Opinion Statement
Five years after adjuvant endocrine treatment for estrogen receptor (ER)-positive breast cancer, patients have a 2 to 20 % risk of metastatic relapse during the next 5 years. Extended adjuvant endocrine therapy seems to further lower this. In UZ Leuven, extended endocrine therapy is now discussed unless the tumor was a grade 1–2, pT1N0, ER-positive, progesterone receptor (PR)-positive, HER2-negative lesion. After 5 years of adjuvant tamoxifen treatment for ER-positive breast cancer, we encourage women to take another 5 years of tamoxifen. If the tumor was lymph node-positive at diagnosis and patients are menopausal after the first 5 years of tamoxifen, we advise to take prolonged treatment with an oral aromatase inhibitor (AI). For this particular group, available data for extending endocrine therapy with an AI after 5 years of tamoxifen are strongest and more convincing for letrozole than for anastrozole or exemestane. Under these conditions, letrozole is reimbursed for 3 years in Belgium. If women are postmenopausal at diagnosis and already used an oral AI at any time during the first 5 years, we discuss an extra 5 years of tamoxifen. Results from ongoing clinical trials will tell us whether in these cases prolonged AI use is better than tamoxifen so that therapy can be adapted. Benefit from extended adjuvant endocrine therapy is likely larger with better compliance and potential side effects of extended endocrine therapy need to be discussed. Therefore, when advising extended adjuvant endocrine treatment, a balance should always be made between relapse risk and treatment tolerance/compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women with an early invasive, estrogen receptor (ER)-positive breast cancer are always advised to take adjuvant endocrine therapy as this lowers relapse. A meta-analysis of a large series of randomized controlled trials (RCTs) with tamoxifen starting 30 years ago was recently updated for its 5-year effect [1, 2]. The conclusion was that daily intake of 20 mg of tamoxifen for 5 years led to 13.2 % fewer recurrences and 9.2 % fewer deaths at 15 years [1, 2]. It did not affect non-breast cancer mortality despite small absolute increases in thromboembolic and uterine cancer mortality (both only in women older than 55 years). This standard of care has, a decade or so ago, been changed in postmenopausal women with the introduction of the three oral aromatase inhibitors (AI), anastrozole, letrozole, and exemestane. Several RCTs tested in many different ways the hypothesis that adjuvant AIs, 5 years continuous or 2–3 years in consecutive sequence with 2–3 years of tamoxifen, are better than 5 years of tamoxifen. This led to an updated ASCO clinical guideline [3]. Considering all such RCTs, a recently presented intent-to-treat meta-analysis of 36,889 postmenopausal women with an ER-positive invasive breast cancer compared the following three groups of 5 years of adjuvant endocrine treatment: [A] continuous AI versus tamoxifen; [B] sequential 2–3 years of tamoxifen then 2–3 years AI versus tamoxifen alone; and [C] continuous AI versus sequential 2 years tamoxifen followed by 3 years AI [4]. There were fewer recurrences with continuous AI therapy in groups A and C as compared to the tamoxifen containing arms in both groups and with sequential tamoxifen followed by AI as compared to the tamoxifen alone arm in group B. Recurrence reductions with AI over tamoxifen were mainly seen during the period AI was used. In group A, recurrence rate ratio (RR) was no longer significant beyond 5 years in the AI group as compared to the tamoxifen alone group (RR 0.90; 0.79–1.04), but overall, breast cancer death was lower (RR 0.86 [0.76–0.97], p = 0.014). Also in group B, recurrence RR was no longer significant beyond 5 years in the AI group in comparison with the tamoxifen alone group (RR 0.97; 0.86–1.09), but overall, breast cancer mortality was lower (RR 0.84 [0.73–0.97], p = 0.015). Patients in group C had the lowest benefit of 5 years treatment with AI. While recurrence RR was lower in the AI versus tamoxifen treatment group during years 0–1 (RR 0.75 [0.62–0.89]), recurrence RR was similar during years 2–4 when both groups received AI (RR 0.99 [0.85–1.15]), and also after treatment completion beyond 5 years (RR 0.96 [0.76–1.21]). Still, this approach in group C translated in 1.1 % fewer 5 years recurrences with continuous AI (9.6 %) than sequential tamoxifen followed by an AI (10.7 %) (RR 0.90 [0.81–1.00], p = 0.042). There were less breast cancer deaths, but this was not significant (RR 0.89 [0.77–1.02], p = 0.097). Not many differences in proportional recurrence reduction by age, nodal status, tumor grade, or progesterone receptor (PR) status could be observed in the three comparisons. Overall, there were less endometrial cancers (0.2 versus 0.6 %, RR 0.37 [0.27–0.51]) but more fractures (8.1 versus 5.9 %, RR 1.40 [1.27–1.53]) in the AI group compared with the tamoxifen group. In both groups, a similar amount of non-breast cancer deaths was observed [4].
