Abstract
Introduction
Non-Invasive Ventilation (NIV) is a crucial therapy for managing acute exacerbations of Chronic Obstructive Pulmonary Disease (COPD) with hypercapnic respiratory failure. Research has shown that NIV can decrease the rate of endotracheal intubation, length of hospital and Intensive Care Unit stays, and mortality. There are three main strategies for weaning patients off NIV: gradual reduction of NIV duration, gradual reduction of NIV pressure support, and immediate cessation of NIV.
Aim
To compare the rate of successful withdrawal of COPD patients with acute hypercapnic respiratory failure, one group will use a stepwise reduction of duration of NIV, while the other group will use a stepwise reduction of pressure support.
Materials and methods
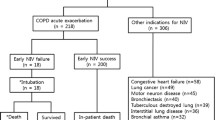
This study was a prospective observational study conducted at the Department of Pulmonary Medicine, Institute of Chest Diseases, Government Medical College, Kozhikode, over a period of 15 months. The study population consisted of all COPD patients admitted to the Pulmonary Medicine ward or ICU with acute hypercapnic respiratory failure who were managed with non-invasive ventilation (NIV) without the need for invasive mechanical ventilation. Exclusions included patients requiring NIV for respiratory diseases other than COPD, those with significant comorbidities like acute left ventricular failure or fluid overload states as in chronic kidney disease, COVID-19 positive patients, patients on home NIV, patients who needed intubation early in treatment, and patients unwilling to participate in the study. The sample size was 140. Initial NIV settings and other management decisions prior to enrolment in the study were made by the treating physician according to standard protocols. Once weaning criteria were met (i.e., arterial pH > 7.35, SpO2 ≥ 90% at an FiO2 ≤ 50%, respiratory rate ≤ 25 breaths per minute, heart rate ≤ 120 beats per minute, systolic BP > 90 mm Hg, and no signs of respiratory distress), patients were assigned to either group 1 or group 2 by purposive sampling. Group 1: stepwise reduction of duration of NIV use, with a reduction to 16 h per day on day 1 of enrolment, 12 h on day 2 (including 6–8 h of nocturnal NIV), 6–8 h on day 3, and NIV withdrawal on day 4. Group 2: stepwise reduction of pressure support, with pressure support reduced by 2–4 cm every 4–6 h until Inspiratory Positive Airway Pressure is < 8 cm H2O and Expiratory Positive Airway Pressure is < 4 cm H2O, followed by NIV withdrawal. The clinical outcome was classified as either improved or weaning failure. Improved was defined as an objective or subjective sense of improvement. Weaning failure was defined as the presence of any of the following: respiratory rate ≥ 25/minute or increase of ≥ 50% from baseline, heart rate ≥ 140/minute or increase of ≥ 20% from baseline, SpO2 ≤ 90% on FiO2 ≥ 50%, arterial pH ≤ 7.35, or respiratory distress. Data was collected using a pro forma that included demographic details, smoking status, GOLD COPD category, comorbidities, and vital signs. ABG parameters, NIV settings at the time of hospital admission, at the time of study enrolment, and 48 h after weaning were also recorded. Independent sample t-test was used to test the statistical significance of the difference between means of variables between the two groups. Pearson Chi square test and Fisher’s exact test were used to compare categorical variables between the groups. A p-value of < 0.05 was considered statistically significant.
Results
NIV was successfully withdrawn in 56/70 (80%) and 50/70 (71.4%) patients in Groups 1 and 2, respectively. This difference was not statistically significant. The length of hospital stay was longer in the stepwise reduction of duration group (Group 1), but this was not statistically significant.
Conclusion
On comparison of two methods of NIV withdrawal, it was found that neither method is superior to the other in terms of weaning failure, intubation rates, and average length of hospital stay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estimated mean incidence of COPD is 13% in the general population aged above 40 years, and its prevalence is rising globally [1]. High mortality rates are reported from low and middle income countries related to COPD exacerbations [2]. Exacerbations negatively impact disease progression, hospitalization, and readmission rates, and overall health. Most often, exacerbations are caused by bacterial infections, viral respiratory infections, and environmental triggers such as pollution and temperature fluctuations leading to the need for additional support measures like non-invasive ventilation to tide over the acute crisis. Standard treatment for severe COPD exacerbations includes systemic steroids, antibiotics, rapid-acting bronchodilators, and controlled oxygen therapy. Acidotic hypercapnic respiratory failure, which is an independent predictor of mortality, develops in about 20% of these individuals or is already present [3]. Non-invasive ventilation (NIV) has revolutionized the management of hypercapnic respiratory failure. Acute respiratory acidosis, oxygenation status, respiratory rate, work of breathing, and the degree of dyspnea have all been found to improve with the use of NIV in several randomized control trials.
