Opinion statement
Despite a decreasing incidence in the USA, gastric cancer is highly prevalent worldwide. Furthermore, gastric cancer remains highly lethal with median survival of less than 1 year for metastatic disease. The backbone of therapy against metastatic gastric cancer remains cytotoxic chemotherapy, but recent advances in the molecular understanding of gastric cancer have renewed hope within that targeted agents can be leveraged to improve survival and reduce toxicity. For example, in patients with human epidermal growth factor-2 (HER2)-positive gastric cancer, the addition of trastuzumab to frontline chemotherapy improves survival. In the second line, oncologists can now administer a vascular endothelial growth factor (VEGF) receptor inhibitor, ramucirumab, as a single agent or in combination with chemotherapy, and the immune checkpoint inhibitor pembrolizumab is approved in multiple settings dependent on the Programmed Death Ligand 1 (PD-L1) status. For patients with metastatic disease, our approach to standard of care in the first-line setting is a 5FU/platinum doublet with trastuzumab for HER2-positive tumors. In the second-line setting, most patients receive ramucirumab + paclitaxel, but those that are MSI high receive pembrolizumab. For squamous cell carcinoma of the esophagus with high PD-L1 status (combined positive score (CPS) ≥ 10), we recommend pembrolizumab in the second line. While for PD-L1 ≥ 1% gastroesophageal adenocarcinoma, we do not recommend pembrolizumab before the third-line setting, although this may change in the near future for CPS ≥ 10. The future landscape for targeted therapy in gastric cancer is promising. Numerous clinical trials evaluating the combination immune therapy with molecularly targeted agents are generating much excitement. Moreover, genomic data from The Cancer Center Genome (TCGA) and Asian Cancer Research Group (ACRG) classifications is being used to identify molecular subtypes to enable future clinical trials to include biomarker-enriched patient populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains a common cancer worldwide with an annual incidence of over 1,000,000 cases and an estimated of 783,000 deaths in 2018 [1]. More than 70% of these cases occur in developing countries, especially East Asia [2]. Despite decreased incidence in many developed nations, gastric cancer remains a significant public health problem in the USA. Moreover, the incidence of gastroesophageal junction (GEJ) centered adenocarcinoma has increased dramatically in Western countries over the past 4 decades [1]. The vast majority of gastric cancer are adenocarcinomas, which have historically been subdivided into intestinal and diffuse types according to the Lauren classification [3].
Gastric cancer is a complex and heterogeneous disease because it arises from multiple interactions of genetic, environmental, and host factors. Radical surgery remains the only curative modality for localized disease, but most patients are advanced at the time of diagnosis. Despite the use of multi-agent chemotherapy, the median overall survival (mOS) for metastatic gastric cancer remains less than 1 year [4]. However, recent advances in high throughput technologies, including microarrays and next generation sequencing, have led to a surge in the discovery of new molecular markers, intracellular pathways, and molecular subtypes of gastric cancer. To improve upon the Lauren classification, insights from molecular findings by TCGA have been leveraged to classify gastric adenocarcinoma into 4 distinct molecular subtypes: (a) Epstein-Barr virus (EBV) positive, (b) microsatellite unstable tumors, (c) genomically stable tumors, and (d) tumors with chromosomal instability [5]. Similarly, the ACRG also analyzed gene expression data from 300 primary gastric tumors and proposed four subtypes of molecular classification for gastric cancer: (a) microsatellite stable (MSS)/epithelial-mesenchymal transition (EMT), (b) MSI, (c) MSS-TP53-active, and (d) MSS-TP53 negative [6]. These novel classification systems, based on the molecular characteristics, have allowed the identification of pathways that contribute to carcinogenesis and underline several driver genes relevant for each gastric cancer subtype, which can be used as therapeutic targets [7].
In this article, we discuss updates in the molecularly targeted agents of advanced gastric cancer and review emerging targeted therapies showing promise and summarize significant clinical trials. The goal is that a better understanding of the molecular landscape of gastric cancer will lead to improvements in survival. Given the dynamic nature of this field, this review is not meant to be all-inclusive but rather to report the major established treatments and those under investigation.
