Abstract
Recent environments, such as restingas, present high habitat heterogeneity, with different vegetation types throughout its geographic range. In this study, we provided the first assessment of how the composition and abundance of non-volant small mammals are influenced by the habitat heterogeneity of the different vegetation types in the restinga of the Paulo César Vinha State Park and the Environmental Protection Area of Setiba, state of Espírito Santo. We used live traps in 24 sampling sites distributed over four vegetation types from April to October 2019 to capture mammals. We found a pattern in the assemblage’s composition, showing a difference in richness and abundance of species among vegetation types. We recorded 21 species of small mammals. The restinga forest showed the highest richness and abundance of species, in contrast the beach vegetation zone showed the lowest richness and abundance. Our results pointed to a high richness of small mammals for the two protected restinga areas in general. This was the first study with non-volant small mammals in the restinga environment that assessed the habitat’s heterogeneity and how species change their composition in different vegetation types. We noted the veracity of the hypothesis of habitat heterogeneity, with higher richness and abundance of species in vegetation types with higher heterogeneity. In addition to verifying the specificity of habitat of species recorded exclusively in forest environments. These results represent the first step to assist in actions for the conservation of mammals in a coastal environment with high anthropogenic pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The habitat heterogeneity’s hypothesis (MacArthur and MacArthur 1961), postulates that more heterogeneous environments would provide more resources, potentially increasing the number of niches, maintaining a higher species diversity than comparatively simpler environments (Bazzaz 1975). In different vegetation types, the levels of luminosity, humidity and temperature vary between environments according to the levels of habitat structural heterogeneity (Li and Reynolds 1994). These factors can be determinant to favor or not the occurrence of species in different sites, because they are basic characteristics for a species be successful to obtain food resources, reproduction, and nesting (August 1983). The habitat heterogeneity plays an important role in structuring assemblages (Tews et al. 2004; Ferreguetti et al. 2017), as a result, species richness and abundance can be proportional to the heterogeneity and complexity of the local environment (Grelle 2003; Sukma et al. 2019).
The Atlantic Forest has a heterogenous set of ecosystems and one of the most biodiverse biomes in Brazil, with a high degree of endemism and a high threat of extinction to local species due to the intense anthropic action (Escarlate-Tavares et al. 2016). Currently, the Atlantic Forest has approximately 26% of native vegetation cover (Rezende et al. 2018) and one of its geological formations are the restingas (Araújo et al. 2017). The restingas are formed by a vegetation types mosaic that despite being a highly threatened environment due to intense urban expansion (Rocha et al. 2003), present a high diversity (Bigarella 2001).
The restingas are coastal plains environments with predominantly sandy terrains formed mainly during regressions and transgressions at sea level (Coutinho 2016), harboring sets of different plant communities such as trees, shrubs, and herbaceous vegetation (Magnago et al. 2011). The restingas present an intense degradation process resulted in an accentuated alteration and loss of this habitat (Rocha et al. 2004). A limitation in monitoring the restinga habitat loss rate is the lack of information about the location and extension of the restinga remnants and about the main degradation factors in each one. Additionally, we also lack information on the conservation state of these remnants to support actions for their protection. The extensive presence along the Brazilian coast and despite being located in areas with comparatively high human density, the restingas are still relatively poorly-understood scientifically in several aspects of their biodiversity and state of conservation (s). The restinga environment has a high habitat heterogeneity, as there are different vegetation types, such as forest formations, shrubs vegetation, open Clusia formation, and beach vegetation zone (Magnago et al. 2011).
One of the first steps towards conservation is knowing the assemblage’s composition and structure and evaluating how they respond to the environment variation in the landscape (Santos 2003). Considering the importance of knowing the mammalian composition in such a threatened coastal environment (such as the restingas) to assist in conservation measures, we assessed how the richness and abundance of non-volant small mammals would be influenced by habitat heterogeneity in each of the four major vegetation types. Specifically, we aimed to: (1) identify the species composition in the different vegetation types of Paulo César Vinha State Park (PEPCV) and Setiba EPA; (2) compare the species richness and abundance in the different vegetation types of PEPCV and Setiba Environmental Protection Area (Setiba EPA); and (3) define which vegetation type would have the highest richness and abundance of species. Our hypothesis was that there would be higher richness and abundance in forest environments that are supposed to be more heterogenous, therefore having a higher variability of niches that, in turn, could potentially hold more species, compared to open formations that would be less heterogenous.