Non-invasive mechanical ventilation is indicated in AECOPD if at least one of the following is present:
-
(i)
Respiratory acidosis PaCO2 > 6 kPa or 45 mmHg and arterial pH < 7.35
-
(ii)
Severe dyspnea with clinical signs suggestive of respiratory muscle fatigue, increased work of breathing, or both, such as use of respiratory accessory muscles, paradoxical motion of abdomen, or retraction of the intercostal spaces.
-
(iii)
Persistent hypoxemia despite supplemental oxygen therapy
This intervention has dramatically decreased the rate of endotracheal intubation, which in turn has reduced the incidence of ventilator associated pneumonia, hospital stay length, and overall mortality [4, 5]. As in the case of invasive mechanical ventilation, it is preferable to keep NIV sessions short to lower the risk of both NIV-related issues and other complications brought on by a prolonged hospital stay. The spontaneous/timed mode is often chosen to support all breaths and provide a minimum respiratory rate if the patient hypoventilates. Starting IPAP (Inspiratory Positive airway pressure) is typically set at 9 cm of H2O and can be increased in increments of 2 cm of H2O to a maximum of 20–30 cm of H2O, based on improvement in dyspnea, respiratory rate, tidal volume, and patient-ventilator synchrony. EPAP (Expiratory Positive airway pressure) is usually set at 3-5 cm of H2O and can be increased up to 8 cm of H2O if oxygenation remains insufficient. Hyperoxygenation is harmful in the self-ventilating patient with acute hypercapnic respiratory failure [6]. The target oxygenation is determined by the underlying disorder. For COPD the target saturation is 88–92%. Studies have shown that there is no difference in inspired O2 content whether delivered directly into the NIV mask or into the ventilator tubing close to the mask [7]. The mean FiO2 achieved was 31% at 1 L/min, 37% at 2 L/min, 40% at 3 L/min and 44% at 4 L/min. Flow rates > 4 L/min provided only minimal additional increase. In situation of higher inspiratory pressure, high flow rates do not show significant benefits due to increased leak. High flow rates also resulted in delayed triggering of the ventilator promoting patient ventilator asynchrony. After NIV is initiated, the patient should be closely monitored for the first few minutes to troubleshoot any initial problems. Some patients may not initially tolerate NIV due to patient discomfort or ventilator dyssynchrony (i.e., the phases of ventilator breath do not match that of the patient) [8]. Presence of Auto-PEEP, mask leak, anxiety etc. contributes to dyssynchrony. Some studies have shown adequate sedation with good NIV tolerance by using infused dexmedetomidine to a standard protocol of, as and when needed bolus intravenous midazolam and fentanyl [9]. Sedation should always be used with close monitoring in an ICU setting. Once the patient is comfortable, vital signs, oxygenation, and mental status should be closely monitored for the next one to two hours. After an NIV trial of 2–4 h, blood gases were reanalysed. If patient demonstrated improvement in clinical signs and symptoms and gas exchange, NIV was continued. If a patient does not improve or deteriorates following a one-to-two-hour trial of NIV, the patient should then be considered to have failed NIV and be promptly intubated. There is no universal protocol for weaning and it is generally individualized and depends on the patient's response to decreased support. Commonly used weaning criteria include inspiratory positive airway pressure (IPAP) less than or equal to 16 cm H2O, expiratory positive airway pressure (EPAP) less than or equal to 8 cm H2O, arterial pH greater than or equal to 7.35, oxygen saturation (SpO2) greater than 90% on FiO2 less than or equal to 50%, respiratory rate less than or equal to 25/min, heart rate less than or equal to 120/min, systolic blood pressure greater than or equal to 90 mm Hg, and no signs of respiratory distress such as agitation, diaphoresis, or anxiety. There are three possible weaning strategies that can be used for NIV weaning – i)stepwise reduction of duration of NIV use, ii)stepwise reduction in pressure support of NIV, and iii)immediate withdrawal of NIV. There is limited data on various techniques of withdrawal of NIV support. It is therefore crucial to find the ideal NIV weaning technique.