Until recently in our institution, all premenopausal and selected low-risk (pT1N0 grade 1) postmenopausal ER-positive breast cancer patients were advised to take 5 years of tamoxifen. We added ovarian function suppression (OFS) for the first 2–3 years to the 5 years of tamoxifen in women ≤35 years of age at diagnosis. Postmenopausal women with a more aggressive tumor than a pT1N0, grade 1, ER-positive breast cancer either received 5 years of a non-steroidal AI (if a higher risk for early relapse so if the tumor was pN0 and PR-negative, or pN2-3, or if there were at least two risk factors: tumor size larger than 2 cm, lymphovascular invasion, grade 3, HER2-positive or pN1) or the consecutive combination of 2–3 years tamoxifen followed by exemestane or anastrozole during the rest of the first 5 years (if patients were not at higher risk for early relapse but the tumor is more aggressive than grade 1 pT1N0). Patients with toxicity or those not tolerating either tamoxifen or AI were switched to the other compound with ideally at least 2 years of an AI if they had a higher relapse risk.
Years ago, clinicians started testing the value of extended (beyond 5 years) adjuvant endocrine treatment in RCTs [5•, 6•, 7–14] since women with an early ER-positive breast cancer have, following the most optimal 5 year adjuvant endocrine treatment schedule, between 2 and 20 % risk of metastatic recurrence during the next 5 years. Unfortunately, we do not have reliable biomarkers for benefit from longer than 5 years treatment. Initial risk of recurrence, however, seems informative for relapse risk estimates and decision making at 5 years. Multi-gene signatures are also able to predict recurrences beyond the first 5 years but whether they are predictive for prolonged therapy is as of today unknown [15–29].
Many but not all of the RCTs studying extended endocrine therapy have been reported [5•, 6•, 7–14]. The available data of some of the trials are mature enough for changing our daily clinical practice in a subset of patients coming off the 5-year treatment [5•, 10, 11]. This was also incorporated in recent guidelines and agreed upon during the St-Gallen 2015 consensus meeting.
In UZ Leuven, late relapse risk is evaluated in the beginning of breast cancer diagnosis using initial tumor stage and biology. Prognostic tumor characteristics at diagnosis and patient characteristics (menopausal status) help to decide who and how we continue to treat with endocrine agents beyond 5 years. At the end of the 5 years, we also consider previous symptoms and side effects to be expected from prolonged endocrine treatment. We inform patients that benefits might be modest and side effects persistent; we only propose prolonged treatment if we judge benefits outweigh side effects. If patients’ symptoms on previous endocrine therapy were tolerable, we propose intermediate to high-risk patients (any >pT1 or >pN0 or grade 3) prolonged endocrine therapy. There is evidence from RCTs for benefit after 5 years of tamoxifen for an extra 5 years of tamoxifen or letrozole (the latter one only if menopausal after 5 years). Data on the efficacy of extended endocrine therapy are missing in women already exposed to an AI during any period in the first 5 years. Recent ASCO guidelines advise in some of these particular cases to administer an AI for 5 or 3 extra years [30••]. Three additional years of an AI is proposed to patients who already received 2 years of an AI during the first 5 years of their adjuvant treatment. An extra 5 years of tamoxifen might be a pragmatic approach if such patients have a residual relapse risk beyond 5 years as prolonged AI use is not reimbursed in Belgium if an AI was already given during any period in the first 5 years. If AIs are better in this setting based on new data to come over the next years, such endocrine treatment can still be adjusted.
