Opinion statement
Several studies have investigated the prognosis of soft tissue sarcomas and the influence of a variety of factors, such as size, histology subtype, malignancy grade, site, margins, on overall survival, recurrence-free survival, incidence of local and distant spreading. The impact of genomic and expression profiling on long-term outcomes of patients with sarcomas has been also evaluated in order to fill the knowledge gap of this heterogeneous disease. Nomograms represent a prognostic tool that extends the standard staging systems on an individualized basis, taking into account tumor- and patient-related factors. They are used to assist the health provider and the patients in the decision-making process, for patient counseling, treatment decision-making, follow-up scheduling, and clinical trial eligibility determination. None of the available nomograms include molecular characterization of sarcomas. In the future, omics signatures might be incorporated into prognostic nomograms possibly improving their performance. In the present review, we focus on the complexity of prognostic and predictive factors for extremity and trunk wall as well as for retroperitoneal soft tissue sarcomas, while exploring the available prognostic models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STSs) usually present as solid masses, generally surrounded by a pseudocapsule of variable thickness in continuity with the surrounding normal tissues. The mainstay of sarcoma treatment is surgery, whose aim is to resect the tumor surrounded by healthy tissue while avoiding positive surgical margins [1].
Despite an optimal surgery, patients operated for STS remain at risk for tumor recurrence, both locally or at distant sites. The quality of the surgical margins is the main determinant of the risk of local recurrence (LR), together with tumor grade and histological subtype. The identification of patients at higher risk for LR is important to identify patients who might benefit from the administration of perioperative radiotherapy, which has been proved to be able to lower the risk of LR in prospective RCT [2,3,4].
The risk for distant metastases (DM) is mainly related to tumor biology. There is a wide difference in the incidence of DM across different histologies. Also, tumor grade and size significantly impact this risk. The role of chemotherapy in reducing the metastatic potential of localized STS is less clearly defined. Several RCTs failed to clearly prove an association between CTx administration and a reduction of the metastatic risk. Nevertheless, these trials enrolled a heterogeneous population of patients where the chemotherapy effect might have been diluted and the use of risk stratification might help identify a subgroup of patients at high risk of tumor recurrence that might benefit from CTx.
In other words, each STS has its own propensity to spread locally and/or distantly. Furthermore, the same histological subtype, in different locations, carries a different risk.
Retroperitoneal sarcomas tend to show a local pattern of recurrence, while extremity STS mainly spread to the lungs.
The computation of the individual risk of tumor recurrence in STS needs to factor, simultaneously, a number of variables related to the tumor itself (i.e., histology, grade, site), the patient (i.e., age), and the treatment (i.e., quality of surgery).
Since surgery is the only potentially curative option in patients with STS, most of the instruments available to predict the risk of tumor recurrence are meant to be used at time of surgery of the primary tumor. However, the risk of tumor recurrence is not static. It changes with time after surgery according to baseline characteristics and the occurrence of oncological events. It is indeed intuitive that a patient who recurs will be at higher risk of further recurrence as compared with patients who have been treated for their primary tumor and never recurred. Over the past 2 years, several instruments were developed to estimate the dynamic risk of tumor recurrence.
In the present review, we will explore the instruments available to stratify patients with STS in risk categories, to estimate a personalized risk at time of surgery of the primary tumor and during the follow-up. We will focus on extremity as well as retroperitoneal STS, while we will not discuss risk estimation in gastrointestinal stromal tumor.
Risk factors for tumor recurrence
In STS, the relative contribution of each prognostic factor on the risk of tumor recurrence is related to tumor site. Also, the prognostic implication of a tumor recurrence per se is site-dependent. In example, local recurrence in extremity STS is most of the time manageable with re-resection. On the contrary, a local recurrence in the retroperitoneum may easily become not resectable and ultimately be responsible for the patient death. For these reasons, we will discuss prognostic factors separately for extremity and retroperitoneal STS.
Retroperitoneal sarcoma
Retroperitoneal sarcomas include a limited spectrum of histologic types: the most common are liposarcoma and leiomyosarcoma which account for approximately 80% of all cases. Solitary fibrous tumor (SFT), Malignant Peripheral Nerve Sheath Tumors (MPNST) and Undifferentiated Pleomorphic Sarcomas (UPS) make up most of the remaining 20%.
A deep knowledge of the natural history of these tumors has been achieved only recently, over the past 15 years, when the collection of patients in single or multicentric prospective databases has generated high-quality data. Through the analysis of these series, it became evident that even within retroperitoneal STS we can identify very heterogeneous subgroups, mainly based upon histology and grade, with different prognosis and pattern of recurrence.
On the one end of the spectrum, there are well-differentiated liposarcomas (WDLPS) that are characterized by a low but steady tendency to recur locally even several years after surgery and a negligible metastatic potential. This reflects into a LR rate of about 22% at 5 years and 35% at 8 years. The survival curve is the most favorable across all the histologies even if it does not plateau due to the risk of late recurrences [5•].
SFT represent about 6% of all STS and generally show an indolent behavior with a low tendency to recur locally or distantly. This does not apply to dedifferentiated or undifferentiated SFT that can be high grade and have an aggressive behavior. The curves in the studies likely represent a combination of the different variants, with the classic, low grade, variant being the most common.
