Abstract
Background
Carpal tunnel syndrome (CTS) is the most widespread form of nerve entrapment neuropathy results from increase compression pressure of the median nerve at the wrist under the transverse carpal ligament.
Aims
To compare ultrasound (US)-guided median nerve steroid injection and pulsed radiofrequency (PRF) on pain intensity, functional status, and patient satisfaction in the treatment of CTS.
Methods
A total of 90 hands of 59 patients who underwent steroid injection at the level of proximal carpal tunnel or PRF for CTS were retrospectively analyzed. Demographic and clinical characteristics of the patients were recorded. The pain severity was assessed using the Numerical Rating Scale (NRS), and the functional status and clinical outcomes were assessed using the Boston Carpal Tunnel Questionnaire (BCTQ) before the procedure and at Week 1, Month 1, and Month 3 after the procedure. Time to pain relief was evaluated at week 1. Patient satisfaction was evaluated at Month 3.
Results
There was no significant difference in the NRS and BCTQ scores between the two treatment methods (p > 0.05 for both). In addition, a significant decrease in the NRS and BCTQ scores were detected at all follow-ups compared to baseline in treatment groups (p < 0.001). The mean time to pain relief was significantly shorter in the PRF group (p < 0.001). Patient satisfaction was similar at Month 3 between the treatment methods (p > 0.05).
Conclusions
Our study results suggest that both US-guided steroid injection to the median nerve and PRF are effective and safe methods in the short-term in the treatment of CTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) is the most widespread form of nerve entrapment neuropathy results from increase compression pressure of the median nerve at the wrist under the transverse carpal ligament [1, 2]. It accounts for approximately 90% of all entrapment neuropathies [3]. Its prevalence ranges from 2.7 to 5.8% in the adult population with a lifetime incidence of about 10% [4]. It is characterized by clinical signs and symptoms, paresthesia, numbness, tingling in the median nerve sensory distribution, and thenar atrophy [3]. These symptoms usually become worse at night or in the morning [5]. The diagnosis of CTS is made using patient history, clinical signs, and electrophysiological study findings [6].
Treatment of CTS includes analgesics, physical therapy modalities, splints, steroid injections, and surgery [7]. Local corticosteroid injection is a simple and effective method which is commonly used in daily practice with satisfactory short- and mid-term outcomes [4, 8, 9]. Several studies have shown that steroid injection under the guidance of ultrasound (US) is associated with a higher efficacy rate [10, 11]. There is an increasing number of evidence suggesting that steroid injection is a favorable alternative to surgery and that it can be used as a bridging therapy until surgery [12]. However, potential side effects of steroid injection limit its use in the clinical practice, and alternative methods have been sought in the treatment of CTS [13].
Pulsed radiofrequency (PRF) delivers electric bursts to the target nerve and/or tissue by preserving the adjacent structures and using a temperature not exceeding 42 °C at the tip of the electrode [14]. In recent years, the use of PRF has been increasingly adopted in the treatment of peripheral neuropathic pain [13]. However, there is a limited number of data regarding the PRF application to the median nerve in CTS. In a recent single-blind, randomized-controlled study, US-guided PRF in addition to night splint and night splint alone was compared and US-guided PRF yielded more favorable outcomes for pain relief in CTS patients [6].
To the best of our knowledge, there is no study comparing steroid injection to US-guided PRF in patients with CTS in the literature. In the present study, we, for the first time, aimed to compare the treatment efficacy of US-guided median nerve steroid injection and PRF in the treatment of CTS.
Materials and methods
Study design and study population
This single-center, retrospective study was conducted at the department of pain medicine of a tertiary care center between January 2018 and January 2020. A total of 131 patients (179 hands) who were diagnosed with CTS based on clinical symptoms, physical examination, and electrophysiological study findings and underwent US-guided corticosteroid + local anesthetic injection at a standard dose or PRF to the median nerve were screened. A total of 59 patients who fulfilled the inclusion criteria were included in the study (Fig. 1).