Anti-HER2
HER2, also known as known an erythroblastosis oncogene B2 (ERBB2), is a proto-oncogene encoded by ERBB2 on chromosome 17. The subsequent protein product is a membrane-bound tyrosine kinase receptor that promotes cell proliferation and apoptosis suppression leading to tumorigenesis [8,9,10]. HER2 protein overexpression and/or gene amplification (HER2 positivity) is found in approximately 13–22% of gastric or GEJ cancers [11,12,13]. Testing HER2 overexpression using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) is recommended for all patients with advanced gastric adenocarcinoma [14].
Trastuzumab
Trastuzumab is a recombinant humanized IgG1 monoclonal antibody directed against the extracellular domain of HER2. The drug binds with high affinity and interrupts HER2-mediated cell signaling pathways and induces antibody-dependent cytotoxicity toward tumor cells that overexpress HER2 [15]. In 2010, the phase III ToGA (Trastuzumab for Gastric Cancer) trial demonstrated a mOS benefit of adding trastuzumab to first-line chemotherapy in patients with HER2-positive (IHC3+ or FISH over expression) locally advanced, recurrent, or metastatic gastric or GEJ adenocarcinoma [16•]. The general characteristics of patients in the trastuzumab plus chemotherapy and chemotherapy-alone groups were similar, including the chemotherapy regimen (capecitabine and cisplatin vs fluorouracil and cisplatin) and primary tumor site (stomach vs GEJ). The study reported that patients receiving trastuzumab plus chemotherapy compared with chemotherapy alone had an improved mOS (13.8 months versus 11.1 months, HR 0.74, CI 0.60–0.91, P = 0.0046). The overall response rate (ORR) in the trastuzumab plus chemotherapy group was 47% versus 35% with chemotherapy alone. The incidence of adverse events (AE) was similar in both groups. Pre-planned and post hoc exploratory analyses of subgroups of patients revealed that the mOS was longer in patients with higher HER2 expression.
In 2010, the FDA approved trastuzumab as a front-line treatment in combination with chemotherapy in patients with HER2-positive gastric cancer.
Trastuzumab emtansine
Trastuzumab emtansine is an antibody-drug conjugate comprised of trastuzumab linked by a stable linker to the tubulin inhibitor emtansine. Intracellular release of cytotoxic emtansine-containing catabolites in HER2-positive cells induces mitotic arrest and apoptosis [17]. However, in 2017 the GATSBY trial failed to show a survival benefit in patients with previously treated advanced HER2-positive gastric cancer receiving trastuzumab emtansine versus taxane alone (mOS 7.9 months versus 8.6 months, HR 1.15, CI 0.87–1.51, P = 0.86) [18].
Pertuzumab
Pertuzumab is a humanized monoclonal antibody that has mechanisms of action complementary to trastuzumab but binds to a different epitope on the HER2 receptor protein [19, 20]. In 2014, the phase II JOSHUA trial reported durable responses when combining a higher dose of pertuzumab (840 mg) with chemotherapy, but the subsequent phase III JACOB trial in 2018 failed to show improved OS in patients with previously untreated HER2-positive metastatic gastric or GEJ cancers [21, 22].
Lapatinib
Lapatinib is a potent ATP-competitive inhibitor of both epidermal growth factor receptor (EGFR) and HER2 [23]. In 2011, the Southwest Oncology Group (SWOG) performed a phase II trial of lapatinib as first-line therapy in patients with advanced HER2-positive gastric cancer and found modest single-agent activity with 9% response rate [24]. This led to a large phase III LOGiC trial where untreated advanced HER2-positive GEJ adenocarcinoma randomly assigned to chemotherapy (capecitabine and oxaliplatin) plus lapatinib versus chemotherapy plus placebo [25]. The results showed no improved OS with the addition of lapatinib (12.2 months versus 10.5 months, HR 0.91; 95% CI 0.73–1.12). In 2014, researchers in Japan published the phase III TyTAN trial that similarly showed no improvement in OS with the addition of lapatinib to paclitaxel in the second line for Asian patients with HER2-amplified advanced gastric cancer [26].
Trastuzumab deruxtecan
Trastuzumab deruxtecan is a novel HER2-targeted antibody-drug conjugate comprised of a humanized monoclonal antibody attached by a cleavable peptide-based linked to a topoisomerase I inhibitor payload. In 2019, a phase I study was done to assess the safety, tolerability, and activity of trastuzumab deruxtecan in HER2-advanced gastric or GEJ cancer [27]. Doses of 5.4 mg/kg and 6.4 mg/kg administered intravenously every 3 weeks were selected based on observed preliminary anti-tumor activity [28]. Trastuzumab deruxtecan showed preliminary anti-tumor activity with 43.2% of patients achieving an objective response. The median time to response was 1.4 months (CI 1.3–1.6), and the median duration of response was 7.0 months (CI 4.4–16.6). In a post hoc analysis, anti-tumor activity was also observed in the subgroup of patients who previously received irinotecan. These results support further investigation of trastuzumab deruxtecan for HER2-poistive gastric or GEJ cancer, and a phase 2 study is ongoing (NCT04014075).