Dedifferentiated liposarcomas (DDLPS) can be further stratified in two subgroups by tumor malignancy grade: G2 DDLPS show a high tendency to recur locally (5-year LR rate is about 43%) and a relatively low metastatic potential (5-year DM rate of about 10%), G3 DDLPS show both a high tendency to recur locally (5-year LR rate 36%), and at distant sites (5-year DM rate 30%).
Leiomyosarcomas (LMS) are mainly high-grade tumors that arise from large veins such as the inferior vena cava, renal veins, gonadal veins, or iliac veins. They harbor the highest metastatic potential across all the histological subtypes with about half of them that will eventually metastasize in 5 years from surgery. On the contrary, their risk of LR is relatively low (approximately 10% at 5 years) after a proper surgical treatment. Notably, their post-relapse outcome tend to be less severe than other histological subtypes, likely due to the availability of several potentially active drugs in advanced leiomyosarcoma, such as anthracyclines, dacarbazine, gemcitabine, trabectedin, and pazopanib.
MPNST and UPS make up the majority of the remaining RPS. They show a high risk of both local and distant recurrence and usually require a multimodality treatment.
With regard to other tumor-related characteristics, also tumor size, and multifocality significantly impact the risk of tumor recurrence, with larger and multifocal tumors generally being at higher risk of local recurrence.
Among treatment-related prognostic factors, completeness of resection holds sway [6]. Incomplete resections are associated with a poor outcome and this is one of the several reasons why RPS should be managed only within reference centers. The quality of surgical margins has repeatedly shown a prognostic implication in RPS, with more aggressive surgical approach being retrospectively associated with a better outcome. Nonetheless, the lack of a standardized sampling protocol and the presence of a vast tumor surface have made the microscopic pathological assessment of the surgical margins less reliable compared with extremity STS or compared with epithelial malignancies. As such, most of the surgical series only distinguish between R0/1 vs R2 resections, with R2 resections strongly impacting survival. An effort of standardizing the pathological sampling technique and reporting is ongoing within the Transatlantic Australasian Retroperitoneal Sarcoma Working Group (TARPSWG).
Tumor rupture has also been investigated as a prognostic factor and proved to be associated with LR in a large multicentric series [7]. As a principle, RPS should be resected with the best-quality surgical margins possible, balancing the need to resect adjacent organs with the expected surgical morbidity.
The use of preoperative radiotherapy was recently explored in an EORTC randomized controlled trial (STRASS trial) showing no difference in abdominal recurrence-free survival (primary endpoint) between patients receiving or not receiving preoperative radiotherapy. However, in an unplanned sensitivity analysis of the subgroup of patients with liposarcoma, 3-year ARFS was higher in patients with liposarcoma treated with preoperative radiotherapy (71.6% vs 60.4%). In particular, radiotherapy showed signs of efficacy in WDLPS and G2 DDLPS.
Chemotherapy in retroperitoneal sarcoma has never been studied in a randomized fashion. A randomized control trial of neoadjuvant chemotherapy vs surgery alone in patients with malignancy grade 3 DDLPS and malignancy grade 2 and 3 LMS (STRASS2 trial) will be coordinated by EORTC and will likely start accruing in 2020.
Extremity STS
Soft tissue sarcomas occur more commonly in the extremities. Here, the histological variety is broader than in the retroperitoneum and the risk of tumor-related death is mainly due to the occurrence of DM, more commonly to the lungs. Patient age, tumor size, malignancy grade, histologic subtype, and depth have been widely considered to be the most relevant prognostic factors for overall survival.
The three-tier grading system of the French Federation of Cancer Centers (FNCLCC) has been largely studied by the French group about 20 years ago [8] and proved to be able to predict DM and tumor mortality more efficiently compared with the two-tier grading system of the National Cancer Institute [9]. The FNCLCC grading system is not applicable to all extremity STS, since some histologies behave aggressively regardless of the tumor morphology (i.e., angiosarcoma), and is not applicable at time of local recurrence but only with primary tumors. Moreover, grading a STS on the biopsy might underestimate the real grading both for sampling variability and because the core needle biopsy aim towards non-necrotic areas, thus tumor necrosis might be underestimated. High-grade extremity STS tend to be associated to a higher risk of LR, DM, and DSD.
The histological classification continues to be updated over time and the new version of the WHO that will be shortly released will include about 100 histotypes overall [10, 11, 12••, 13••]. In extremity ESTS, histologic subtype has both prognostic and predictive implications. There are histotypes with a high risk of local failure (i.e., myxofibrosarcoma) and others with a high systemic risk (i.e., synovial sarcoma, angiosarcoma, and leiomyosarcoma, Fig. 1). In addition, there are histologic subtypes sensitive to chemotherapy (i.e., myxoid liposarcoma, synovial sarcoma, etc.) and others where conventional chemotherapy is completely inactive (i.e., clear cell sarcoma, alveolar soft part sarcoma, etc.). The same variability applies to radiotherapy. As such, a correct diagnosis formulated by dedicated pathologists is crucial to both risk estimation and treatment planning.