Inclusion criteria were as follows: age 18–65 years, presence of paresthesia and pain in median nerve distribution, severity of pain based on Numerical Rating Scale (NRS) ≥ 4, persistent symptoms for 3 months or longer, mild to moderate CTS confirmed by nerve conduction study, and having complete follow-up data for 3 months. Exclusion criteria were as follows: having severe CTS, previous CTS surgery, and having steroid injection or PRF to the median nerve two or more.
The presence of CTS was evaluated using nerve conduction studies, and classification was made based on the American Academy of Neurology Summary Statement [15]. Accordingly, (i) mild CTS was defined as the presence of prolonged median distal sensory latency (DSL) (> 3.5 ms) and normal median distal motor latency (DML), (ii) moderate CTS as prolonged DSL and DML (> 4.2 ms), and (iii) severe CTS as the absence of median sensory nerve action potential or absent compound muscle action potentials.
For all procedures, a written informed consent was obtained from all patients. The study protocol was approved by the institutional Ethics Committee (No: 2021/106). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Data collection and assessment
Demographic and clinical characteristics of the patients including age, sex, body mass index (BMI), symptom duration, symptom severity, affected side, and dominant hand and procedural data and treatment outcomes were retrieved from the hospital database. The pain severity was assessed using the NRS (0 to 10, verbal), and the symptom severity and functional status were assessed using the Boston Carpal Tunnel Questionnaire (BCTQ) before the procedure and at Week 1, Month 1, and Month 3 after the procedure. The BCTQ is a self-assessment tool which contains two subscales: Symptom Severity Scale (SSS) and Functional Status Scale (FSS) [16]. Both subscales have a total of 19 items including 11 in the SSS and 8 in the FSS. Each subscale is scored on a 5-point Likert scale from 1 (no or mild symptoms/best functional capacity) to 5 (severe symptoms/the worst functional capacity). Higher scores indicate more severe symptoms or poor functional capacity. Patient satisfaction was evaluated using a 5-point Likert scale (1 = very dissatisfied, 5 = very satisfied) at three months. Time to pain relief was obtained from the follow-up forms of the patients. It was defined as the day when the NRS score decreased by 40% or more relative to the pre-procedure NRS score and it was evaluated at week 1.
In addition, procedure-related complications such as bleeding, ulnar artery, ulnar nerve or median nerve injury, transient pain over the median nerve due to the compression caused by the injection fluid, and increased paresthesia and postoperative complications such as steroid-induced skin lesions and infection at the injection site were noted.
In our clinic, the same treatment algorithm is applied to both hands of patients who are diagnosed with bilateral CTS and scheduled for steroid injection or PRF. In addition, NRS pain scores, BCTQ scores, and patient satisfaction are separately assessed for each hand and documented.
Treatment protocol
The patient was placed in the sitting position with the forearm supinated and wrist slightly flexed (dorsal flexion). Both treatment methods were performed under the sterile conditions. The skin was cleaned with povidone-iodine, and the US probe was covered with a sterile drape. Both treatment methods were carried out by a single physician under the guidance of US (Esaote 10–18 MHz linear probe, MyLab 30 Gold, Italy) vial in-plane technique to the median nerve using an ulnar approach at the level of proximal carpal tunnel [17].
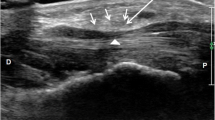
During the procedure, the transducer was inserted to the proximal carpal tunnel via the transverse route. The median nerve was detected beneath the pisiform, ulnar artery, ulnar nerve, flexor tendons, and flexor retinaculum (Fig. 2). The needle was placed parallel to the transducer using the in-plane technique from the ulnar side in both treatment methods. The median nerve was reached through the ulnar artery and ulnar nerve. After bleeding control for steroid injection, a mixture containing dexamethasone 8 mg and 0.5 cc of bupivacaine 0.5% was injected under the median nerve. The median nerve was separated from the underlying structures by hydrodissection during the injection (Fig. 2).