Margetuximab
Margetuximab is an anti-HER2 antibody that is derived from the parent antibody of trastuzumab and binds to HER2. However, five amino acid substitutions engineered into the margetuximab IgG1 Fc domain yield increased binding compared with trastuzumab [29]. In 2017, investigators reported the phase I study of margetuximab in patients with HER2-positive solid tumors, including gastric cancer [30]. Sixty patients were evaluated for tumor response, and seven (12%) patients had a confirmed PR, and 31 (52%) had stable disease (SD), and AEs were mild to moderate. Thus, the results indicate that single-agent margetuximab is well tolerated with promising activity in patients with HER2-expressing tumors. In 2018, a follow-up phase I/II trial tested margetuximab in advanced HER2-positive, PD-L1 unselected gastric cancer in combination with immune checkpoint inhibitor (ICI) pembrolizumab [31]. Of 38 evaluable patients, 7 (18.4%) patients achieved PR and 11 (28.9%) SD. In conclusion, margetuximab plus pembrolizumab is well tolerated with promising preliminary anti-tumor activity in the second line. A subsequent phase II study is currently underway with margetuximab in combination with ICI with or without chemotherapy as first-line treatment for patients with HER2-positive gastric cancer (NCT04082364).
Anti-EGFR
The EGFR family consists of four members: EGFR (HER1), HER2, HER3, and HER4. EGFR activation by one of its ligands initiates diverse downstream signaling pathways that result in cell proliferation and cancer development [32]. EGFR is overexpressed in more than 30% of gastric and esophageal adenocarcinoma and associated with a poor prognosis [5].
Cetuximab and panitumumab
Cetuximab and panitumumab are recombinant human-mouse and human chimeric EGFR IgG1 and IgG2 monoclonal antibodies, respectively. There have been two negative phase III trials investigating cetuximab and panitumumab in combination with chemotherapy. In 2013, both the EXPAND (cetuximab) and REAL3 (panitumumab) trials failed to show a survival benefit in unselected, untreated patients with advanced gastroesophageal adenocarcinomas when combined with chemotherapy [33, 34]. However, small clinical studies selecting gastroesophageal cancers with EGFR amplification for treatment with anti-EGFR therapies reveal high response rates and prolonged survival [35]. This suggests that these EGFR-targeted agents may have a role in the management of properly selected patient populations although larger studies are needed.
Anti-VEGF
Angiogenesis is essential for the growth and metastasis of cancers, and thus the inhibition of angiogenesis has understandably received considerable attention in gastric cancer. VEGF induces tumor angiogenesis by promoting endothelial cell proliferation and increasing vascular permeability [36]. It has been reported that gastric tissue often expresses high levels of VEGF and correlated to more advanced disease and poor outcome [37, 38].
Bevacizumab
Bevacizumab is a monoclonal antibody targeting VEGF-A and has been shown to result in tumor growth inhibition when given as monotherapy or in combination with cytotoxic chemotherapy [39]. In 2010, the phase III trial AVAGAST evaluated the efficacy of adding bevacizumab to capecitabine-cisplatin in the first-line treatment for advanced gastric cancer [40]. Although the mOS was 12.1 months with bevacizumab plus chemotherapy versus 10.1 months with placebo plus chemotherapy, the difference was not statistically significant (HR 0.87; CI 0.73–1.03; P = 0.1002). However, the bevacizumab arm did show an improvement in progression free survival (PFS) (6.7 months versus 5.3 months) and ORR (46% versus 37.4%) compared with placebo. In 2015, another phase III AVATAR study from China also found that bevacizumab combined with capecitabine/cisplatin did not significantly improve OS for advanced gastric cancer [41].