Material and methods
Study area
We conducted the study in two protected areas (hereafter PA), the Paulo César Vinha State Park (PEPCV) and the Setiba Environmental Protection Area (Setiba EPA) located in the Espírito Santo state, southeastern Brazil. The two PAs are separated only by the “Rodovia do Sol” (ES-060 road) connecting the Guarapari and Vila Velha municipalities. The PEPCV (20°33′-20°38’S, 40°23′-40°26’W) covers an area of approximately 1500 ha, and the Setiba EPA (20°32′-20°39’S, 40°22′-40° 32’W) has approximately 12,960 ha (IEMA 2016a, b).
These two PAs present four different vegetation types, as following: beach vegetation zone (BVZ, closer to the sea, with herbaceous vegetation), shrub vegetation zone (SVZ, with low shrubs, usually cacti and bromeliads), open Clusia formations (OCZ, composed of higher shrubs, high density of bromeliads and presence of tree species, Clusia spp.) and restinga forests (RF, dominated by tree species) (Oprea et al. 2009; Oliveira et al. 2017; Fig. 1).
The RF, with a predominance of trees, which can be classified in terms of canopy coverage (open or closed) and also in terms of flooding (Lima et al. 2017), OCZ that manifests interleaving islands of vegetation with white and sandy soil with sparse vegetation and high salinity (Pereira et al. 2004) with a predominance of Clusia spiritusanctenses and Clusia hilariana species (IEMA 2016a, b). Shrub vegetation zone that basically consists of thickets with a height of around 1.5 m and in the areas between thickets we find outcropping of sandy sediment (Martins 2012) and BVZ that occurs after the reptive vegetation of the tide line, in this formation we can observe a sudden transformation to the bush vegetation (Braz et al. 2013) (Fig. 1).
Data sampling
We collected data weekly from April to October 2019. We defined 14 sampling sites in the PEPCV and 10 sampling sites in the Setiba EPA (Fig. 2) with 500 m between sampling sites. In each sampling site, we defined six sampling stations that consisted in a 10 × 10 m plot with a minimum distance of 50 m between stations. In each sampling station, we installed five live-traps, one Tomahawk (size: 45 X 21 X 21 cm) and four Shermans (size: 25 X 8 X 9 cm). We arranged the live-traps as follows: four Sherman traps at the corners of the sampling station plot (two placed 1.5 m high on lianas or tree trunks in the understory and two placed on the ground) and a Tomahawk trap in the center placed on the ground. All stations were sampled five times.
We used a bait composed of banana, sardines, ground peanuts and cornmeal, mixed until it became a homogeneous paste. We left the live-traps open for three consecutive nights in each campaign and checked every morning. For each captured animal, we recorded the date of capture, the sampling site at which it was captured, and identified the species. We marked the individuals with numbered ear tags to make it possible to recognize if the individual had been recaptured. After all the information was collected, the individual was released in the same site where it was captured.
For identification, we used mammalian guides (Emmons and Feer 1997; Pine 1999; Bonvicino et al. 2008). To classify species in terms of endemism, we used the annotated list of mammals by Paglia et al. (2012) and, for the degree of threats, we used the International Union for Conservation Red List (IUCN 2019) and the Brazilian List of Threatened Fauna (ICMBio 2018).
Data analysis
We used the Non-Metric Multidimensional Scaling (NMDS) to order the data of composition and number of individuals captured (abundance) of the non-volant mammals’ species in the PEPCV and Setiba EPA. We used the Bray-Curtis index in the NMDS to estimate the dissimilarity in species composition and the number of individuals captured in each sampling site. This analysis aimed to assess the existence of some pattern ordering the mammalian assemblage. We performed the analyzes in the R version 3.4.4 program using a “metaMDS” function in the Vegan package version 2.5–4 for community analysis (Oksanen et al. 2013). We used a one-way analysis of variance (ANOVA) to assess whether there were differences in the richness and abundance of small mammals, among vegetation types using the R version 3.4.4 program.
Results
We recorded 170 individuals of 21 species, 12 species of the Order Rodentia and nine species of the Order Didelphimorphia (Table 1). Among the recorded species, five are endemic to the Atlantic Forest. Among the endemic species of the Atlantic Forest are the rodents Phyllomys pattoni, Hylaeamys laticeps, Trinomys setosus, and the Brazilian Common Opossum Didelphis aurita and the Brazilian Gracile Opossum Gracilinanus microtarsus (Table 1). Hylaeamys laticeps is listed as almost threatened on the IUCN list.