Lun et al. contrasted the withdrawal of NIV gradually versus immediately [10]. The gradual withdrawal group had 35 patients and the immediate withdrawal group had 25 patients, respectively. The procedure for gradual withdrawal was as follows: on the day of randomization (day 0), the length of NIV usage was lowered to 16 h; on days 1 through 3, it was reduced to 12, 8, and 0 h, respectively. Following this strategy, the stepwise and rapid withdrawal groups experienced weaning success rates of 74% and 56%, respectively. Although the difference was not statistically significant, it appeared that a progressive withdrawal approach led to greater weaning success (p – 0.139). In a study conducted by Kavitha Venkatnarayanan etal, patients with AECOPD and HcRF who improved on NIV were randomised into one of the three groups: i) abrupt discontinuation)stepwise reduction of pressure support, iii)or stepwise reduction of duration of NIV [11]. The likelihood of a successful withdrawal was compared between the groups. They came to the conclusion that it was possible for individuals with COPD exacerbations to immediately stop using the NIV after their respiratory failure had improved. The probability of NIV weaning failure did not increase with an abrupt removal. The decision to discontinue NIV seems tricky, as there is paucity of data available. Hence in this study the 2 methods of NIV weaning which are followed in our institute i.e., step wise reduction of duration of NIV and stepwise reduction of pressure support were compared to pick out the best option.
Materials and methods
This prospective observational study was conducted in the Department of Pulmonary Medicine, Government Medical College, Kozhikode during a 15 months (June 2021 – May 2022) period. The study population included all patients diagnosed with AECOPD as defined by criteria of GOLD 2020, who satisfied the inclusion criteria were recruited for the study. The study obtained approval from the Institutional Ethics Committee (IEC approval number: GMCKKD/RP 2021/IEC/194).
Sample size
The minimum sample size was calculated as 140 with 70 patients in each group based on the reference study by Lun et al., the success rate of step wise withdrawal group was 74% [10]. Sampling method followed was purposive sampling.
Sample size calculated by the formula;
Hence n = 140 Final sample size = 140 with 70 in each group.
Inclusion criteria
1. COPD patients admitted in the Pulmonary Medicine ward or ICU with acute HcRF (arterial pH ≤ 7.35 and PaCO2 ≥ 45 mmHg) who were managed with NIV without the need for invasive mechanical ventilation. 2. Patients who satisfied the weaning criteria which is adopted from the earlier study [10] – arterial pH ≥ 7.35, oxygen saturation (SpO2) > :90% on FiO2 ≤ 50%, respiratory rate ≤ 25/min, heart rate ≤ 120/min, systolic blood pressure ≥ 90 mm Hg, and no signs of respiratory distress such as agitation, diaphoresis, or anxiety. 3. Patient who signed written informed consent.
Exclusion criteria
1. Patients who required invasive mechanical ventilation for acute exacerbation of COPD. 2. Patients on home NIV prior to admission. 3. Those who required NIV for respiratory failure due to diseases other than COPD 4. Covid 19 positive patients 5. Patients with significant comorbidity like acute left ventricular failure or fluid overload states like secondary to chronic kidney disease.
Study Procedure
Patients were enrolled in the study once they had recovered from acute respiratory failure and satisfied weaning criteria as evidenced by all of the following: Patients on NIV with inspiratory positive airway pressure (IPAP) ≤ 16 cm H2O and expiratory positive airway pressure (EPAP) ≤ 8 cm H2O. The weaning criteria adopted from the earlier study [10] were – arterial pH ≥ 7.35, oxygen saturation (SpO2) > 90% on FiO2 ≤ 50%, respiratory rate ≤ 25/min, heart rate ≤ 120/min, systolic blood pressure ≥ 90 mm Hg, and no signs of respiratory distress such as agitation, diaphoresis, or anxiety. Portable NIVs were used. Initial NIV settings, pressure changes as well as other management decisions, prior to enrolment in the study, were left to the discretion of the treating physicians, and the study group was not involved in the same till the patients satisfied inclusion criteria for weaning. All the patients received nursing care and medical management as per the standard institutional protocol by the same team of doctors throughout the study. Patients' data were collected from the time of admission; however, the study investigator was not involved in patient management until they satisfied the inclusion criteria. The clinical outcomes were categorized as A) Improved B) Weaning failure. “Improved” is clinically defined as subjective sense of improvement and objective improvement in dyspnea scoring. The criteria of "weaning failure" included the appearance of any one of the following features – respiratory rate > 25/min or increase of > 50%; heart rate > 140/min or increase > 20%; SpO2 < 90% on FiO2 of 50%; arterial blood pH < 7.35; or respiratory distress. Appearance of any one of these within 48 h of withdrawal was considered as a weaning failure. Such patients were restarted on NIV with pressures increased to previously tolerated levels [11].