Incidence of distant metastasis by histologic subtype for extremity soft tissue sarcoma in the series of 3752 primary localized extremity sarcomas, derived from [12] (LMS: Leiomyosarcoma; SS: Synovial Sarcoma; UPS: Undifferentiated Pleomorphic Sarcoma; PLPS: pleomorphic Liposarcoma; MLPS: Myxoid Liposarcoma; MFS: Myxofibrosarcoma; MPNST: Malignant Peripheral Nerve Sheath Tumor; VS: Vascular Sarcoma).
Tumor size is an important prognostic variable for both OS and DM. On the contrary, it does not seem to affect the incidence of LR [7].
Historically, STS involving the fascia layer (deep STS) were thought to be associated with a worse oncological outcome. Indeed, in example, tumor depth was one of the criteria used to identify “high risk” patients in chemotherapy trials [14,15,16]. More recently, its prognostic role has been questioned even if it is still included in some prognostic tools [10, 11, 17, 18].
In the setting of extremity ESTS, the quality of surgery, and in particular the microscopic margin status, is the main determinant of the risk of LR. A microscopically positive surgical margin (R1) has been constantly associated with a higher risk of tumor recurrence, with a magnitude that is a function of the clinical context in which the positive margin occurs. In particular, an unexpected positive margin at a wide local excision or at a re-excision after an inadequate surgery is associated with a significantly higher risk of LR while an expected positive margin on a clinical structure in patients treated with perioperative radiotherapy is associated with the same risk of LR that would have been observed if the clinical structure was resected upfront [1]. R1 margins are not clearly associated with a higher incidence of distant metastases while the impact of surgical margin status on survival is more complex. Indeed, surgical margins are both an indicator of inadequate surgery and of biologic aggressiveness per se. Probably the margin status gain prognostic implications on survival especially in those patients that escape the early biological risk and in those patients with tumor located close to the abdomen/trunk where losing the local control might directly impact survival [19].
Radiotherapy in extremity STS has a very well-defined role in decreasing the risk of LR, which has been proved by RCTs [4]. It can be delivered both preoperatively (usually 50 Gy) or postoperatively (usually 60 Gy) with the same efficacy but with higher toxicity when delivered in the postoperative setting [20]. Interestingly, different histologies show different radiosensitivities. In a multicentric series of more than 3700 patients with extremity STS, the LR risk associated with the lack of radiotherapy administration was higher for myxoid liposarcoma, angiosarcoma, and myxofibrosarcoma [21].
The role of chemotherapy in the localized setting has been explored in several RCTs that failed to clearly prove a benefit of chemotherapy on the oncological outcome with studies being formally negative but consistently pointed towards a possible benefit in high-risk patients [22, 23]. In particular, the larger RCT on chemotherapy in the localized setting that failed to prove a benefit in the chemotherapy arm was recently reappraised using a different risk stratification showing that high-risk patients gained a survival benefit from CTx [24••]. Besides, the first trial coordinated by the Italian Sarcoma Group, which was characterized by the selection of a higher risk population, was closed early due to the observation of a benefit from standard anthracycline-based chemotherapy compared with surgery alone. However, its statistical significance was lost at a longer FU. As a result of this uncertainty, the administration of adjuvant/neoadjuvant chemotherapy is extremely variable among different sarcoma centers. Finally a recent neoadjuvant chemotherapy study comparing standard anthracycline + ifosfamide chemotherapy with histology-tailored chemotherapy in a high-risk population of localized STS of the extremities and trunk wall failed to show the superiority of histology-tailored chemotherapy in the 5 histologic subtypes included in the study (undifferentiated pleomorphic sarcoma, leiomyosarcoma, synovial sarcoma, high-grade myxoid liposarcoma, and malignant peripheral nerve sheath tumor), showing an overall survival benefit in favor of standard chemotherapy. The results of this study along with the reinterpretation of the EORTC study discussed above have led to a broader use of adjuvant/neoadjuvant chemotherapy in a selected high-risk population.
Again, the chemosensitivity varies across histologies, with synovial sarcoma, angiosarcoma, and myxoid liposarcoma showing a better response to standard anthracycline-based chemotherapy or histology-tailored chemotherapy regimens compared with other histologic subtypes.
Most important, all the studies above highlight how the correct and precise prediction of the risk is critical to understand the role of adjuvant/neoadjuvant therapies. Failing to harmonize the risk of death translates in a risk of failure of the trial overall.
Defining the risk of tumor recurrence
AJCC/UICC staging system
Until the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM classification [25], malignancy grade, tumor size, tumor depth, lymph node involvement, and distant metastasis were used to stratify patients in different stages. With a better understanding of the natural history of STS, it became clear that a single staging system was not granular enough to properly stratify all disease sites. With the 8th edition, new site-specific staging systems for STS of the trunk and extremities, retroperitoneum, head and neck, and abdomen and thoracic visceral organs have been developed; tumor size was modeled as a 4-tier categorical variable, and the N1 disease was defined as stage IV. While recognizing the role of personalized prognostic tools in determining a risk probability tailored to the single tumor of the single patient, the AJCC still does not differentiate between different histologic types. However, histology is a very strong determinant of patient prognosis in STS and patients within a same disease stage may have a completely different prognosis based on histology (Figs. 1, 2).