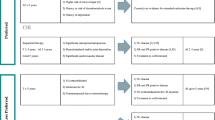
We here present our current in-house policy of extended adjuvant endocrine therapy by menopausal status after 5 years of endocrine therapy (Table 1). We have to stress that there is no proper definition for “menopause” in breast cancer patients with amenorrhea whether or not on tamoxifen and especially if adjuvant chemotherapy was given. This is in particular important when prescribing AIs for patients under age 52 as AIs are only active if no residual follicle is remaining. In this age group, biochemistry (estradiol, inhibine, anti-mullerian hormone levels) nor ovarian ultrasonography is reliable to define menopause and start on an AI. We evaluate menopausal status of patients who we propose this treatment as well as which type of adjuvant endocrine therapy. We also refer to ongoing trials and potential side effects in extended anti-hormonal therapy trials as these should be considered when informing patients on prolonged anti-estrogen therapy.
Premenopausal after 5 years of tamoxifen
For those ending 5 years of tamoxifen, two recently reported trials, one published (ATLAS) and one presented during ASCO 2013 (aTTom) support extending endocrine therapy [5•, 6•]. Both trials confirm one but oppose two earlier trials studying extended tamoxifen treatment beyond 5 years of tamoxifen [7–9]. The three early trials of continuing adjuvant tamoxifen to 10 years versus stopping tamoxifen at 5 years recruited relatively few patients, and two of these trials were negative. The small numbers of patients meant that these adverse results could have been due to the play of chance, so larger trials like ATLAS and aTTom were needed. Al-Mubarak et al. recently published in a systematic review and meta-analysis of these five trials the benefits and harms of 5 additional years of adjuvant tamoxifen compared with only 5 years of adjuvant tamoxifen [31••]. This meta-analysis comprising 21,554 patients concluded that extended adjuvant tamoxifen was not associated with a significant reduction in the risk of recurrence (odds ratio (OR) 0.89 [0.76–1.05], p = 0.17) and all-cause death (OR 0.99 [0.84–1.16], p = 0.88). There was a reduction in risk of recurrence after completion of extended adjuvant tamoxifen which was, considering all trials, not significant. In a subgroup analysis, the absolute risk reduction was 2.1 % in lymph node-negative patients as compared to 4.1 % in lymph node-positive patients at 10 years of follow-up. This difference, however, was not significant. Menopausal status had no effect on breast cancer recurrence.
The two most recent trials in this meta-analysis, ATLAS and aTTom, demonstrated that an extra 5 years of adjuvant tamoxifen is beneficial for women with an ER-positive breast cancer 15 years after initial diagnosis. It decreases the risks of recurrence and death by 3.7 and 2.8 %, respectively, but 15 years of follow-up was required to observe this mortality difference [5•]. In UZ Leuven, 10 years of tamoxifen is proposed unless the tumor is pT1N0 grade 1–2. There are three relevant issues for our daily practice in 2015 regarding these findings in women premenopausal at 5 years of follow-up.
First, most women in both ATLAS and aTTom were postmenopausal and now receive an AI as adjuvant therapy in the first 5 years. We asked the ATLAS investigators if they could provide information on the benefit of extended tamoxifen in ATLAS depending on ovarian function. The authors provided reassurance that the new strategy of extending 5 years of tamoxifen is appropriate also in the younger age group [32, 33]. The ATLAS findings therefore represent an important step forward in adjuvant treatment for women with ER-positive breast cancer, and we agree that they are very likely to pertain to all eligible women.