Ramucirumab
Ramucirumab is a recombinant human immunoglobulin G1 (IgG1)-neutralizing monoclonal antibody specific for VEGF receptor-2 (VEGFR2). In 2014, the phase 3 REGARD trial investigated ramucirumab versus placebo in the second-line setting in patients with advanced gastric or GEJ cancer [42]. The mOS was 5.2 months with ramucirumab group and 3.8 months with placebo (HR 0.77, CI 0.6–0.99; P = 0.047). The rates of AE were similar between the two groups, though rates of hypertension were higher in the ramucirumab group. A second phase III study, RAINBOW, investigated ramucirumab plus paclitaxel versus placebo plus paclitaxel in the second-line in patients with advanced gastric or GEJ cancer [43•]. This study revealed that the combination of ramucirumab with paclitaxel significantly improved mOS to 9.6 months vs 7.4 months with paclitaxel (HR 0.80; CI 0.68–0.96, P = 0.017), mPFS 4.4 months vs 2.9 months, and ORR 28% vs 16% and established the new standard second-line treatment for the majority of patients with advanced gastric cancer. The most common grade ≥ 3 treatment emergent adverse events (TEAEs) included neutropenia, leukopenia, hypertension, and fatigue, which had higher incidence in the ramucirumab plus paclitaxel arm.
In 2016, investigators evaluated the addition of ramucirumab to chemotherapy (mFOLFOX6) in the first-line setting [44]. A total of 168 patients were randomized to mFOLFOX6 plus ramucirumab or placebo, and although patients in the investigational arm experienced higher disease control rate (85% vs 67%), no difference was observed in mPFS (6.4 vs 6.7 months) and mOS (11.7 vs 11.5 months). In 2019, the phase 3 RAINFALL evaluated 645 patients with untreated advanced gastric of GEJ adenocarcinoma and randomized them to receive cisplatin plus capecitabine with or without ramucirumab [45]. The addition of ramucirumab to chemotherapy, however, did not improve OS (mOS 11.2 months in the ramucirumab group vs 10.7 months in the placebo group; HR 0.962, CI 0.801–1.156, P = 0.6757). Therefore, at this time there is no role for ramucirumab in the first-line setting for gastric or GEJ cancers.
Ramucirumab is FDA approved as a single agent, or in combination with paclitaxel, for the treatment of patients with advanced or metastatic gastric or GEJ cancer with disease progression on or after prior fluoropyrimidine or platinum-containing chemotherapy.
Apatinib, regorafenib, sunitinib, sorafenib
Another strategy for targeting VEGF is using small molecule tyrosine kinase inhibitors (TKIs), which inhibit the function of the VEGF receptor. For example, apatinib is a small molecular TKI targeted toward VEGFR-2 that has been investigated in China in a phase 3 trial [46]. Chinese patients were randomized to apatinib vs placebo, and mOS with monotherapy apatinib was 6.5 months vs 4.7 months with placebo. Apatinib was approved in October 2014 by the China Food and Drug Administration for metastatic gastric or GEJ adenocarcinoma after second-line chemotherapy, but is not available in the USA.
There have been a series of early-phase clinical trials evaluating the activity of other TKIs, notably regorafenib. In 2016, the phase II INTEGRATE trial evaluated the activity of regorafenib in advanced gastric adenocarcinoma against best supportive care [47]. Patients were randomly assigned to regorafenib or matching placebo, and median PFS was 2.6 months with regorafenib versus 0.9 months in placebo (HR 0.40, CI 0.28–0.59, P < 0.001). A non-statistically significant increase in mOS was observed (5.8 months vs 4.5 months, HR 0.74, CI 3.4–5.2, P = 0.147), although the study was not powered for this end point. A phase 3 study is currently underway (INTEGRATE II NCT02773524) to determine whether regorafenib improves OS compared with placebo in the third line for patients with advanced gastric or GEJ cancer.
In 2019, investigators reported the REGONIVO trial, a phase 1b trial of previously treated advanced gastric or colorectal cancer patients with regorafenib plus nivolumab [48]. In the trial, two cohorts of a total of 50 patients with previously treated advanced or metastatic gastric or colorectal cancer were enrolled and received regorafenib plus nivolumab. The majority of patients (98%) had MSS disease, 59% had negative PD-L1 expression, and 14% had received prior treatment with an immune checkpoint inhibitor. The ORR was 40% with a disease control rate of 88%. Specifically, the ORR was 44% in patients with gastric cancer and 36% in patients with colorectal cancer. The combination of regorafenib plus nivolumab was determined to have a manageable safety profile and encouraging anti-tumor activity and warrants further investigation in a larger cohort; a phase III trial in gastric cancer is planned.