Disease-free survival by histologic subtype for retroperitoneal soft tissue sarcoma in the series of 1007 primary localized RPS, derived from [5] (LMS: Leiomyosarcoma; MPNST: Malignant Peripheral Nerve Sheath Tumor; SFT: Solitary Fibrous Tumor; UPS: Undifferentiated Pleomorphic Sarcoma; WD LPS: Well Differentiated Liposarcoma; DD LPS G1-2: Dedifferentiateed Liposarcoma grade 1 and 2; DD LPS G3: Dedifferentiated Liposarcoma grade 3).
Prognostic nomograms
All the prognostic variables discussed in the sections above have to be considered when we want to estimate the risk of tumor recurrence or the risk of tumor-related death.
Traditionally, patients with extremity STS that were deep, high grade and larger than 5 cm were considered to have “high-risk” tumors. This definition for example has been constantly adopted to select patients to be enrolled in trial of adjuvant/neoadjuvant chemotherapy. On the one hand, it is true that patients with extremity STS falling within this group carries a higher risk compared with smaller or lower grade tumors. On the other hand, it is also true that different histologies are associated with a different risk and that, if we consider survival as the endpoint of our risk estimation, patient’s age is also important. In example, a 10-cm angiosarcoma of the extremity is associated with a DM probability of 63% at 5 years. On the contrary, the DM probability of a G3 myxoid liposarcoma is approximately half (31% at 5 years). With this example, we understand that categorizing patients with STS on the basis of some, but not all, prognostic variables, results in a substantial heterogeneous population. This is why the prognostication in patients with STS has moved towards personalized prognostic tools.
Prognostic nomograms are able to estimate the probability of survival or tumor recurrence by taking into account simultaneously the relative contribution of all the relevant determinants of that specific endpoint. Covariates to be included in nomograms might be tumor-, patient-, or treatment-related factors, as well as biomarkers.
The first prognostic nomogram for patients with STS was developed and internally validated at Memorial Sloan Kettering Cancer Center, New York, in 2002. This nomogram was built upon a database of 2136 prospectively followed adult patients treated at a single institution and predicted the probability of 12-year sarcoma specific death was [11]. Age at diagnosis, tumor size (< 5, 5 to 10, or > 10 cm), malignancy grade (binomial), histologic subtype (fibrosarcoma, leiomyosarcoma, liposarcoma, malignant fibrous histiocytoma, malignant peripheral nerve tumor, synovial, or other), depth (binomial), and site (upper extremity, lower extremity, visceral, thoracic or trunk, retro-intra-abdominal, or head or neck) were the prognostic factors included in the nomogram. Subsequently, this tool underwent several external validations on both institutional series and population-based registries showing good discrimination and calibration [10, 17, 18].
Later, several nomograms have been developed, specific for a particular site (i.e., for extremity and retroperitoneal STS) [10, 12••, 13••, 26,27,28,29], or a specific histotype (i.e., nomograms for liposarcoma [30], synovial sarcoma [31], and uterine leiomyosarcoma [32]).
Once a nomogram is developed, its performance is commonly measured in terms of discrimination and calibration. Discrimination is the ability of a nomogram to assign, given a random pair of patients, the worse prognosis to the patients who will be observed to die first. It is usually measured with the Harrel C index, which goes from 0.5 (nomogram without any discriminative ability) to 1 (nomogram with perfect discrimination). Calibration reflects how the nomogram predictions are close to the observed outcome. Both calibration and discrimination can either be tested on the same cohort used for a nomogram development (“internal validation”) or on an independent cohort (“external validation”).
Retroperitoneal sarcoma
The first two prognostic nomograms for patients with RPS were developed at Istituto Nazionale Tumori (INT), Milan, Italy, and M.D. Anderson Cancer Center, Houston, USA [27, 28]. The first [27] was a histology-specific nomogram able to predict OS at 5 and 10 years after surgery for primary RPS. The prognostic factors included in the nomogram were age and tumor size (modelled as a continuous variable), malignancy grade, histologic subtype (LPS, LMS, MPNST, SFT, other), completenes of surgical resection. This nomogram predicted 5 and 10-yr OS. The second [28], including age, type of presentation (primary versus recurrent), tumor size (considered a binomial variable with a cutoff of 15 cm) and number (single versus multifocal), completeness of resection and histologic subtype (WD-LPS versus DD-LPS versus others), was capable of stratifying patients based on probability of OS. Both Anaya’s and Ardoino’s nomograms [27, 28] only predicted OS without considering the risk of local and distant recurrence. Neither of these nomograms underwent external validation so their applicability outside of the developing centers is unknown.
In order to overcome this limitation a prospective changing nomogram predicting both OS and disease-free survival (DFS) was developed few years later by a collaboration between three major institutions, INT, M.D. Anderson Cancer Center, and University of California Los Angeles (Figs. 3, 4) and externally validated by the Institut Goustave-Roussy [33]. The main strengths of these nomograms are in the large multicenter sample size, the external validation, and the variables included, such as age, size, histologic subtype, multifocality, malignancy grade and completeness of surgical resection. The inclusion of both grade and histologic subtype as two different covariates did improve the complexity of the model and the predictions ability.
The role of surgery for retroperitoneal sarcoma after first local relapse was evaluated by Raut et al. [29•] developing and externally validating nomograms estimating DFS and OS from second surgery. Nomograms included age at second surgery, multifocality, grade, completeness of second surgery, histologic subtype, chemotherapy/radiotherapy at first surgery, and number of organs resected at first surgery and showed good calibration and discriminative ability (see below).