Second, young women have the highest rates of death from early breast cancer. Based on extrapolations from studies in postmenopausal women, AIs might also be better than tamoxifen in younger women but in that case, premenopausal women need to be rendered menopausal. Indeed, in premenopausal women with a high relapse risk receiving adjuvant chemotherapy, OFS with exemestane improves disease-free survival (DFS), as compared with tamoxifen alone or OFS plus tamoxifen in SOFT [34]. This advantage of OFS plus exemestane was not seen in low-risk premenopausal breast cancer patients where physicians decided not to give chemotherapy; tamoxifen users in the trial had an excellent 5-year prognosis independent of the choice of anti-hormonal therapy. Also, Pagani et al. reported that OFS plus exemestane improved DFS by 3.8 % as compared with OFS plus tamoxifen (hazard ratio 0.72 [0.60–0.86], p = 0.0002) [35]. The advantage of an AI was not observed in low-risk patients in both TEXT (exemestane) and ABCSG-12 (anastrozole). The latter study compared anastrozole with tamoxifen mainly in non-chemotherapy treated premenopausal women with OFS [36]. In ABCSG-12, tamoxifen was not inferior to AI for DFS, and it was even superior for overall survival. It should be acknowledged that this study only treated patients for 3 years, that serum follicle stimulating hormone levels were not only higher in the anastrozole as compared to the tamoxifen group but also predictive for relapse, and that body mass index predicted for higher residual estradiol levels in these women with an artificially suppressed ovarian function using gonadotropin-releasing hormone agonist (GnRH-a) [37, 38]. The potential benefit of extended AI or tamoxifen treatment for postmenopausal and premenopausal patients, respectively, after 5 years of treatment with OFS plus tamoxifen/AI is unknown. Long-term follow-up in SOFT and TEXT is critical to improve decision making about extended treatment. The recently updated ABCSG-12 trial, studying 3 years of adjuvant endocrine therapy with OFS plus tamoxifen or anastrozole with or without zoledronic acid showed an excellent prognosis between year 5 and 8 but still, 20 % of all metastatic events appeared during this follow-up period [39].
Third, is there a place for OFS with tamoxifen or an AI after 5 years of endocrine therapy if high-risk patients remain premenopausal? In this setting, one study evaluated the efficacy, safety, and tolerability of AI plus OFS with GnRH-a after 5 years of tamoxifen in premenopausal breast cancer survivors [40]. This was a small phase II study from three participating sites in the USA, including the Dana-Farber Cancer Institute. Due to poor accrual over 3.5 years, however, the study was closed early with only 16 patients who began treatment. Moreover, most patients stopped treatment early as a result of toxicity. Common adverse events that led to stop of treatment were hot flashes, vaginal dryness, and arthralgia. This study’s poor accrual suggests that young women may not be highly motivated to pursue lengthier courses of endocrine therapy and that future studies of this approach with OFS plus an AI after 5 years of tamoxifen may be challenging. We do not extend OFS beyond 5 years in our center for these reasons.
After 5 years of tamoxifen treatment: postmenopausal or premenopausal at diagnosis but postmenopausal after that period
Patients postmenopausal after 5 years of tamoxifen treatment are switched from tamoxifen to another 3–5 years of letrozole as this reduces the risk of recurrence and improves overall survival especially in lymph node-positive disease [41]. MA-17 tested 5 years of treatment extension and most guidelines now advise to give 5 extra years of letrozole, but reimbursement in Belgium is only for 3 years. The cohort “premenopausal at diagnosis but postmenopausal at year 5” was a small group in MA-17. A retrospective subgroup analysis showed that these patients benefited most from extended letrozole therapy after 5 years on tamoxifen [42]. Similar findings as in the MA-17 trial were also found in other trials like ABCSG-6a with anastrozole and ATENA and NSABP B-33 with exemestane [12–14].
In our hospital, women postmenopausal after 5 years of diagnosis with a low residual risk for breast cancer relapse (grade 1–2, pT1N0, ER-positive, PR-positive, HER2-negative lesion without lymphovascular invasion) stop their treatment after 5 years of tamoxifen as its risks might outweigh its benefit. Women in this category with a higher residual relapse risk but lymph node-negative will receive an additional 5 years of tamoxifen as AIs are not reimbursed in Belgium if lymph nodes are negative whereas tamoxifen is. In these women, it is unknown whether extended use of tamoxifen beyond 5 years of tamoxifen is inferior to an AI as such trials have not been done. In a cross-trial comparison of ATLAS and AI studies, it was found that in patients pre- or postmenopausal at diagnosis but postmenopausal after 5 years switching from tamoxifen to letrozole was superior to extended tamoxifen treatment [43••]. These results are similar as in the metastatic and early breast cancer settings where AIs are superior to tamoxifen as well. Higher efficacy, however, might also lead to a worse quality of life. Patients with letrozole intolerance can therefore switch again to tamoxifen, which they were able to cope with during the initial 5 years. Selection between tamoxifen and letrozole on the base of avoidance of specific potential side effects (e.g., thrombosis or endometrial hyperplasia for tamoxifen and osteoporosis and arthralgia for AIs) is a reasonable approach. If patients have a period without therapy after the 5 years of tamoxifen, MA-17 showed that delayed letrozole was better than no letrozole [11]. LATER is an ongoing trial to further test this hypothesis.