Other agents such as sunitinib and sorafenib have had more mixed results with promising results in single-arm studies, but randomized trials showing no appreciable benefit over chemotherapy [49, 50].
Anti-mTOR
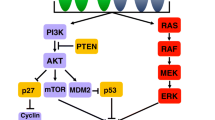
Mammalian target of rapamycin (mTOR) belongs to the PI3K-related kinase family, which primarily regulates cell growth and proliferation via the PI3K/Akt/mTOR signaling pathway [51]. Several preclinical studies have indicated dysregulation of mTOR activity in gastric cancer cell models, identifying mTOR as a potential therapeutic target [52, 53].
Everolimus
Everolimus is an oral mTOR inhibitor. Early-phase trials of everolimus in the treatment of advanced gastric cancer revealed a disease control rate of 56% without any radiographic responses [54]. In 2013, the subsequent phase III trial, GRANITE-1, found that everolimus did not improve the survival of advanced gastric cancer patients who failed previous chemotherapy compared with placebo. The mOS with everolimus was 5.4 months versus 4.3 months with placebo (HR 0.90, P = 0.124) [55]. Thus, at this time everolimus has no role in the management of gastroesophageal cancers outside the context of a clinical trial.
Anti-HFG/MET
The mesenchymal-epithelial transition (MET) factor receptor is a receptor tyrosine kinase for hepatocyte growth factor (HGF). Dysregulation of the HGF/MET pathway promotes tumor growth and metastasis [56]. In gastric cancer, MET expression is reported in 26–74% of cases and gene amplification in 2–23% of cases [57, 58].
Rilotumumab and onartuzumab
Rilotumumab is a fully human monoclonal antibody that selectively targets the ligand of the MET receptor, HGF. In 2017, the phase III RILOMET-1 trial reported no improvement in OS with the addition of rilotumumab to chemotherapy in patients with untreated MET-positive gastric or GEJ adenocarcinoma [59]. In fact, mOS was 8.8 months in the rilotumumab group compared with 10.7 months with placebo (HR 1.34, CI 1.1–1.64, P = 0.003).
Onartuzumab is a recombinant, fully humanized, monovalent monoclonal antibody that binds to the extracellular domain of MET. In 2014, the phase III MET-Gastric trial reported no improvement in OS with the addition of onartuzumab to chemotherapy in patients with untreated MET-positive gastroesophageal adenocarcinomas [60]. Median OS was 11.3 months for the placebo plus chemotherapy arm versus 11.0 months for onartuzumab plus chemotherapy arm (HR 0.82, 95% CI 0.59–1.15, P = 0.24). In the post hoc exploratory subgroup analyses, there was no difference in the ORR between the treatment arms in the MET 2+/3+ subgroups. In summary, rilotumumab and onartuzumab do not have a role in the management of gastroesophageal cancers.
PARP inhibitors
Dysregulation of the DNA damage response creates genomic instability that promotes tumorigenesis [61]. Ataxia-telangiectasia mutated (ATM) protein is a key activator of the DNA damage response to double-strand breaks [62]. ATM deficiency has been associated with sensitivity to poly(ADP-ribose) polymerase (PARP) inhibitors, and up to 22% of patients with metastatic gastric cancer may harbor low or detectable ATM expression [63, 64].
Olaparib
Olaparib is an oral poly(ADP-ribose) polymerase (PARP) inhibitor that blocks DNA base excision repair and causes synthetic lethality in tumors with homologous recombination repair deficiencies [65]. In 2017, the phase III GOLD study performed in Asia failed to show a statistically significant improvement in survival with the addition of olaparib to paclitaxel in both the overall and ATM-negative metastatic setting [66]. The mOS was 8.8 months in the olaparib group versus 6.9 months with placebo (HR 0.79, 95% CI 0.64-1.00; P = 0.026), with the pre-specified significance set at 0.025 instead of the more common 0.05.
Immunotherapy
In the past several years, the ICIs have revolutionized the treatment for many cancers. One such checkpoint is programmed death 1 (PD1), which is an inhibitory receptor expressed mainly on activated T cells [67]. In tumor cells, inhibition of PD-1 prevents PD-1 from binding to its ligands, PD-L1 and PD-L2, thus restoring anti-tumor immunity [68]. Studies have shown overexpression of PD-L1 in gastric cancer, making the inhibition of PD-1 an exciting target in this disease group [5].
