Opinion statement
Denosumab is a RANK ligand inhibitor approved for the treatment of giant cell tumor of bone. While the role of denosumab in the setting of advanced and unresectable disease is well established, its role in surgically resectable disease is currently under discussion. Several prospective and retrospective series on neoadjuvant therapy in potentially resectable tumor with high morbidity surgery reported a relapse rate of 10–20% after resection and 30–40% after curettage. At the same time, less morbid surgery has obvious clinical advantages for the patient, and several studies have shown the efficacy of denosumab in downgrading of the surgical procedure. Currently, the role of neoadjuvant denosumab in operable GCTB is limited to selected cases in which a diffuse reactive bone formation and peripheral ossification can make an easier surgical procedure, for example, in tumors with a large soft tissue component. A planned resection may become less morbid when preoperative denosumab is administered. Whenever a segmental resection is thought to be indicated at diagnosis, denosumab may be considered in the neoadjuvant setting. A preoperative course of 6 months is considered safe and effective. Two case scenarios are presented and critically discussed. Because of the high recurrence rates after denosumab treatment followed by curettage, we discourage the use of denosumab when curettage is considered feasible. In this setting, a short course of preoperative denosumab (2–6 months) may be considered for highly selected cases, for example in pathological fractures. The role of adjuvant denosumab needs further investigation. Long-term disease control has been reported in case of non-surgical lesions, even after treatment interruption, but there is no consensus on ideal treatment duration and dosage for these scenarios. In all cases, multidisciplinary discussion with oncology, pathologist, radiologist, and surgeons is mandatory. Patient’s comorbidities, dental conditions, and preferences, including family planning, should always be taken into account.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giant cell tumor of bone is a benign, locally aggressive neoplasm that commonly afflicts young adults within the epiphyseal equivalent of long bones. It most commonly arises in the distal femur, proximal tibia, and distal radius. Clinically, patients present with pain and joint dysfunction. Radiographically, there is progressive lucency and cortical expansion or destruction, with or without a soft tissue component.
It is composed of two predominant cell types: neoplastic mononuclear stromal cells and reactive osteoclast-like giant cells. The stromal cells adopt a mostly round shape but can take on an oval or elongated appearance.
The giant cells express receptor activator of nuclear kappa B (RANK). The mononuclear stromal cells express RANK ligand, a mediator of osteoclast differentiation and activation.
Denosumab is a human monoclonal antibody that specifically inhibits RANKL-mediated formation and activation of multinucleated osteoclasts or giant cells [1,2,3]. This drug has been used in the treatment of giant cell tumors of bone (GCTB), showing clinical, radiological, and histopathological responses [4,5,6,7,8]. Over the last decade, denosumab has been studied extensively for GCTB. Yet, many controversies still exist regarding its safety, efficacy, and treatment indications. This review addresses these issues and presents the current standard for denosumab treatment in GCTB.
Natural history
Most of patients present with giant cell tumor of bone considered a stage 3 lesion within the MSTS, or Enneking, classification. It is a benign, locally aggressive lesion. The unique diagnostic and terminology features of giant cell tumor are the seemingly conflicting nature of a benign lesion that also has the ability to spread systemically. The most common site of second-site involvement is the lung. The formal defined process of metastases follows a stepwise pattern that includes invasion of the basement membrane and introduction into the blood stream and then extravasation out of the bloodstream into a distant organ. It is unclear if pulmonary foci of giant cell tumor follow these same steps. This has led some to refer to these foci as emboli rather than metastatic disease.
There are reports in the literature of sarcomatous degeneration of giant cell tumor, although this is rare [9,10,11,12,13]. These reports are further complicated by the difficulty in initial histologic diagnosis in challenging cases. It could be reasonably argued that some of the cases described as undergoing sarcomatous degeneration may have actually been sarcomas originally and the histologic diagnosis was inaccurately reported due to sampling error or other diagnostic limitations.