Nomograms able to predict disease-specific death (DSD), LR, and DR at 3, 5, and 15 years after surgery of primary RPS and demonstrating good agreement between the predictions made and the actual outcomes were developed by the Memorial Sloan Kettering Cancer Center. The DSD nomogram included histology, radiation, number of organs resected, completeness of surgical resection, and size; the LR nomogram did not include radiation but had location, age and vascular resection, while the DR nomogram included only histology, organs resected, radiation, vascular resection, and size [6]. None of these Memorial Sloan Kettering Cancer Center nomograms was ever externally validated.
The nomogram from Gronchi et al. has been included in the app Sarculator (http://www.sarculator.com).
Extremity STS
The first nomogram dedicated to patients with extremity STS was published in 2005 and aimed to predict the 10-year sarcoma-specific death using age, size, depth, site, histologic subtype and malignancy grade as prognostic variables [10].
Few years later, the MSKCC group developed a nomogram quantifying local recurrence at 3 and 5 years after limb-sparing surgery in patients not receiving adjuvant radiation [26]. In this highly selected cohort of patients treated at a single institution, age, size, margin status, malignancy grade and histology were analyzed for prognostic significance with respect to local recurrence. The single institution cohort, the absence of an external validation, the limited histologic classification, and perhaps more importantly, age, size, and grade expressed as dichotomous variables largely limit the clinical meaning of the nomogram that can be applied in a very limited cohort of patients.
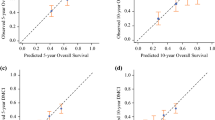
The following nomograms, developed at Istituto Nazionale Tumori, Milan, Italy [12••, 13••], predict 5- and 10-year OS (Figs. 5, 6) after surgery for primary ESTS [12••]. They include age, size (modeled as a continuous variable), FNCLCC malignancy grade, and histologic subtype and have a Harrell C index of 0.77 in the developmental cohort and 0.70–0.76 in the validation cohorts. The 5- and 10-year distant recurrence nomogram, including size, FNCLCC malignancy grade, and histologic subtype, have a Harrell C index of 0.76 in the developmental cohort and 0.65–0.77 in the validation cohorts. These nomograms are included in the same app above called Sarculator.
Nomogram for 5- and 10-year OS after resection of a primary soft-tissue sarcoma of the extremities. Reprinted from The Lancet Oncology, Volume 17, Issue 5, Callegaro D, et al., “Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis,” pages 671-680, ©2016, with permission from Elsevier.
Nomogram for 5- and 10-year distant recurrence after resection of a primary soft-tissue sarcoma of the extremities. Reprinted from The Lancet Oncology, Volume 17, Issue 5, Callegaro D, et al., “Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis,” pages 671-680, ©2016, with permission from Elsevier.
Besides informing patients about their risk, the utility and validity of these nomograms have been recently shown by Pasquali et al. [24••]. Sarculator was used to stratify patients enrolled in a prospective RCT according to the individualized baseline risk to test the specific benefit of adjuvant chemotherapy in a high-risk population. Patients with extremity and trunk wall STS included in the EORTC-STBSG 62931 RCT [22, 23], which failed to prove the superiority of adjuvant doxorubicin plus ifosfamide over observation, were analyzed. Most patients were included in the high predicted OS group (> 66%), 68 in the intermediate and 52 in the low predicted OS group (< 51%). Interestingly, high-risk patients, i.e., those with a predicted OS < 51%, benefitted the most from adjuvant treatment, halving the risk of death, while no effect was detected in patients with a predicted OS > 51%. Chemotherapy also proved to have a positive impact on DFS of patients with predicted OS < 51%, leading to a 21% 8-year absolute risk reduction for adjuvant chemotherapy compared to observation. When categorizing patients in two groups, using a cutoff of 60% of predicted OS, reduction on the risk of death and recurrence in high-risk patients treated with doxorubicin plus ifosfamide were confirmed.
The sole nomogram predicting LR was developed by van Praag et al. in 2017 [34]. The PERsonalised SARcoma Care (PERSARC) model predicts both OS and LR at 3, 5, and 10 years. Size, margins, and radiotherapy were found to correlate with LR and incorporated into the nomogram. The model built and internally validated by van Praag et al. showed good calibration and discrimination, with a C-index of 0.677 and 0.696 for OS and LR.
Dynamic risk estimation
Prognostic nomograms usually predict survival or the occurrence of an event at time of surgery. After surgery, the individual prognosis will change on the basis of three factors. First, baseline prognostic variables might have a time-dependent effect. This means that determinants of patient prognosis at baseline might no longer be able to influence the residual prognosis as time goes by after surgery. Second, the event history: patients who will experience a LR or DM during FU will do worse as compared with patients who will remain disease free. Third, the longer the time interval from surgery, the lower the chance of a tumor to recur. When all of these factors are combined in a nomogram, the prognosis prediction can be computed not only at surgery but also at various time points during follow-up. In other words, the nomogram becomes dynamic.
There are currently two dynamic nomograms available for patients with extremity STS [13••, 35] and a third one for patients with RPS has been presented at Connective Tissue Oncology Society 2019 meeting.