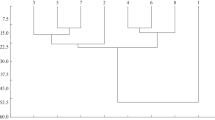
The non-volant small mammals’ composition from PEPCV and Setiba EPA differed between the four vegetation types, constituting three main groups regarding the dissimilarity between the sampling sites (Fig. 3). The areas with the lowest dissimilarity to each other were the SVZ and OCZ, which constituted a grouping and, the most dissimilar to each other, were the RF and BVZ areas, constituting two distinct groups (Fig. 3).
Non-Metric Multidimensional Scaling (NMDS) showing the dissimilarity between the sample areas in the composition of non-volant small mammals recorded in the Paulo César Vinha State Park and the Environmental Protection Area of Setiba, Guarapari, Espírito Santo, Brazil. Caption: SVZ = Shrub vegetation zone; OCZ = open Clusia zone; RF = Restinga forest and BVZ = beach vegetation zone
We found a significant difference in the richness (ANOVA, F = 13.8, p = <0.001, degree of freedom = 20) and in the abundance of species (ANOVA, F = 13.87, p = <0.001, degree of freedom = 20) in the four different vegetation types studied (Fig. 4). The richness and abundance of the species captured were higher in RF when compared to OCZ, SVZ and BVZ (Fig. 4).
We found that small mammals assemblage differed in the different vegetation types, with higher species richness in the Restinga Forest (RF). Approximately 61% of the species found in the RF were also present in Shrub Formations (OCZ and SVZ). In the BVZ, only two species were captured, Didelphis aurita and Cavia fulgida (Fig. 5). Only D. aurita was captured in all vegetation types. We captured seven species exclusively to the forest formation, they were the Marmosa paraguayana, Caluromys philander, Gracilinanus microtarsus, Monodelphis americana, Necromys lasiurus, Rhipidomys mastacalis and Phyllomys pattoni (Fig. 5).
Discussion
We recorded a high species richness in the sampled areas of PEPCV and Setiba EPA, with a total of nine marsupials and 12 rodents distributed in four different vegetation types. Our results indicate a high species richness for both PAs, when compared with other studies carried out in other restinga areas of Brazil. Of the 21 species found in PEPCV and Setiba EPA, five are endemic to the Atlantic Forest, which represents 23.8% of the rate of endemism of small mammals to this biome among local species (Paglia et al. 2012). This result represents about 16% of all species of non-volant small mammals occurring in the Atlantic Forest (Paglia et al. 2012; Graipel et al. 2017) and 43.75% of species of the Rodentia Order and Didelphimorphia of the State of Espírito Santo listed by Moreira et al. (2008). A study at the Morro Grande Forest Reserve, in the State of São Paulo (Pardini and Umetsu 2006) recorded eight species of marsupials and 15 of rodents. On the other hand, in fragments of restinga forest in Rio Grande, in the coastal plain of Rio Grande do Sul, recorded three species of marsupials and eight rodents (Quintela et al. 2012). Another study conducted in the restinga of the Acaraí State Park in the south of Brazil recorded 126 individuals of small mammals, one species of the Order Didelphimorphia and three species of the Order Rodentia (Balieiro et al. 2015). In the Restinga de Jurubatiba National Park, Bergallo et al. (2004) recorded four species of marsupials and seven species of rodents in five vegetation types.
The small mammals’ composition differed between the vegetation types of PEPCV and Setiba EPA. Only the Didelphis aurita was captured in all vegetation types. This may be related to the fact that this opossum is an animal with a high ecological plasticity and with omnivorous feeding habits and can be found in the most different types of habitat from the interior of forests to urban areas (Graipel and dos Santos-Filho 2006). Cavia fulgida was recorded in all vegetation types except for forest environments. In fact, it has been argued that the species is terrestrial and inhabit tunnels in low and closed vegetation, such as grasslands (Silva 1994), which is in line with what was found in PEPCV and Setiba EPA. Of the species recorded exclusively in the forest environment, the species Rhipidomys mastacalis and Phyllomys pattoni, are exclusively arboreal rodents (Bonvicino et al. 2008; Paglia et al. 2012). Necromys lasiurus is a terrestrial rodent that occupies open and forested formations in the Cerrado, but also transits the ecotone Atlantic Forest-Cerrado (Bonvicino et al. 2008). The marsupials recorded only in forest environments were Caluromys philander, Gracilinanus microtarsus and Marmosa paraguayana, which are arboreal species (Rossi et al. 2012; Graipel et al. 2017). In forest formations, we also register Monodelphis americana, which is a species of semi-fossorial marsupial (Vieira et al. 2012).