Treatment
Prior to the introduction of denosumab, the management of localized disease has historically been surgical. The extent and nature of surgery is dependent on multiple factors, including the age of the patient, anatomic location and extent of the lesion, and soft tissue extension. The goal of surgery is to remove the entirety of the tumor through either an extended curettage, which implies extending the zone of the osseous or soft tissue bed past the perimeter of the lesion or resection of the tumor. The choice is largely dependent on location. As most of these lesions occur near joints in the appendicular skeleton, the goal is to spare the native joint. For cases within the axial skeleton (spine, skull base, pelvis, sacrum), the proximity of vital anatomic structures can relabel these tumors unresectable. Unresectable does not mean non-operable; it simply changes the nature of the intervention from one attempting to completely remove the neoplastic cells to one in which debulking is preferential. In these cases, additional adjuvants have been utilized, such as bisphosphonate-loaded cement [14]. En bloc resection is an option and has shown decreased local recurrence rates [15]. If en bloc resection is chosen, joint reconstruction is typically undertaken with a megaprosthesis. Megaprostheses result in lower joint function when compared to joint preservation, and their survival decreases over time. In light of this, joint preservation procedures are preferred.
Surgery is typically either an extended curettage or resection. Curettage includes mechanical curettage and also adjuvant therapy, which includes argon beam, hydrogen peroxide, polymethylmethacrolate, ethanol, or liquid nitrogen. Adjuvant therapies are utilized to decrease the risk of local recurrence. There are multiple reports in the literature indicating improvements in local recurrence, but there are no randomized trials comparing them. Local recurrence rates are high with ranges from 20 to 50% without local use of adjuvants [16,17,18]. Recurrence increases local morbidity due to bone loss and soft tissue damage from prior procedures. Challenging anatomic sites include the spine, pelvis, and peri-articular regions in which curettage would either destabilize the joint or create significant incongruity. Non-operative management is pursued when there is unreasonable expected surgical morbidity. This is surgeon- and patient-dependent and must be made on a case by case basis.
The introduction of denosumab has altered the perception of this pathology as well as the way surgical decision-making is approached. In 2013, denosumab was approved by the FDA for use in patients with giant cell tumor who were determined to be either inoperable, when surgery would result in unacceptable morbidity or in metastatic disease. This approval was based on the results of multiple phase II trials. Several studies reported substantial clinical, radiological, and histological response to denosumab treatment [4, 19••, 20] (Table 1). Pain reduction, improved function, and mobility are typical for patients treated with denosumab [5, 8]. Radiological changes included reduction in tumor size, central sclerosis and bone formation, peripheral bone formation, and shrinkage of soft tissue components [3, 6, 21, 22]. Also, complete healing of pathological fracture was seen during the course of denosumab treatment [7]. Histologically, a marked regression or complete absence of multinucleated giant cells is seen, and reduction of the spindle/stromal cell population has been reported. Reactive, woven bone formation and abundant collagen matrix are described, and at the periphery of the lesions, osteoid formation is generally observed [1, 3, 7, 21, 23]. Although these changes were consistently present in most studies on denosumab, this was not reflected in reduction of local recurrence, in the neoadjuvant setting [24, 25•, 26,27,28]. A possible reason for this is that reactive collagen matrix and osteoid formation within the neoplasm that is seen after denosumab treatment make it more difficult to distinguish tumor from normal tissue. Consequently, tumor tissue can be left behind during intralesional excision/curettage and will eventually give rise to recurrent disease after denosumab treatment interruption [29]. Although there are no results from randomized trials, comparing surgical treatment of GCTB with and without neoadjuvant denosumab, recurrence rates in the neoadjuvant cohorts are very similar to historical data on GCTB treated only surgically [25•, 30, 31]. A systematic literature review reports a pooled weighted recurrence rate of 9% (95% CI 6–12%) [25•]. Long-term results of a phase II trial with denosumab in more than 500 cases showed the results for 157 surgically treated patients: the recurrence rate was 27% overall, 34% for patients treated by curettage, and 12% for those who underwent resection [19••].
Attention has now turned to the use of denosumab in cases for which surgery should not result in undue morbidity. An open label phase II trial investigated the influence of neoadjuvant denosumab on surgical downstaging in primary and recurrent GCTB. Patients were included if planned surgery would produce significant functional compromise. They found that most patients either had no surgery or a less morbid surgery than planned. Joint preservation was achieved in 96% of patients for whom joint replacement was anticipated. There was a 15% local recurrence rate for those who underwent surgery. For the patients who underwent surgery, they were on denosumab for a median time of 14.2 months [22]. Several other studies mention the advantage of surgical downstaging after preoperative denosumab treatment [7, 21, 27, 28]. However, with very high recurrence rates in the cohort of patients treated with denosumab and curettage, some authors [24] discourage intralesional surgery after denosumab treatment, especially in hands and foot [32]. On the other hand, peripheral ossification of an initially completely lytic tumor with cortical thinning can make a segmental resection technically easier and less morbid [7, 27, 33•].