Postmenopausal at diagnosis and already treated with an AI
In postmenopausal patients treated with 2 to 5 years of an AI, there is no evidence for extended anti-hormonal therapy with tamoxifen beyond the 5 years of anti-hormonal therapy. In ATAC and BIG 1–98 residual relapse risk between years 5 and 10 was 10 % [44, 45]. Women with an AI during the first 5 years were not included in available trials with extended AI therapy, but such trials are ongoing (NSABP B-42, DATA, ABCSG-16/SALSA, LEAD, IDEAL, MA-17R, see Table 2). The benefit from extended letrozole therapy for 5 years was taken for granted by MINDACT and SOLE investigators. MINDACT opted for 7 instead of 5 years of adjuvant letrozole [46] whereas SOLE opted for 5 additional years of continuous or interrupted use of letrozole after 5 years of any anti-hormonal therapy. Outcome data from both trials are awaited. The recently published ASCO guidelines advise continuing 2–3 more years of an AI for postmenopausal women receiving already 3–2 years AI treatment after 2–3 years of tamoxifen. This was already included in their previous guidelines, not as a result of an RCT but as a reasonable option based on safety data for 5 years of an AI alone or after 5 years of tamoxifen [30••]. In the meanwhile in UZ Leuven, high-risk patients (lymph node-positive and no comorbidity) who already took an AI during the first 5 years of treatment are counseled for an extra 5 years of tamoxifen. Postmenopausal women who cannot tolerate an AI are advised to take 10 years of tamoxifen, as mostly, AI intolerance appears within the first 6 months of therapy. Further, patients with intolerance of tamoxifen can be given 5 years of AI, also suggested in the ASCO guidelines but not (yet) tested in RCTs.
Frequency of metastatic relapse in the first 5 years as well as beyond 5 years in UZ Leuven
In UZ Leuven, primary operable, ER-positive, HER2-negative breast cancer patients diagnosed between January 2000 and December 2004 were analyzed for metastatic relapse during the first 5 years as well as beyond 5 years (Table 3). All patients were treated according to standard of care and follow-up was at least 10 years. Frequency of metastatic relapse was investigated according to menopausal status (age 50 or younger versus older than 50 years), PR status, lymph node status, and grade. In the cohort age 50 or younger, 462 patients were included. Lymph node status was unknown for 4 and grade for 1 of these patients. In absolute numbers, we found that those with a PR-positive lesion more often relapsed during the first 5 years except when the tumor was grade 1–2 with positive lymph node status. Further, patients with PR-negative disease more frequently developed metastasis during years 6–10. None of the patients with PR-negative, lymph node-negative breast cancer relapsed during years 0–5. Also, none of these patients with a grade 3 tumor relapsed during years 6–10. Some of the subgroups, however, contained only a small number of patients. In the cohort older than age 50, PR status was unknown for 7 patients and lymph node status for 21 of 1082 patients that were included. In absolute numbers, PR-negative breast cancer patients had more recurrences during the first 5 years as compared to years 6–10, except for those who had a lymph node-negative, grade 1–2 lesion, doing well in both time periods. Further, PR-positive breast cancer patients more frequently developed metastasis during years 6–10 versus years 0–5 when lymph nodes were positive at diagnosis. Among PR-positive breast cancer patients with pN0, more recurrences were detected during the first 5 years versus years 6–10 only in the group with grade 3 disease. Based on these numbers, it could be suggested to administer extended endocrine treatment only to subgroups that seem to be at higher risk during years 6–10 when taking PR status, lymph node status, and grade into account.