The higher richness found in the forest environment could be related to the greater complexity of the vertical space, expanding the availability of different niches, increasing resources and protection against terrestrial predators (Vieira et al. 2012). Banasiak and Shrader (2016) suggest that communities of small mammals prefer more complex environments, because in addition to the higher resource’s availability, these environments still offer protection against predation through their vegetation cover. A study in South Africa found differences in the composition of small mammals in different habitats and recorded a higher richness and abundance in forest areas (Simelane et al. 2018). Our results recorded this same pattern because forest environments provide higher plant diversity, thus presenting the highest richness and abundance of all other formations evaluated. In the areas of OCZ and SVZ, a similar pattern of richness and abundance was observed, and this can be explained due to the similarity of this environments, because both are composed of thickets of vegetation (Martins 2012). However, in the BVZ sampling sites we recorded the lowest richness and abundance, with only plant species that are adapted to the actions of the winds, increased salinity, and greater water restriction, with a very characteristic undergrowth (reptile) prevailing (Martins 2012). These factors reduce the chances of rodents and arboreal marsupials transiting in these environments because the ideal conditions for these species occur are vegetation types with the presence of a more diversified vegetation with lianas that form an understory. In the Jurubatiba sandbank, Bergallo et al. (2004), found similar results regarding the habitat heterogeneity and the richness of species.
The results obtained in this study indicate a high small mammals’ richness for two PAs of restinga, which are important environments for conservation due to their intense exploitation for sand extraction and high real estate speculation due to urban expansion. These results represent the first step to assist in actions for the conservation of mammals in a coastal environment with high anthropogenic pressure. Our results corroborate the hypothesis of habitat heterogeneity in the restinga environments. In addition, we verified the plasticity of the species D. aurita and the specificity of habitat of species recorded exclusively in forest environments. We also noticed that although the forest vegetation types showed the higher richness in the resting, some species occurred only in open vegetation formations, emphasizing the importance of the irreplaceable nature of different vegetation types for the conservation of species. We hoped that our results will be able to contribute with important data on the distribution of the species of small mammals of PEPCV and Setiba EPA, helping in the decision making for actions related to the conservation of this ecosystem.
References
[ICMBio] Instituto Chico Mendes de Conservação da Biodiversidade (2018) Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. 1ª ed. Brasília (DF): ICMBio/MMA. Volume II – Mamíferos
[IEMA] Instituto Estadual De Meio Ambiente E Recursos Hídricos (2016a) Unidade de Conservação - Área de Proteção Ambiental de Setiba. [cited 2019 Sept 25]. Available from: https://iema.es.gov.br/APA_Setiba
[IEMA] Instituto Estadual De Meio Ambiente E Recursos Hídricos (2016b) Unidade de Conservação – Parque Estadual Paulo César Vinha. 2016. [cited 2019 Sept 25]. Available from: https://iema.es.gov.br/PEPCV
[IUCN] International Union for Conservation of Nature (2019) The IUCN red list of threatened species. Version 2019-1. [cited 2019 Sept 20]. Available from: https://www.iucnredlist.org/
Araújo RC, Santos SLO, Magenta M, Ramires M (2017) Avaliação do estado de conservação de duas áreas de restinga no litoral de São Paulo, Brasil. In: Encontro Nacional de Pós-Graduação - ENPG. Vol. 6. Universidade Santa Cecília, Santos - SP, Brasil. [cited 2019 Sept 29]. Available from: https://ojs.unisanta.br/index.php/ENPG/article/view/1140