Statistical analysis
Data collection was done using a pro forma which included demographic details, smoking status, GOLD COPD category, co morbidities and vitals. ABG parameters, NIV settings at the time of hospital admission, at the time study enrolment and 48 h after weaning were noted. Independent sample t-test was used to test statistical significance of difference between means of variables among two independent groups. Pearson Chi-square test and Fisher’s exact test were used for comparing categorical variables between groups. A p value of < 0.05 was considered significant.
Results
Group 1- stepwise reduction of duration of NIV use. Group 2- stepwise reduction of pressure support (Tables 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11).
Discussion
When it comes to the choice of the best protocol for withdrawal of NIV once the patient recovers from respiratory failure is not yet fully answered. The three possible methods of doing it are, first, withdraw NIV immediately once the patient's respiratory failure has recovered and monitor closely for failure. Second, gradually reduce the IPAP and EPAP at a defined interval and once patient requires minimum pressure support (IPAP < 8 cm of H2O and EPAP < 4 cm of H2O) withdraw the NIV. Third, the duration of NIV use may be reduced gradually and then withdrawn completely. The primary objective of our study was to compare the two methods of NIV weaning which is commonly followed in our institute i.e. stepwise reduction of duration of NIV and stepwise reduction of pressure support in patients recovered from acute hypercapnic exacerbation of COPD. Majority of study subjects belonged to the age group of the study population included 55 males (78.6%) in group 1 and 56 males (80%) in group2. Majority of the patients belonged to the GOLD Group C or D. When comorbidities were compared both groups had Type II diabetes mellitus as the most common one followed by hypertension. While the patients were enrolled, the mean pH was 7.27 ± 0.02 and 7.26 ± 0.026 in group 1 and group 2 respectively. Mean PaCO2 was higher in group2 (81 ± 18.89) compared to group1 (77.12 ± 17.92) and was statistically significant with a p value of 0. 012.Initial NIV settings were similar in both groups (group 1- IPAP 19.27 ± 4.92, EPAP 6.23 ± 0.95 and in group 2-IPAP 19.39 ± 4.86, EPAP 6.21 ± 0.98).
At the time of weaning, mean pH was 7.42 ± 0.04 and 7.40 ± 0.06 in group 1 and group 2 respectively. Mean PaCO2 was higher in group 2 (54.43 ± 5.96) compared to group1 (50.24 ± 6.97) and was not statistically significant. NIV settings at the time of weaning were (group 1- IPAP 17.14 ± 2.94, EPAP 5.86 ± 0.77 and in group 2-IPAP 17.38 ± 2.96, EPAP 5.64 ± 0.62). In a similar study conducted by Venkatnarayanan et al. [11] comparing three strategies of NIV weaning, 90 patients (90.6% men) with a mean age of 59.9 ± 8.3 years (SD) were included. The mean (SD) PaCO2 and pH values were 7.23 ± 0.04 and 84.4 ± 12.0 mm Hg, respectively, at the time of admission. With maximum inspiratory and expiratory positive airway pressures of 17.6 ± 2.7 cm H2O and 7.4 ± 1.4 cm H2O, respectively, NIV was given for 31.6 ± 9.2 h prior to randomization. After the analysis of our data it was found that successful withdrawal of NIV was higher (56- 80%) in group 1 (stepwise reduction of duration of NIV use) compared to step wise reduction of pressure support group (50- 71.4%) but was statistically not significant (p value – 0.237). Length of stay (in hospital days) were comparable in both groups (7.41 ± 3.48 and 7.49 ± 3.274 respectively, p value – 0.981). Number of intubated patients also were comparable with a p value of 0.681 (2 and 4 respectively). In a study conducted by Lun et al. which compared stepwise reduction in the duration of use with immediate withdrawal of NIV and reported a success rate of 74.3% and 56%, respectively [10]. Another study conducted by Sellares et also compared immediate withdrawal and additional 3 days of nocturnal NIV support after recovery from the respiratory failure. They also reported that the success of withdrawal was comparable between the groups (83% and 87%; P = 0.56) [12]. However, the immediate withdrawal group spent the lowest time on NIV and in the hospital. Immediate withdrawal of NIV was not done in our study because it is not followed in our institute, which is one of the main limitation of our study. As these results imply that immediate withdrawal of NIV is feasible in AECOPD patients without any additional risk of weaning failure. The lesser duration of hospital stay may translate into lesser risk of hospital-acquired infections and NIV-associated complications. Total number of failed weaning patients in our study was 34 (24%), out of which 6 patients finally required intubation, NIV use was extended for 18 patients, home NIV was arranged for 6 patients and home oxygen for 4 patients. Patients with lower GCS scores at admission indicate a more severe disease process. Additionally, they had greater PaCO2 levels at admission, enrolment, and withdrawal—a finding that was observed in both of the two groups. From the aforementioned findings, it can be concluded that patients with a higher PaCO2 have a higher risk of failing NIV withdrawal, making them likely candidates for stricter monitoring and most probably a more progressive withdrawal. The above conclusions, however, must be verified in studies that are sufficiently powered and have a bigger sample size. NIV was successfully withdrawn in 56/70 (80%), 50/70 (71.4%), patients in Groups 1 and 2 respectively. This difference was not statistically significant. Among the patients who failed NIV weaning (n = 34), 18 patients were subsequently withdrawn from NIV and discharged. Six patients continued to require NIV support and were discharged on home NIV. Length of hospital stay was longer in stepwise reduction of duration group (Groups 1) but was statistically not significant. Two patients in group 1 and four patients in group 2, finally ended up in intubation.