The first dynamic prediction tool for patients with high-grade STS of the extremity was developed by the group of Leiden in 2018 [35]. This model is able to predict the chance of surviving an additional 5 years throughout the first 5 years of follow-up based upon baseline variables (age, tumor size, histology, tumor depth, RTx administration, surgical margins status) and upon the occurrence of LR/DM. The model was well calibrated and was able to discriminate between high- and low-risk patients at internal validation but was not externally validated.
In 2019, the Sarcoma Team at INT in Milan, Italy, developed a dynamic prognostic nomogram to predict 5-year overall survival at different times during the first 3 years of follow-up for primary extremities sarcomas (Fig. 7) [13••]. The nomogram predictions are based on baseline variables (patient age, tumor size, FNCLCC grade, histology) and on the event history (no events vs LR vs DM). As compared with the previous dynamic nomogram, this is built on a larger cohort of more than 3700 patients, it is valid for patients with extremity STS of all grades, adopts a more updated and granular histological classification and was successfully externally validated. Indeed, the performance of the nomogram was good in terms of both discrimination and calibration when it was tested on an independent series of almost 900 patients.
Dynamic nomograms for 5-year OS after resection of a primary soft-tissue sarcoma of the extremities. Reprinted from EClinicalMedicine, Volume 17, Callegaro D, et al., “Development and external validation of a dynamic prognostic nomogram for primary extremity soft tissue sarcoma survivors,” 100215, ©2019, with permission from Elsevier.
Dynamic prognostic tools are useful for patient counseling in clinic, to help the physician understanding the prognostic implication of a local or a distant recurrence on the single patient and they can help modeling the follow-up schedule based on the dynamic risk.
A further effort in tailoring the FU schedule to the patient dynamic risk has been recently carried out by the PERSARC group with the creation of two models predicting risks of LR and DM over the first 5 years of follow-up. By using flexible parametric competing risk regression modeling, these models are able to estimate the personalized 3–6-month risks for LR or DM in patients surgically treated for high-grade extremity soft tissue sarcoma, thus allowing the physician and the patient to tailor the follow-up strategies to the risk of tumor recurrence. This model was externally validated with a Harrell C Index of 0.68 for LR and 0.77 for DM. At external calibration, the LR model tended to underestimate the patient risk while the DM model was overall well calibrated.
All these prognostic models have been incorporated in digital prediction tools: the first and the third in the PERSARC app, the second in the Sarculator app.
Recurrent disease
Outcome of recurrent tumors was evaluated for RPS that tends to recur locally and distantly, despite radical surgery. Two large multi-institutional studies evaluated pattern of recurrence and outcome of patients with recurrent RPS [5•, 36]. In the study by Gronchi et al. [5•], 1007 patients treated at 6 European and 2 North American institutions between January 2002 and December 2011 were included. Within a follow-up of 58 months 5-, 8-, and 10-year LR were 25.9%, 31.3%, and 35%, respectively, showing a progressive increasement in the rate of local recurrence overtime. Differently the crude index of DR remained steady at 21% overtime, meaning that the biologic aggressiveness of sarcomas with a distant pattern of recurrence tends to manifest within the first 5 years after surgery with a median time to DR of 14 months. Age, size, completeness of surgical resection, malignancy grade, tumor rupture, multifocality, administration of radiotherapy, and histologic subtype were associated with LR, while only size, grade, multifocality, and histologic subtype were correlated with the risk of developing DR. Patterns of recurrence proved to vary on the basis of the histology, in particular when evaluating tumors with completely different behavior such as WD LPS and leiomyosarcoma, treatment policies (i.e., compartmental resections and administration of radiotherapy) influenced LR of WD LPS without affecting OS, whereas differences in the use of adjuvant systemic therapies did not impact LR, DR, or OS outcomes of leiomyosarcomas. Solitary fibrous tumor and DD LPS showed a pattern of local and distant relapse laying between WD LPS and leiomyosarcoma.
One year later [36], the Trans-Atlantic Australasian RPS Working Group queried the same dataset in order to define survival of relapsing patients. Median and 5-year OS after LR were 33 months and 29% after LR, 25 months and 20% after DR, and 12 months and 14% after both LR and DR. The majority of patients experiencing LR had liposarcomas (80%), but only time to LR and time to surgery for LR had a significant impact on OS after local relapse. Differently, histology and time interval to DR correlated with OS of patients with distant disease, mostly represented by patients with DD LPS and leiomyosarcoma. Interestingly patients receiving surgery for LR (48%) and DR (36%) had a better long-term outcome compared with patients treated conservatively.