Side effects of extending anti-estrogen therapy
Most side effects of continuing tamoxifen for an additional 5 years are seen after menopause and include postmenopausal symptoms such as hot flashes and night sweats, and vaginal dryness, discharge, or irritation. In premenopausal patients, the most common side effect is irregular menses. Extended tamoxifen treatment also increases the risk for endometrial cancer and thromboembolic disease as shown in the ATLAS trial [5•]. Toxicities induced by AIs are AI-induced musculoskeletal syndrome (AIMSS) including arthralgia, myalgia, joint stiffness, paresthesia and carpal tunnel syndrome, osteoporosis and fractures, and probably also cardiovascular risks. MA.17 showed that especially osteoporosis was frequently diagnosed with prolonged letrozole treatment [47]. Koch et al. reported that breast cancer survivors had comparable general health and overall quality of life 10 years after diagnosis as compared with controls and that mainly the youngest survivors reported relevant restrictions [48]. However, the IDEAL trial showed that there was a high non-compliance to extended endocrine treatment due to toxicities [49]. After 2.5 years of extended letrozole therapy, 18.4 % of 1215 patients stopped treatment of which 85.1 % discontinued because of toxicities. Therefore, benefits and risks of the proposed extended endocrine therapy should always be discussed with the patient, together with her preferences and available alternatives. Since patients have 5 years of experience with tamoxifen and/or AI, the decision to continue with either one can integrate the patients’ own experience during the last 5 years. If patients are well informed and aware of potential toxicity associated with their therapy, they can identify and intervene or seek help when necessary.
Conclusion
After 5 years of any endocrine treatment schedule, extended adjuvant endocrine therapy should be discussed with women whose tumor was not low grade, pT1N0, ER-positive, PR-positive, HER2-negative, and if there was no lymphovascular invasion. We encourage these women to take another 5 years of tamoxifen unless they did not take an AI, any time during the first years, and they were lymph node-positive at diagnosis. In the latter case, women are advised to take letrozole for another 3 years. Most benefit is larger with better compliance, and a balance should be made between relapse risk and treatment tolerance/compliance.
References and Recommended Reading
Recently published papers of particular importance have been highlighted as: • Of importance•• Of major importance
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Burstein HJ, Prestrud AA, Seidenfeld J, Davidson NE, et al. American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96.
Forbes JF, Dowsett M, Bradley R, Ingle JN, Aihara T, Bliss JM, et al. Patient-level meta-analysis of randomized trials of aromatase inhibitors (AI) versus tamoxifen (Tam). ASCO Annual Meeting 2014.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. Important trial on extending initial tamoxifen treatment with another 5 years.
Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31:abstr 5. Important trial on extending initial tamoxifen treatment with another 5 years.
Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond 5 years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst. 1996;88:1828–33.
Stewart HJ, Prescott RJ, Forrest AP. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93:456–62.
Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than 5 years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–90.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–71.
Jin H, Tu D, Zhao N, Shepherd LE, Goss PE. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol. 2012;30:718–21.
Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845–53.
Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–71.
Markopoulos C, Dafni U, Misitzis J, Zobolas V, Tzoracoleftherakis E, Koukouras D, et al. Extended adjuvant hormonal therapy with exemestane has no detrimental effect on the lipid profile of postmenopausal breast cancer patients: final results of the ATENA lipid substudy. Breast Cancer Res. 2009;11:R35.
Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–11.
Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015;33:916–22.
Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, Greil R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109:2959–64.
Mittempergher L, Saghatchian M, Wolf DM, Michiels S, Canisius S, Dessen P, et al. A gene signature for late distant metastasis in breast cancer identifies a potential mechanism of late recurrences. Mol Oncol. 2013;7:987–99.
Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14:1067–76.
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–92.
Metzger Filho O, Ignatiadis M, Sotiriou C. Genomic Grade Index: an important tool for assessing breast cancer tumor grade and prognosis. Crit Rev Oncol Hematol. 2011;77:20–9.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, et al. Comparison of PAM50 risk of recurrence score with Oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–90.
Zhang Y, Schnabel CA, Schroeder BE, Jerevall PL, Jankowitz RC, Fornander T, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013;19:4196–205.