Limitations
Small sample size, selection bias especially being a single center study is the major limitation of the study. Immediate withdrawal of NIV was not compared with the two groups. The differences in outcome due to NIV model variability was also not taken into account.
Conclusion
On comparison of two methods of NIV withdrawal, it was found that no method is superior to the other in terms of weaning failure, intubation rates and average length of hospital stay.
References
Blanco I, Diego I, Bueno P et al (2019) Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur Respir J 54(1):1900610. https://doi.org/10.1183/13993003.00610-2019. PMID: 31000678
Viniol C, Vogelmeier CF (2018) Exacerbations of COPD. Eur Respir Rev 27(147):170103. https://doi.org/10.1183/16000617.0103-2017. PMID: 29540496; PMCID: PMC9488662
Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP et al (2011) Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J 37(3):508–515. https://doi.org/10.1183/09031936.00043710. Epub 2010 Jul 1 PMID: 20595157
(1999) Clinical indications for non-invasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation--a consensus conference report. Chest 116(2):521–34. https://doi.org/10.1378/chest.116.2.521. PMID: 10453883
Nava S, Navalesi P, Conti G (2006) Time of non-invasive ventilation. Intensive Care Med 32(3):361–370. https://doi.org/10.1007/s00134-005-0050-0. Epub 2006 Feb 14 PMID: 16477416
Austin MA, Wills KE, Blizzard L et al (2010) Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ 18(341):c5462. https://doi.org/10.1378/chest.116.2.521. PMID: 20959284; PMCID: PMC2957540
Padkin AJ, Kinnear WJ (1996) Supplemental oxygen and nasal intermittent positive pressure ventilation. Eur Respir J 9(4):834–836. https://doi.org/10.1183/09031936.96.09040834. PMID: 8726952
Carteaux G, Lyazidi A, Cordoba-Izquierdo A et al (2012) Patient-ventilator asynchrony during noninvasive ventilation: a bench and clinical study. Chest 142(2):367–376. https://doi.org/10.1378/chest.11-2279. PMID: 22406958
Devlin JW, Al-Qadheeb NS, Chi A et al (2014) Efficacy and safety of early dexmedetomidine during noninvasive ventilation for patients with acute respiratory failure: a randomized, double-blind, placebo-controlled pilot study. Chest 145(6):1204–1212. https://doi.org/10.1378/chest.13-1448. PMID: 24577019
Lun CT, Chan VL, Leung WS et al (2013) A pilot randomized study comparing two methods of non-invasive ventilation withdrawal after acute respiratory failure in chronic obstructive pulmonary disease. Respirology 18(5):814–819. https://doi.org/10.1111/resp.12080. PMID: 23490403
Venkatnarayan K, Khilnani GC, Hadda V et al (2020) A comparison of three strategies for withdrawal of noninvasive ventilation in chronic obstructive pulmonary disease with acute respiratory failure: Randomized trial. Lung India 37(1):3–7
Sellares J, Ferrer M, Anton A et al (2017) Discontinuing noninvasive ventilation in severe chronic obstructive pulmonary disease exacerbations: a randomised controlled trial. Eur Respir J 50(1):1601448. https://doi.org/10.1183/13993003.01448-2016. PMID: 28679605
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadanandan, A.M., George, S., Mohamed Nambipunnilath, S. et al. A comparison of two weaning strategies for non-invasive ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. Ir J Med Sci (2024). https://doi.org/10.1007/s11845-024-03724-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11845-024-03724-3