The only nomograms predicting DFS and OS for patients undergoing surgery for recurrent RPS were developed by the TARPSWG group [29•], filling the knowledge gap on the role of surgery for first relapse in locally recurrent RPS. Twenty-two centers were included between 2002 and 2011 in order to generate and validate nomograms with a concordance index of 0.70 and 0.67, respectively, for OS and DFS. Pattern of recurrence and OS was found to correlate with the histotype, in particular liposarcomas had the highest rate of second local recurrence (6-year crude cumulative index ranging from 60.2 of WDLPS and 70.9% of grade 3 DDLPS), while leiomyosarcoma were more likely to spread distantly (6-year CCI of 36.3%). Interestingly, even if grade 3 DDLPS had a lower metastatic potential compared with leiomyosarcoma, OS was slightly, probably due to the limited chemotherapeutic agents available for LPS compared with leiomyosarcoma. Apart from the common prognostic factors, such as age, multifocality, grade, completeness of second surgery and histology, the nomograms for recurrent RPS included chemotherapy or radiotherapy at the time of first surgery, and number of organs resected at the first surgery. Interestingly, the higher the number of organs resected at initial surgery, the worse was OS and DFS, meaning that patients with an aggressive tumor presentation, requiring extensive surgery ab initio, are less likely to achieve good long-term oncological outcomes. The number of organs resected is a proxy of tumor biology in this case and should not be confused as endorsing a limited resection to achieve a better long-term outcome. On the other hand, patients undergoing extended surgery and experiencing recurrence are less like to be rescue due to worse tumor biology.
The predictive value of molecular signatures
The impact of genomic and expression profiling on long-term outcomes of patients with sarcomas has been evaluated in order to fill the knowledge gap of this heterogeneous disease. At the genetic level, sarcomas without a known translocation are distinguished in two groups: those with a complex genomic profile accounting for the 80% of sarcomas, and represented by undifferentiated sarcomas, LMS, UPS, and pleomorphic rhabdomyosarcomas [37,38,39], and those with a simple genetic profile (20%) with many limited amplifications represented by dedifferentiated LPS [40]. A complexity index in sarcomas (CINSARC) composed of 67 genes related to mitosis and chromosome management, was developed by Chibon et al., and proved its superiority compared with the FNCLCC malignancy grade. The limit of the CINSARC established on frozen samples has been overcome using next generation sequencing (NGS) [41] or NanoString on formalin-fixed, paraffin-embedded (FFPE) [42] blocks and CINSARC remained an independent prognostic signature for metastatic outcome. Genomic Grade Index (GGI) [41], a 108-gene signature previously developed in early-stage breast cancer, has been also applied to the prognostication of STS with good correlation with metastatic-free survival. The major limit of the genomic tests is that they have not been prospectively validated in independent series. Further clinical validations are warranted in both retrospective and prospective series. Similarly, functional validations of relevant genes that could provide new therapeutic targets are also needed.
The predictive value of other features
The prognostic ability of other tools such as radiomic features have been studied in several diseases. In oncology, radiomic tools have been integrated in prognostic nomograms and adopted to better stratify patients based on their risk of death/recurrence or response to treatment. However, the data on sarcoma patients’ stratification are still very preliminary to be adopted in clinical practice.
Conclusions
The individual probability of tumor recurrence or death computed with prognostic nomograms or by using omics signatures is critical to inform patients about their prognosis, at time of surgery or later on during follow-up by means of dynamic nomograms. Estimating an individual risk may also assist the health provider and the patients in the decision-making process. In addition, given the growing evidence that high-risk patients are the ones who benefit the most from perioperative chemotherapy, nomograms might identify those high-risk patients, as recently shown by Pasquali et al. [24••], and improve patient stratification in clinical trials. In STS, in the lack of large numbers of patients, it is of utmost important testing hypotheses on homogeneous risk cohorts.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gundle KR, Kafchinski L, Gupta S, Griffin AM, Dickson BC, Chung PW, et al. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol. 2018;36(7):704–9.
Palassini E, Ferrari S, Verderio P, de Paoli A, Martin Broto J, Quagliuolo V, et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian sarcoma group/grupo Espanol de investigacion en sarcomas randomized clinical trial: three versus five cycles of full-dose epirubicin plus ifosfamide. J Clin Oncol. 2015;33(31):3628–34.
Beane JD, Yang JC, White D, Steinberg SM, Rosenberg SA, Rudloff U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014;21(8):2484–9.
Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203.
• Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. 2016;263(5):1002–9 This paper is important because it underlines the importance of histologic subtype in predicting patterns of recurrence after resection of primary retroperitoneal sarcoma.
Tan MC, Brennan MF, Kuk D, Agaram NP, Antonescu CR, Qin LX, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263(3):593–600.
Gronchi A, Bonvalot S, Le Cesne A, Casali PG. Resection of uninvolved adjacent organs can be part of surgery for retroperitoneal soft tissue sarcoma. J Clin Oncol. 2009;27(12):2106–7 author reply 2107-2108.
Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869–77.
Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350–62.
Mariani L, Miceli R, Kattan MW, Brennan MF, Colecchia M, Fiore M, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103(2):402–8.
Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–6.
•• Callegaro D, Miceli R, Bonvalot S, Ferguson P, Strauss DC, Levy A, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671–80 These two nomograms for overall survival and distant-metastasis in patients with sarcomas of the extremity are included in the ‘Sarculator’, a freely online available applications, for sarcomas of the retroperitoneum and of the extremity.
•• Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of a dynamic prognostic nomogram for primary extremity soft tissue sarcoma survivors. EClinicalMedicine. 2019;17:100215 This nomogram allows physician to update the individual survival prediction during follow-up on the basis of baseline variables, time elapsed from surgery and first-event history.
Ferrari S, Ruggieri P, Cefalo G, Tamburini A, Capanna R, Fagioli F, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112–8.
Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845–52.