August PV (1983) The role of habitat complexity and heterogeneity in structuring tropical mammal communities. Ecology 64(6):1495–1507
Balieiro P, Behs D, Graipel ME, Dornelles SS, Tiepolo LM, Cremer MJ (2015) Riqueza de pequenos mamíferos não voadores em florestas de restinga do Sul do Brasil. Mastozoología Neotropical 22(2):367–373
Banasiak N, Shrader AM (2016) Similarities in perceived predation risk prevent temporal partitioning of food by rodents in an African grassland. J Mammal 97(2):483–489
Bazzaz FA (1975) Plant species diversity in old-field successional ecosystems in southern Illinois. Ecology 56(2):485–488
Bergallo HG, Martins-Hatano F, Raíces DS, Ribeiro TTL, Alves AG, Luz JL, Mangolin R, Mello MAR (2004) Os mamíferos da Restinga de Jurubatiba. Pesquisas de longa duração na Restinga de Jurubatiba. Ecologia, História Natural e Conservação. São Carlos, Editora Rima, 374p, 215-230
Bigarella JJ (2001) Contribuição ao estudo da planície litorânea do Estado do Paraná. Braz Arch Biol Technol:65–110
Bonvicino CR, Oliveira JD, D’Andrea PS (2008) Guia dos roedores do Brasil, com chaves para gêneros baseadas em caracteres externos. Centro Pan-Americano de Febre Aftosa-OPAS/OMS, Rio de Janeiro, p 120
Braz DM, Jacques EDL, Somner GV, Sylvestre LDS, Rosa MMTD, Pereira-Moura MVL, Amorim TA, Filho PG, Couto AVS (2013) Restinga de Praia das Neves, ES, Brasil: caracterização fitofisionômica, florística e conservação. Biota Neotropica 13(3):315–331
Coutinho LM (2016) Biomas brasileiros. Oficina de Textos, São Paulo
Emmons L, Feer F (1997) Neotropical rainforest mammals: a field guide
Escarlate-Tavares F, Valença-Montenegro MM, Jerusalinsky L (2016) Plano de Ação Nacional para Conservação dos Mamíferos da Mata Atlântica Central. Série Espécies Ameaçadas. Brasília, Brasil: Instituto Chico Mendes de Conservação da Biodiversidade, ICMBio
Ferreguetti AC, Tomas WM, Bergallo HG (2017) Differences in the mammalian habitat use in a mosaic of vegetation types of an Atlantic rain-forest reserve, Brazil. Mastozoología Neotropical 24(2):355–364
Graipel ME, dos Santos-Filho M (2006) Reprodução e dinâmica populacional de Didelphis aurita Wied-Neuwied (Mammalia: Didelphimorphia) em ambiente periurbano na Ilha de Santa Catarina, Sul do Brasil. Biotemas 19(1):65–73
Graipel ME, Cherem JJ, Monteiro-Filho EL, Carmignotto AP (2017) Mamíferos da Mata Atlântica. Revisões em Zoologia: Mata Atlântica, 391-482
Grelle CEV (2003) Forest structure and vertical stratification of small mammals in a secondary Atlantic forest, southeastern Brazil. Stud Neotropical Fauna Environ 38(2):81–85
Li H, Reynolds JF (1994) A simulation experiment to quantify spatial heterogeneity in categorical maps. Ecology 75(8):2446–2455
Lima GP, Lacerda DMA, Lima HP, de Almeida Jr EB (2017) Caracterização fisionômica da Restinga da Praia de Panaquatira, São José de Ribamar, Maranhão. Rev Bras Geogr Fís 10(6):1910–1920
Macarthur RH, Macarthur JW (1961) On bird species diversity. Ecology 42:594–598
Magnago LFS, Martins SV, Pereira OJ (2011) Heterogeneidade florística das fitocenoses de restingas nos estados do Rio de Janeiro e Espírito Santo, Brasil. Revista Árvore 35(2):245–254
Martins MLL (2012) Fitofisionomia das formações vegetais da restinga da Área de Proteção Ambiental (APA) de Guaibim, Valença, Bahia, Brasil. Rev Bras Biociências 10(1):66
Moreira DDO, Coutinho BR, Mendes SL (2008) O status do conhecimento sobre a fauna de mamíferos do Espírito Santo baseado em registros de museus e literatura científica. Biota Neotropica, 8(2), 0-0
Oksanen J, Kindt R, O’Hara B (2013) The vegan package. Commun Ecol Packag 10:631–637
Oliveira JCF, Winck GR, Pereira-Ribeiro J, Rocha CFD (2017) Local environmental factors influence the structure of frog communities on the sandy coastal plains of southeastern Brazil. Herpetologica 73(4):307–312. https://doi.org/10.1655/Herpetologica-D-16-00075.1