Sgroi D, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Erlander MG, et al. Comparative performance of Breast Cancer Index (BCI) vs. Oncotype Dx and IHC4 in the prediction of late recurrence in hormonal receptor-positive lymph node-negative breast cancer patients: a TransATAC study. Cancer Res. 2012;72:S1–9.
Dubsky P, Brase JC, Fisch K, Jakesz R, Singer C, Greil R, et al. The EndoPredict score identifies late distant metastases in ER+/HER2− breast cancer patients. Cancer Res. 2012;72:S4–3.
Sestak I, Cuzick J. Markers for the identification of late breast cancer recurrence. Breast Cancer Res. 2015;27:10.
Knauer M, Filipits M, Dubsky P. Late recurrences in early breast cancer: for whom and how long is endocrine therapy beneficial? Breast Care. 2014;9:97–100.
Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69. This paper gives an overview of the optimal duration of endocrine treatment according to ASCO guidelines.
Al-Mubarak M, Tibau A, Templeton AJ, Cescon DW, Ocana A, Seruga B, et al. Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PLoS One. 2014;9:e88238. This paper gives an overview of all benefits and harms of extended tamoxifen treatment compared to only 5 years of adjuvant tamoxifen treatment based on the 5 main studies on this topic.
Neven P, Joensuu H, Osborne K, Cardoso F, Loibl S, Linn S, et al. Tamoxifen therapy for patients with breast cancer. Lancet. 2013;381:2078.
Davies C, Gray R, Pan H, Peto R, ATLAS collaborative group. Tamoxifen therapy for patients with breast cancer—authors’ reply. Lancet. 2013;381:2078–9.
Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–46.
Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–18.
Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91.
Pfeiler G, Königsberg R, Filipcic L, Greil R, Stoeger H, Singer CF, et al. Follicle stimulating hormone (FSH) as a surrogate parameter for the effectiveness of endocrine therapy with or without zoledronic acid in premenopausal patients with breast cancer: An analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2014;32:5s.
Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29:2653–9.
Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26:313–20.
Ruddy KJ, DeSantis SD, Barry W, Guo H, Block CC, Borges V, et al. Extended therapy with letrozole and ovarian suppression in premenopausal patients with breast cancer after tamoxifen. Clin Breast Cancer. 2014;14:413–6.
Goss PE, Ingle JN, Pater JL, Martino S, Robert NJ, Muss HB, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–55.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Livingston RB. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol. 2013;24:355–61.
Strasser-Weippl K, Badovinac-Crnjevic T, Fan L, Goss PE. Extended adjuvant endocrine therapy in hormone-receptor positive breast cancer. Breast. 2013;22:S171–5. This is a cross-trial comparing extended tamoxifen treatment with extended AI treatment based on the ATLAS trial and important AI studies.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41.
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12:1101–8.
Cardoso F, Van’t Veer L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ. Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol. 2008;26:729–35.
Mann BS, Johnson JR, Kelly R, Sridhara R, Williams G, Pazdur R. Letrozole in the extended adjuvant treatment of postmenopausal women with history of early-stage breast cancer who have completed 5 years of adjuvant tamoxifen. Clin Cancer Res. 2005;11:5671–7.
Koch L, Jansen L, Herrmann A, Stegmaier C, Holleczek B, Singer S. Quality of life in long-term breast cancer survivors—a 10-year longitudinal population-based study. Acta Oncol. 2013;52:1119–28.
Fontein DB, Nortier JW, Liefers GJ, Putter H, Meershoek-Klein Kranenbarg E, van den Bosch J. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. Eur J Surg Oncol. 2012;38:110–7.
Compliance with Ethics Guidelines
Conflict of Interest
Kathleen Van Asten, An Poppe, Kevin Punie, Lynn Jongen, Anneleen Lintermans, Hans Wildiers, and Patrick Neven declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Van Asten, K., Poppe, A., Punie, K. et al. The UZ Leuven Policy for Extended Adjuvant Anti-estrogen Therapy in Women With Early Estrogen Receptor-Positive Breast Cancer. Curr. Treat. Options in Oncol. 16, 31 (2015). https://doi.org/10.1007/s11864-015-0349-1
Published:
DOI: https://doi.org/10.1007/s11864-015-0349-1