Nivolumab and ipilimumab
Nivolumab is a fully human IgG4 monoclonal antibody inhibitor of PD-1. In 2017, the phase 3 ATTRACTION-2 trial evaluated the efficacy of third-line nivolumab versus placebo in Asian patients with advanced gastric or GEJ cancer regardless of PD-L1 expression [69]. Patients were randomly assigned 2:1 to receive nivolumab or placebo, and the results revealed improved mOS with nivolumab of 5.26 months vs 4.14 months with placebo (HR 0.63; 95% CI 0.51–0.78; P < 0.001). Furthermore, the nivolumab 12-month OS was 26.2% vs 10.9% with placebo. These results led to the approval of nivolumab in Japan regardless of PD-L1 status. Nivolumab is not currently approved for gastric cancer in the USA.
In 2018, the open-label CheckMate-032 phase I/II study evaluated the efficacy and safety of nivolumab versus nivolumab plus ipilimumab (anti-CTLA4) in patients with chemotherapy-refractory gastric, esophageal and GEJ cancers in the United States and Europe [70]. Patients with disease progression after at least one chemotherapy received either 3 mg/kg nivolumab alone (NIVO3) every 2 weeks or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (NIVO1 + IPI3) or nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (NIVO3 + IPI1) every 3 weeks. The ORR was 12% with NIVO3, 24% with NIVO1 + IPI3, and 8% with NIVO3 + IPI1. Median duration of response was 7.1 months in the NIVO3 group, 7.9 months in the NIVO1 + IP3 group and not yet reached in the NIVO3 + IPI1 group. Grade 3/4 TRAEs were reported in 17%, 47%, and 27% of patients receiving NIVO3, NIVO1 + IPI3, and NIVO3 + IPI1, respectively. Results from the CheckMate-032 study demonstrated that nivolumab and nivolumab plus ipilimumab provide clinically meaningful and durable anti-tumor activity with an expected safety profile in heavily pretreated Western patients with advanced gastric, esophageal and GEJ cancers. The open-label, phase III CheckMate 649 trial will evaluate nivolumab plus ipilimumab with and without chemotherapy in the first line in patients with gastric or GEJ cancer (NCT02872116).
In Japan, nivolumab is approved for gastric cancer after conventional chemotherapy without regard to specific biomarker expression. To date, nivolumab is not approved in the USA for the treatment of gastric cancer.
Pembrolizumab
Pembrolizumab is a humanized IgG4 monoclonal antibody that binds to PD-1, preventing the interaction of PD-1 with PD-L1 and PD-L2. In 2018, an open-label, single-arm, phase II trial KEYNOTE-059 evaluated the safety and efficacy of pembrolizumab in patients with previously treated gastric of GEJ cancer [71]. The single-arm study enrolled 259 patients and revealed that pembrolizumab monotherapy elicited an ORR of 11.6% with an mOS of 5.6 months in the entire patient population and for PD-L1-positive subgroup, the ORR was 15.5% and mOS 5.8 months. These findings led to the approval of pembrolizumab in the third-line setting for PD-L1-positive gastric and GEJ adenocarcinoma.
The phase III KEYNOTE-061 trial in the second-line setting revealed that pembrolizumab did not significantly improve OS when compared with paclitaxel for advanced gastric or GEJ cancer with PD-L1 positive (combined positive score (CPS) ≥ 1) [72•]. The mOS was 9.1 months with pembrolizumab and 8.3 months with paclitaxel (HR 0.82, 95% CI 0.66–1.03; P = 0.0421). In contrast to these findings, the phase III KEYNOTE-181 study was a second-line trial, which included metastatic squamous cell carcinoma (SCC) of the esophagus, adenocarcinoma of the esophagus, and Siewert type I and compared pembrolizumab versus investigators’ choice of chemotherapy. The preliminary results for the CPS ≥ 10 patients revealed a mOS survival of 9.3 months with pembrolizumab vs 6.7 months with chemotherapy with no difference in the overall study population [73]. In KEYNOTE-181 the improvement in mOS was primarily driven by SCC patients, which ultimately led to an approval for pembrolizumab for SCC of esophagus CPS ≥ 10 in the second-line setting.