Unresolved problems
It is here that a distinction in tumor extent is important in denosumab duration and relation to timing of surgical intervention. For tumors in which the goal of neoadjuvant denosumab therapy is downstaging and formation of a sclerotic rim to form borders to demarcate the extent of a curettage, consideration should be given to surgery within the first few months of treatment. While a sclerotic rim can be protective, it also may be home to a nidus of neoplastic stromal cells that contribute to increased local recurrence. In contrast, for tumors in which the surgical plan involved wide resection, consideration should be given to extended denosumab therapy in order to provide maximal calcification of the tumor. Response to denosumab is determined based on clinical, radiographic, and histopathologic features.
Another consideration relates to the role of denosumab in the adjuvant setting. The dose in the first proof of principle trial was 120 mg every 28 days with a loading dose on days 8 and 15 of the first month. Patients who had complete tumor resection (cohort 2) received adjuvant denosumab for six doses after surgery.
In the real life use of denosumab, there is a lack of consistency in those administering denosumab in the adjuvant setting and those that do not. The utility of dose, frequency, and duration remains to be defined. Also, while denosumab is generally well tolerated, severe adverse effects have been reported and include osteonecrosis of the jaw (ONJ) (7 cases, 3% in the resectable group), atypical femur fracture (4 cases, < 1%, all occurred after 48 months of denosumab treatment, none in resectable cases), and four (< 1%, two resectable cases) had hypercalcemia that occurred after treatment interruption (all occurred between 4 and 9 months after the last denosumab dose, two cases were recurrent). It is important to underscore that ONJ was mainly observed in patients with prior dental conditions [19••].
Neoadjuvant denosumab case presentation
-
Case 1. A 52-year-old female was referred at our institute for pubic pain. A CT scan was performed, and a large purely lytic lesion, with extension into the soft tissues, was demonstrated in the left pubic rami (Fig. 1). A CT-guided biopsy was performed confirming a GCTB diagnosis. She was started on denosumab within a clinical trial [19••]. CT scan after 3 months on denosumab treatment showed shrinkage of the tumor, peripheral ossification, and increased central matrix production (Fig. 1b). Patient was asymptomatic and continued monthly denosumab administration without any side effect or delay in treatment. CT scan after 31 months showed further improvement (Fig. 1c). She was then proposed for surgery. A resection was performed and she received pre-planned adjuvant denosumab (6 doses). Postoperative CT scan demonstrates resection of the pubic rami. After 5 years of follow-up, patient is well and free of disease recurrence (Fig. 1d).
-
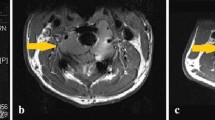
Case 2. We present an 88-year-old male with 1.5 years of progressively worsening right knee pain. Imaging revealed an expansile lucent lesion in the metaphyseal region of the proximal tibia (Fig. 2). Workup revealed a slightly elevated PSA, a significant smoking history (100 pack year), and a normal SPEP. Additionally, he underwent a CT scan of his chest, abdomen, and pelvis which revealed a right lower lobe nodule. A subsequent PET/CT revealed an avid 2.2 cm right lower lobe nodule (SUV 5.1). Fine needle aspiration of his lung nodule revealed a well-differentiated adenocarcinoma, for which he underwent stereotactic radiation as definitive therapy. He underwent biopsy of his tibia lesion, which revealed a giant cell tumor of bone. Treatment options were discussed with the patient regarding his right tibia giant cell tumor. Given the extensive involvement and proximity to the joint surface, as well as his superimposed osteopenia, there was concern that an extended curettage would leave him with inadequate subchondral bone and possibly damage his tibial tuberosity. Alternatively, a proximal tibia replacement was discussed, which would likely require rotational flap coverage and a prolonged rehabilitation. We also discussed medical management with denosumab with the intent of improving his pain and providing consolidation and improved sclerosis of his tumor. He received 4 cycles of 120 mg denosumab subcutaneously, plus additional doses on days 8 and 15 of the initial cycle. He had an excellent clinical and radiographic response. He has continued with medical management and regular follow-up to monitor his clinical and radiographic response as well as his tolerance of denosumab.