Gronchi A, Frustaci S, Mercuri M, Martin J, Lopez-Pousa A, Verderio P, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30(8):850–6.
Eilber FC, Brennan MF, Eilber FR, Dry SM, Singer S, Kattan MW. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101(10):2270–5.
Bagaria SP, Wagie AE, Gray RJ, et al. Validation of a soft tissue sarcoma nomogram using a national cancer registry. Ann Surg Oncol. 2015;22(Suppl 3):S398–403.
Callegaro D, Fiore M, Gronchi A. Personalizing surgical margins in retroperitoneal sarcomas. Expert Rev Anticancer Ther. 2015;15(5):553–67.
O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–41.
Callegaro D, Miceli R, Bonvalot S, Ferguson P, Strauss DC, Levy A, et al. Impact of perioperative chemotherapy and radiotherapy in patients with primary extremity soft tissue sarcoma: retrospective analysis across major histological subtypes and major reference centres. Eur J Cancer. 2018;105:19–27.
Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13(10):1045–54.
Le Cesne A, Ouali M, Leahy MG, et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC phase III clinical trials. Ann Oncol. 2014;25(12):2425–32.
•• Pasquali S, Pizzamiglio S, Touati N, Litiere S, Marreaud S, Kasper B, et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer. 2019;109:51–60 This paper is very important because stratifying patients with Sarculator was able to define soft tissue sarcoma patients that would benefit of adju- vant/neoadjuvant chemotherapy.
American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed: Springer Chicago; 2017.
Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255(2):343–7.
Ardoino I, Miceli R, Berselli M, Mariani L, Biganzoli E, Fiore M, et al. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer. 2010;116(10):2429–36.
Anaya DA, Lahat G, Wang X, Xiao L, Pisters PW, Cormier JN, et al. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol. 2010;21(2):397–402.
• Raut CP, Callegaro D, Miceli R, Barretta F, Rutkowski P, Blay JY, et al. Predicting survival in patients undergoing resection for locally recurrent retroperitoneal sarcoma: a study and novel nomogram from TARPSWG. Clin Cancer Res. 2019;25(8):2664–71 This is the first nomogram predicting disease-free survival and overall survival of patients with local recurrent retroperitoneal sarcoma.
Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381–91.
Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14(24):8191–7.
Zivanovic O, Jacks LM, Iasonos A, Leitao MM Jr, Soslow RA, Veras E, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118(3):660–9.
Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31(13):1649–55.
van Praag VM, Rueten-Budde AJ, Jeys LM, Laitinen MK, Pollock R, Aston W, et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: personalised sarcoma care (PERSARC). Eur J Cancer. 2017;83:313–23.
Rueten-Budde AJ, van Praag VM, van de Sande MAJ, Fiocco M, Studygroup P. Dynamic prediction of overall survival for patients with high-grade extremity soft tissue sarcoma. Surg Oncol. 2018;27(4):695–701.
MacNeill AJ, Miceli R, Strauss DC, Bonvalot S, Hohenberger P, van Coevorden F, et al. Post-relapse outcomes after primary extended resection of retroperitoneal sarcoma: a report from the Trans-Atlantic RPS Working Group. Cancer. 2017;123(11):1971–8.
Idbaih A, Coindre JM, Derre J, et al. Myxoid malignant fibrous histiocytoma and pleomorphic liposarcoma share very similar genomic imbalances. Lab Investig. 2005;85(2):176–81.
Chibon F, Mariani O, Mairal A, Derré J, Coindre JM, Terrier P, et al. The use of clustering software for the classification of comparative genomic hybridization data. An analysis of 109 malignant fibrous histiocytomas. Cancer Genet Cytogenet. 2003;141(1):75–8.
Derre J, Lagace R, Nicolas A, et al. Leiomyosarcomas and most malignant fibrous histiocytomas share very similar comparative genomic hybridization imbalances: an analysis of a series of 27 leiomyosarcomas. Lab Investig. 2001;81(2):211–5.
Coindre JM, Mariani O, Chibon F, Mairal A, de Saint Aubain Somerhausen N, Favre-Guillevin E, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16(3):256–62.
Bertucci F, De Nonneville A, Finetti P, et al. The Genomic Grade Index predicts postoperative clinical outcome in patients with soft-tissue sarcoma. Ann Oncol. 2018;29(2):459–65.
Le Guellec S, Lesluyes T, Sarot E, et al. Validation of the Complexity INdex in SARComas prognostic signature on formalin-fixed, paraffin-embedded, soft-tissue sarcomas. Ann Oncol. 2018;29(8):1828–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gaya Spolverato and Dario Callegaro each declare no potential conflict of interest. Alessandro Gronchi, however, has received research funding from PharmaMar, and has received compensation from Novartis, Pfizer, Bayer, Lilly, PharmaMar, SpringWorks Therapeutics, and Nanobiotix for service as a consultant.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sarcoma
Rights and permissions
About this article
Cite this article
Spolverato, G., Callegaro, D. & Gronchi, A. Defining Which Patients Are at High Risk for Recurrence of Soft Tissue Sarcoma. Curr. Treat. Options in Oncol. 21, 56 (2020). https://doi.org/10.1007/s11864-020-00753-9
Published:
DOI: https://doi.org/10.1007/s11864-020-00753-9