Oprea M, Esbérard CEL, Vieira TB, Mendes P, Pimenta VT, Brito D, Ditchfield AD (2009) Bat community species richness and composition in a Restinga protected area in southeastern Brazil. Braz J Biol 69(4):1073–1079
Paglia AP, Fonseca GAB, Rylands AB, Herrmann G, Aguiar LMS, Chiarello AG, Leite YLR, Costa LP, Siciliano S, Kierulff MCM et al (2012) Lista Anotada dos Mamíferos do Brasil 2ª Edição/Annotated checklist of Brazilian mammals. Arlington, Conservation International
Pardini R, Umetsu F (2006) Pequenos mamíferos não-voadores da Reserva Florestal do Morro Grande: distribuição das espécies e da diversidade em uma área de Mata Atlântica. Biota Neotropica, 6(2): 0-0
Pereira MCA, Cordeiro SZ, Araujo DSDD (2004) Estrutura do estrato herbáceo na formação aberta de Clusia do Parque Nacional da Restinga de Jurubatiba, RJ, Brasil. Acta Bot Bras 18(3):677–687
Pine RH (1999) Neotropical rainforest mammals. A field guide. J Mammal 80(1):304
Quintela FM, Santos MB, Christoff AU, Gava A (2012) Non-volant small mammals (Didelphimorphia, Rodentia) in two forest fragments in Rio Grande, Rio Grande do Sul coastal plain, Brazil. Biota Neotropica 12(1)
Rezende CL, Scarano FR, Assad ED, Joly CA, Metzger JP, Strassburg BBN, Tabarelli M, Fonseca GA, Mittermeier RA (2018) From hotspot to hopespot: an opportunity for the Brazilian Atlantic Forest. Perspect Ecol Conserv 16(4):208–214
Rocha CFD, Bergallo HG, Alves MAS, van Sluys M (2003) A biodiversidade nos grandes remanescente florestais do estado do Rio de Janeiro e nas restingas da Mata Atlântica. RiMa, São Carlos, 160 p
Rossi RV, Brandão MV, Miranda CL, Carmignoto AP, Cherem J (2012) Diversidade morfológica e taxonômica de marsupiais didelfídeos, com ênfase nas espécies brasileiras. Os marsupiais do Brasil: biologia, ecologia e conservação (NC Cáceres, ed.). Ed. UFMS. Campo Grande, 23-72
Santos A (2003) Estimativas de Riqueza em Espécies. Métodos de estudo em Biologia da Conservação e Manejo de Vida Silvestre. Editora da Universidade Federal do Paraná, Paraná
Silva F (1994) Mamíferos silvestres, Rio Grande do Sul. Fundação Zoobotânica do Rio Grande do Sul, Porto Alegre
Simelane FN, Themb’alilahlwa AM, Shapiro JT, MacFadyen D, Monadjem A (2018) Habitat associations of small mammals in the foothills of the Drakensberg Mountains, South Africa. Mammalia 82(2):144–152
Sukma HT, Di Stefano J, Swan M, Sitters H (2019) Mammal functional diversity increases with vegetation structural complexity in two forest types. For Ecol Manag 433:85–92
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31(1):79–92
Vieira EM, Camargo NF, Cáceres N (2012) Uso do espaço vertical por marsupiais brasileiros. Os marsupiais do Brasil: biologia, ecologia e conservação (NC Cáceres, org.). Editora UFMS, Campo Grande, Brazil, 345-362
Acknowledgements
This study was conducted with the research license Process 76444341 - Authorization 003A-2017 provided by the “Instituto Estadual de Meio Ambiente e Recursos Hídricos - IEMA“. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. This study is a portion of the results of the Project “Vivendo na Floresta: Conservação da biodiversidade capixaba”. The authors benefitted from grants provided to HGB (process 307781/2014-3) and to CFDR (302974/2015-6 and 472287/2012-5) from CNPq and through “Cientistas do Nosso Estado” Program from FAPERJ to CFDR (process No. E-26/102.765/2012 and E-26/202.920/2015) to HGB (process E-26/202.757/2017). ACF thanks FAPERJ for the PhD scholarship (FAPERJ nota 10) process No. E-26/202.198/2018 (240022) and for the post-doc scholarship “Programa Pós-Doutorado NOTA 10 - 2020” process No. E-26/201.855/2020 (255804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure statement
We have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Bortolo, G.T., Ferreguetti, A.C., de Souza Rocha, B. et al. Non-volant small mammals in the different vegetation types of two protected restinga areas, Southeastern Brazil. J Coast Conserv 25, 18 (2021). https://doi.org/10.1007/s11852-021-00811-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11852-021-00811-w