There are a few pertinent points related to this case. The first is the location and extent of the tumor. When the surgical intervention is not expected to cause significant morbidity, it should be considered as first line treatment. In this case, the tumor occupied the entire proximal tibia metaphysis and was compromising cortical integrity, leading to pain and reasonable concern for progression to a pathologic fracture. In this situation, a resection procedure would be favored to an extended curettage, in order to perform the best oncologic procedure and decrease the risk of local recurrence. A proximal tibia replacement has a unique complication profile, specifically in terms of wound healing and integrity and function of the extensor mechanism. In this situation, especially in an elderly patient with a concurrent lung adenocarcinoma diagnosis, medical management with denosumab should be strongly considered. The duration of therapy in this situation has not been well defined. We will continue with medical therapy with close monitoring of symptoms. If symptoms continue to be well controlled, consideration could return to surgical intervention if there is sustained radiographic evidence of downstaging.
Fig. 1 A 52-year-old female with a giant cell tumor of bone of the left pubic rami. a The CT scan at diagnosis shows a large purely lytic lesion, with extension into the soft tissues. b CT scan after 3 months on denosumab treatment (120 mg/month) shows shrinkage of the tumor volume, peripheral ossification, and increased central matrix production. c CT scan after 31 months on treatment shows further increase of the previously mentioned radiographic changes. d Postoperative CT scan resection of the pubic rami was performed after 42 months of denosumab treatment.
Fig. 2 Plain X-ray of the knee – AP imaging before (a) and after (b) initiation of treatment. Lateral imaging before (c) and after (d) initiation of treatment. Note the metaphyseal/epiphyseal equivalent location and the cortical expansion and destruction medially and posteriorly at diagnosis. Note the reconstitution of the medial cortical bone as well as thickening of the subchondral bone and intralesional ossification resulting from treatment effect.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thomas DM. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24(4):397–403.2.
Lewiecki EM. Clinical use of denosumab for the treatment for postmenopausal osteoporosis. Curr Med Res Opin. 2010;26(12):2807–12.
Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18(16):4415–24.
Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
Martin-Broto J, Cleeland CS, Glare PA, Engellau J, Skubitz KM, Blum RH, et al. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol. 2014;53(9):1173–9.
Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label,parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–8.
Traub F, Singh J, Dickson BC, Leung S, Mohankumar R, Blackstein ME, et al. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer. 2016;59:1–12.
Deveci MA, Paydaş S, Gönlüşen G, Özkan C, Biçer ÖS, Tekin M. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: prospective study of 14 cases. Acta Orthop Traumatol Turc. 2017;51(1):1–6.
Aponte-Tinao L, Piuzzi NS, Roitman P, Farfalli GL. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473:3050–5.
Broehm JC, Garbrecht EL, Wood J, Bocklage T. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med. 2015. https://doi.org/10.1155/2015/767198.
Alaqaili SI, Abduljabbar AM, Altaho AJ, Khan AA, Alherabi JA. Malignant sarcomatous transformation of benign giant cell tumor of bone after treatment with denosumab therapy: a literature review of reported cases. Cureus. 2018. https://doi.org/10.7759/cureus.3792.
Tsukamoto S, Righi A, Vanel D, Honoki K, Donati DM, Errani C. Development of high-grade osteosarcoma in a patient with recurrent giant cell tumor of the ischium while receiving treatment with denosumab. Jpn J Clin Oncol. 2017;47(11):1090–6.
Park A, Cipriano CA, Hill K, Kyriakos M, McDonald DJ. Malignant transformation of a giant cell tumor of bone treated with denosumab. JBJS Case Connect. 2016. https://doi.org/10.2106/JBJS.CC.16.00024.
Greenberg DD, Lee FY. Bisphosphonate-loaded bone cement as a local adjuvant therapy for giant cell tumor of bone a 1 to 12-year follow-up study. Am J Clin Oncol. 2019;42:231–7.
Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–78.
