moran C, et al. Phase III prospective randomized comparison trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2017;35(15):1695–703 A randomized trial for NETs but results not clinically applicable as Interferon is not used.
Kolby L, Persson G, Franzen S, Ahren B. Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg. 2003;90(6):687–93.
Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association. 2005;3(8):761–71.
• Kulke MH, Ruszniewski P, Van Cutsem E, et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol : official journal of the European Society for Medical Oncology. 2017;28(6):1309–15 Randomized trial completed recently but without positive results that would change practice.
• Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2017;18(12):1652–64 Randomized trial for lung NETs completed recently but results unlikely to change practice.
Kulke MH, Niedzwiecki D, Foster NR, et al. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+ B) in patients (pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). In: Proc Am Soc Clin Oncol; 2015.
Chan JA, Mayer RJ, Jackson N, Malinowski P, Regan E, Kulke MH. Phase I study of sorafenib in combination with everolimus (RAD001) in patients with advanced neuroendocrine tumors. Cancer Chemother Pharmacol. 2013;71(5):1241–6.
Castellano D, Capdevila J, Sastre J, et al. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumour: a phase II study of Spanish neuroendocrine tumour group (GETNE0801). Eur J Cancer (Oxford, England : 1990). 2013;49(18):3780–7.
Xu JM, Wang Y, Chen YL, et al. Sulfatinib, a novel kinase inhibitor, in patients with advanced solid tumors: results from a phase I study. Oncotarget. 2017;8(26):42076–86.
•• Xu J, Li J, Bai C, et al. Surufatinib in advanced well-differentiated neuroendocrine tumors: a multicenter, single-arm, open-label, Phase Ib/II trial. Clin Cancer Res. 2019;25(12):3486–94 Randomized study of a new targeted agent in asian population but impressive activity.
Xu J, Shen L, Zhou Z, et al. LBA76 - efficacy and safety of surufatinib in patients with well-differentiated advanced extrapancreatic neuroendocrine tumors (NETs): results from the randomized phase III study (SANET-ep). Ann Oncol. 2019;30:v911.
Innovation Platform RA Chi-med announces that surufatinib phase III SANET-p study has already achieved its primary endpoint in advanced pancreatic neuroendocrine tumors in China and will stop early. 2020; chi-med.com/surufatinib-phase-iii-sanet-p-study-achieved-primary-endpoint/. Accessed April 23, 2020.
Phase III Study of surufatinib in treating advanced pancreatic neuroendocrine tumors. 2020; https://clinicaltrials.gov/ct2/show/NCT02589821. Accessed April 23, 2020.
Dasari A, Li D, Sung MW, et al. Efficacy and safety of surufatinib in United States (US) patients (pts) with neuroendocrine tumors (NETs). J Clin Oncol. 2020;38(15_suppl):4610.
Verheijen RB, Beijnen JH, Schellens JHM, Huitema ADR, Steeghs N. Clinical pharmacokinetics and pharmacodynamics of pazopanib: towards optimized dosing. Clin Pharmacokinet. 2017;56(9):987–97.
Phan AT, Halperin DM, Chan JA, et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol. 2015;16(6):695–703.
Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE)††2011 ASCO annual meeting: poster session; 2012 ASCO annual meeting: poster session; 2013 ASCO annual meeting: poster session; 2014 ASCO annual meeting: abstract; ESMO 2012 congress Vienna: Oral presentation; European Cancer Congress 2013: poster session; Sociedad Española de Oncología Médica (SEOM) 2013: oral presentation. Ann Oncol. 2015;26(9):1987–93.
Bergsland EK, Mahoney MR, Asmis TR, et al. Prospective randomized phase II trial of pazopanib versus placebo in patients with progressive carcinoid tumors (CARC) (Alliance A021202). J Clin Oncol. 2019;37(15_suppl):4005.
Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308.
Chan JA, Faris JE, Murphy JE, et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol. 2017;35(4_suppl):228.
Gilbert JA, Adhikari LJ, Lloyd RV, Halfdanarson TR, Muders MH, Ames MM. Molecular markers for novel therapeutic strategies in pancreatic endocrine tumors. Pancreas. 2013;42(3):411–21.
Allen E, Mieville P, Warren CM, et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15(6):1144–60.
Strosberg JR, Cives M, Hwang J, et al. A phase II study of axitinib in advanced neuroendocrine tumors. Endocr Relat Cancer. 2016;23(5):411–8.
Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–82.
Roth GJ, Heckel A, Colbatzky F, et al. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120). J Med Chem. 2009;52(14):4466–80.
Bill R, Fagiani E, Zumsteg A, et al. Nintedanib is a highly effective therapeutic for neuroendocrine carcinoma of the pancreas (PNET) in the Rip1Tag2 transgenic mouse model. Clin Cancer Res. 2015;21(21):4856–67.
Iyer RV, Konda B, Owen DH, et al. Multicenter phase 2 study of nintedanib in patients (pts) with advanced progressing carcinoid tumors. J Clin Oncol. 2018;36(15_suppl):4105.
Abdel-Rahman O, Eldin NB, ElHalawani H. Risk of selected gastrointestinal and hepatic toxicities in cancer patients treated with nintedanib: a meta-analysis. Future Oncol. 2016;12(18):2163–72.
Capdevila J, Fazio N, Lopez CL, et al. Final results of the TALENT trial (GETNE1509): a prospective multicohort phase II study of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs). J Clin Oncol. 2019;37(15_suppl):4106.
Funding
This work is supported by Cancer Center Support Grant (CCSG) P30 CA006927.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Namrata Vijayvergia has received research funding from Merck for an MISP clinical trial grant, and has received compensation from Lexicon and Novartis for service as a consultant. Arvind Dasari declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuroendocrine Cancers
Rights and permissions
About this article
Cite this article
Vijayvergia, N., Dasari, A. Targeted Therapies in the Management of Well-Differentiated Digestive and Lung Neuroendocrine Neoplasms. Curr. Treat. Options in Oncol. 21, 96 (2020). https://doi.org/10.1007/s11864-020-00794-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-020-00794-0