At the 2019 ASCO Annual Meeting, Dr. Tabernero presented the results of KEYNOTE-062 [74]. This trial evaluated 3 first-line treatments: (1) pembrolizumab in PD-L1-positive gastric or GEJ adenocarcinomas, (2) chemotherapy, and (3) pembrolizumab plus chemotherapy. The results revealed that pembrolizumab was non-inferior to chemotherapy and perhaps better than chemotherapy in the CPS ≥ 10 subgroup. However, in the chemotherapy plus pembrolizumab arm did not reveal a survival benefit compared with the chemotherapy alone arm in CPS ≥ 10. These findings were surprising and raise the question as to whether adding chemotherapy to immunotherapy has a negative impact on immune stimulation for gastric cancer. At this time more data is needed to draw any definitive conclusions from KEYNOTE-062 and to understand chemo-immunotherapy combinations in this disease.
In the USA, pembrolizumab is approved in the second-line setting for MMRd)/MSI-H gastric cancer and in the third line for gastric adenocarcinoma with PD-L1 ≥ 1%.
Avelumab
Avelumab is a human anti-PD-L1 IgG1 antibody that binds PD-L1 and inhibits its interaction with PD-1. In 2019, two phase I studies, JAVELIN Solid Tumor (NCT01772004) and JAVELIN Solid Tumor JPN (NCT01943461), revealed avelumab to have an acceptable safety profile and promising rate clinical efficacy in patients with advanced gastric cancer and GEJ adenocarcinoma [75, 76]. In 2018, the phase III JAVELIN Gastric 300 compared avelumab versus physician’s choice of chemotherapy as third-line therapy in patients with advanced gastric or GEJ cancer [77]. The trial did not meet its primary end point of OS with mOS 4.6 months in the avelumab arm vs 5.0 months with chemotherapy (HR 1.1, CI 0.9–1.4, P = 0.81). Thus, there is no role for avelumab in the treatment of advanced gastric cancer at this time. However, first-line avelumab maintenance following chemotherapy is currently being investigated in the phase 3 JAVELIN Gastric 100 trial (NCT02625610).
Conclusion
Although chemotherapy has been the backbone therapy for advanced gastric cancer for many years, we are now moving onto an era of more targeted drugs for genes and signaling pathways. Trastuzumab with chemotherapy is now considered to be the standard first-line therapy for HER2-positive gastric cancer. The anti-VEGFR2 inhibitor, ramucirumab, has been approved for the use in the second-line setting. Immunotherapy with checkpoint inhibition holds great promise with the first approved immunotherapy agent, pembrolizumab, in the third-line settings for PDL-1-positive tumors or second-line setting for MSI high tumors and SCC CPS ≥ 10. However, the results with immunotherapy have been mixed which highlights the role for improved biomarkers based on our understanding of molecular pathways and novel immunotherapy combinations with molecularly targeted agents. Despite the advances highlighted in this review, the absolute gains have been modest, with a longevity increase of a few months of survival for agents. Moving forward, development of multi-target drugs in combination with surgery, radiotherapy, and chemotherapy may result in new opportunities for our ultimate pursuit: to improve survival and reduce symptoms.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20(16):4483–90.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Group G, Oba K, Paoletti X, Bang YJ, Bleiberg H, Burzykowski T, et al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. 2013;49(7):1565–77.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–56.
Chivu-Economescu M, Matei L, Necula LG, Dragu DL, Bleotu C, Diaconu CC. New therapeutic options opened by the molecular classification of gastric cancer. World J Gastroenterol. 2018;24(18):1942–61.
Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22(42):6570–8.
Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–4.
Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232(4758):1644–6.
Grillo F, Fassan M, Sarocchi F, Fiocca R, Mastracci L. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol. 2016;22(26):5879–87.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25.
Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35(4):446–64.
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59(6):795–805.
• Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97 This study resulted in the FDA approval of the first HER2 directed therapy in gastric cancer.
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90.
Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–75.
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–6.
Kang YK, Rha SY, Tassone P, Barriuso J, Yu R, Szado T, et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111(4):660–6.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84.
Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–9.
Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, et al. Southwest oncology group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22(12):2610–5.
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC--a randomized phase III trial. J Clin Oncol. 2016;34(5):443–51.
Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49.
Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):827–36.
Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512–22.
Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13(6):R123.
Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-human phase 1 study of margetuximab (MGAH22), an fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61.
Catenacci DVT, Park H, Lockhart AC, Gold PJ, Enzinger PC, Nordstrom JL, et al. Phase 1b/2 study of margetuximab (M) plus pembrolizumab (P) in advanced HER2+ gastroesophageal junction (GEJ) or gastric (G) adenocarcinoma (GEA). J Clin Oncol. 2018;36(4_suppl):140.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–9.
Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):481–9.
Maron SB, Alpert L, Kwak HA, Lomnicki S, Chase L, Xu D, et al. Targeted therapies for targeted populations: anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(6):696–713.
Xu W, Yang Z, Lu N. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. 2016;35:1.
Grigore D, Simionescu CE, Stepan A, Margaritescu C, Balasoiu M, Georgescu CC, et al. Assessment of CD105, alpha-SMA and VEGF expression in gastric carcinomas. Romanian J Morphol Embryol. 2013;54(3 Suppl):701–7.
Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24(2):228–40.
Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671–80.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–76.
Shen L, Li J, Xu J, Pan H, Dai G, Qin S, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2015;18(1):168–76.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
• Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35 This study resulted in the FDA approval of the first VEGF directed therapy in gastric cancer.
Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip PA, Cohn AL, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter phase II trial. Ann Oncol. 2016;27(12):2196–203.
Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35.
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54.
Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol. 2016;34(23):2728–35.
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). Journal of Clinical Oncology. 2020:JCO.19.03296.
Yi JH, Lee J, Lee J, Park SH, Park JO, Yim DS, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer. 2012;106(9):1469–74.
Sun W, Powell M, O'Dwyer PJ, Catalano P, Ansari RH, Benson AB 3rd. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28(18):2947–51.
Sasore T, Kennedy B. Deciphering combinations of PI3K/AKT/mTOR pathway drugs augmenting anti-angiogenic efficacy in vivo. PLoS One. 2014;9(8):e105280.
Li VS, Wong CW, Chan TL, Chan AS, Zhao W, Chu KM, et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29.
Al-Batran SE, Ducreux M, Ohtsu A. mTOR as a therapeutic target in patients with gastric cancer. Int J Cancer. 2012;130(3):491–6.
Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28(11):1904–10.
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31(31):3935–43.
Gherardi E, Birchmeier W, Birchmeier C, Vande WG. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103.
Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85(9):1894–902.
Taniguchi K, Yonemura Y, Nojima N, Hirono Y, Fushida S, Fujimura T, et al. The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c-met), autocrine motility factor receptor, and urokinase-type plasminogen activator receptor. Cancer. 1998;82(11):2112–22.
Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–82.
Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3(5):620–7.
Curtin NJ. PARP inhibitors target ATM+p53-defective gastric cancer. Cell Cycle. 2014;13(20):3161–2.
Kurz EU, Lees-Miller SP. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst). 2004;3(8–9):889–900.
Bang YJ, Im SA, Lee KW, Cho JY, Song EK, Lee KH, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol. 2015;33(33):3858–65.
Kim HS, Kim MA, Hodgson D, Harbron C, Wellings R, O'Connor MJ, et al. Concordance of ATM (ataxia telangiectasia mutated) immunohistochemistry between biopsy or metastatic tumor samples and primary tumors in gastric cancer patients. Pathobiology. 2013;80(3):127–37.
O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–60.
Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1637–51.
Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–9.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–44.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013.
• Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33 This study resulted in the FDA approval of the first immune therapy in gastric cancer.
Kojima T, Muro K, Francois E, Hsu C-H, Moriwaki T, Kim S-B, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J Clin Oncol. 2019;37(4_suppl):2.
Tabernero J, Cutsem EV, Bang Y-J, Fuchs CS, Wyrwicz L, Lee KW, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(18_suppl):LBA4007–LBA.
Chung HC, Arkenau HT, Lee J, Rha SY, Oh DY, Wyrwicz L, et al. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2019;7(1):30.
Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, et al. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN solid tumor JPN trial. Gastric Cancer. 2019;22(4):817–27.
Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN gastric 300. Ann Oncol. 2018;29(10):2052–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Timil Patel does not have any conflicts of interest to disclose. Michael Cecchini has received compensation from AstraZeneca, Eisai, and Agios for service as a consultant. Michael Cecchini has received honoraria from AstraZeneca, Eisai, and Agios.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Upper Gastrointestinal Cancers
Rights and permissions
About this article
Cite this article
Patel, T.H., Cecchini, M. Targeted Therapies in Advanced Gastric Cancer. Curr. Treat. Options in Oncol. 21, 70 (2020). https://doi.org/10.1007/s11864-020-00774-4
Published:
DOI: https://doi.org/10.1007/s11864-020-00774-4