Knochentumoren A, Becker WT, Dohle J, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–7.
Algawahmed H, Turcotte R, Farrokhyar F, Ghert M. High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: a systematic review and meta-analysis. Sarcoma. 2010;2010:586090.
Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79(1):86–93.
•• Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open label, phase 2 study. Lancet Oncol. 2019;20(12):1719–29. Largest phase 2 clinical trial on denosumab for giant cell tumor of bone, including 532 patients, showing long-term efficacy and safety of the treatment.
Ueda T, Morioka H, Nishida Y, Kakunaga S, Tsuchiya H, Matsumoto Y, et al. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol. 2015;26(10):2149–54.
Boye K, Jebsen NL, Zaikova O, Knobel H, Løndalen AM, Trovik CS, et al. Denosumab in patients with giant-cell tumor of bone in Norway: results from a nationwide cohort. Acta Oncol. 2017;56(3):479–83.
Rutkowski P, Ferrari S, Grimer RJ, Stalley PD, Dijkstra SP, Pienkowski A, et al. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22:2860–8.
Girolami I, Mancini I, Simoni A, Baldi GG, Simi L, Campanacci D, et al. Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol. 2016;69(3):240–7.
Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, et al. Denosumab may increase the risk of local recurrence in patients with Giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018;100(6):496–504.
• Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J. Denosumab treatment for giant-cell tumor of bone: a systematic review of the literature. Arch Orthop Trauma Surg. 2019;139(10):1339–49. Systematic review of the literature including 1095 patients, showing clinical and radiologic responses, and less morbid surgery after denosumab.
Scoccianti G, Totti F, Scorianz M, Baldi G, Roselli G, Beltrami G, et al. Preoperative denosumab with curettage and cryotherapy in giant cell tumor of bone: is there an increased risk of local recurrence? Clin Orthop Relat Res. 2018;476(9):1783–90.
Agarwal MG, Gundavda MK, Gupta R, Reddy R. Does denosumab change the giant cell tumor treatment strategy? Lessons learned from early experience. Clin Orthop Relat Res. 2018;476(9):1773–82.
Puri A, Gulia A, Hegde P, Verma V, Rekhi B. Neoadjuvant denosumab: its role and results in operable cases of giant cell tumour of bone. Bone Joint J. 2019;101-B(2):170–7.
Müller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016;14:281.
CDM F, Unni KK, Mertens F, World Health Organization, International Agency for Research on Cancer. Pathology and genetics of tumours of soft tissue and bone. Geneva: IARC Press; 2002. p. 427.
Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006;29(1):96–9 Review.
Chinder PS, Hindiskere S, Doddarangappa S, Pal U. Evaluation of local recurrence in giant-cell tumor of bone treated by neoadjuvant denosumab. Clin Orthop Surg. 2019;11:352–60.
• Rutkowski P, Gaston L, Borkowska A, Stacchiotti S, Gelderblom H, Baldi GG, et al. Denosumab treatment of inoperable or locally advanced giant cell tumor of bone - Multicenter analysis outside clinical trial. Eur J Surg Oncol. 2018;44:1384–90. Multicenter study including 138 patients showing the surgical downstaging effect of denosumab.
Author information
Authors and Affiliations
Contributions
EP had the idea for the article, ES and LBJ performed the literature search and data analysis, and all drafted and/or critically revised the work.
Corresponding author
Ethics declarations
Conflict of Interest
Emanuela Palmerini has received compensation from Daiichi Sankyo, Deciphera, Lilly, and EUSA Pharma for service as a consultant and has received non-financial support from PharmaMar.
Eric Lodewijk Staals declares that he has no conflict of interest.
Louis Baxter Jones declares that he has no conflict of interest.
Davide Maria Donati declares that he has no conflict of interest.
Alessandra Longhi declares that she has no conflict of interest.
R. Lor Randall declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sarcoma
Rights and permissions
About this article
Cite this article
Palmerini, E., Staals, E.L., Jones, L.B. et al. Role of (Neo)adjuvant Denosumab for Giant Cell Tumor of Bone. Curr. Treat. Options in Oncol. 21, 68 (2020). https://doi.org/10.1007/s11864-020-00766-4
Published:
DOI: https://doi.org/10.1007/s11864-020-00766-4