PRF was performed with a NeuroTherm NT1000 (NeuroTherm, Inc., Middleton, MA, USA) radiofrequency generator. The median nerve was visualized using the transverse view of the proximal carpal tunnel through US, as described above. A 22-gauge, 50-mm radiofrequency cannula with a 5-mm active tip was advanced through the in-plane technique, and the median nerve was reached advancing through the ulnar nerve and ulnar artery. The cannula was placed very proximate to the superior of the median nerve (Fig. 3). After positioning the cannula, sensory and motor tests were performed. Using selective stimulation of sensory fibers (50 Hz) below 0.5 V, paresthesia occurred in the distal first four fingers, as confirmed by the patient. During motor stimulation, contraction of the thenar muscles was observed at below 1 V. After the sensory and motor stimulation, the PRF cycles were applied for 120 s with a pulse frequency of 2 Hz and pulse width of 20 ms at 42 °C [6].
All patients were recommended using a wrist splint for 3 months after the procedure. The patients were discharged after no complications were observed in the first hour after the treatment.
Statistical analysis
Descriptive values of the data obtained were calculated as mean, standard deviation (SD), median (25th and 75th quartiles), and mean rank. The differences between two treatment groups (steroid and pulse rf) and the between four periods, and also group X period interaction effects in terms of NRS, SSS, and FDS were evaluated by using F1-LD-F1 design and adjustment pairwise comparisons. The F1-LD-F1 is non-parametric factorial design and takes different names according to different number of factors. The first “F1” is called the between-subjects factor and shows the difference between the treatments applied (treatment effect). The second “F1” is called the within-subjects factor and, in this study, it refers to the difference between the periods. This model also contains interaction effect (treatment X period).
The relationships between the categorical variables were examined with the Pearson chi-square analysis. Independent samples t-test was used to compare the two groups in terms of mean symptom duration and in terms of the average day the pain began to subside. The Mann–Whitney U-test was used to compare the two groups in terms of patient satisfaction. All analyses were performed in R (v.4.1.1) and “nparLD” library used for non-parametric repeated F1-LD-F1 designs. A p value of < 0.05 was considered statistically significant.
Results
Of a total of 90 hands of 59 patients (54 females and 5 males) were included in the study. Twenty-nine patients (45 hands) were included in the steroid injection group, while 30 patients (45 hands) were included in the PRF group (Fig. 1). The mean age was 49.17 (± 8.97) years in the steroid injection group and 47.97 (± 9.48) years in the PRF group (p > 0.05). The mean symptom duration was 10.02 (± 5.99) months in the steroid injection group and 9.11 (± 5.70) months in the PRF group (p > 0.05). Demographic and clinical characteristics of the patients were similar between the groups (Table 1).
There was no significant difference in the NRS and BCTQ (SSS, FSS) between the treatment groups at different time points. Similarly, there was no significant difference in the size and direction of the measurements at all time points, indicating no significant interaction between the treatment and time points for all scales (p > 0.05 for all) (Table 2).
Irrespective of the treatment applied, there was a significant decrease in the NRS, SSS, and FSS scores at all time points (p < 0.001) (Table 2). These results were available for both treatment groups, as there was no significant interaction between the treatment and time points.
Patient satisfaction was similar at three months between the treatment methods (p > 0.05) (Table 3). The mean time to pain relief was significantly shorter in the PRF group (1.41 ± 0.62) than the steroid injection group (2.56 ± 1.14) (p < 0.001) (Table 3).
None of the patients experienced procedure-related complications. Although three patients in the steroid injection group had fullness at the thenar side of the hand and distal fingers due to compression of the injection agent, this complication was transient and resolved spontaneously within 1 h.
Discussion
In our study, we compared the treatment efficacy of US-guided median nerve steroid injection and PRF in the treatment of CTS. Our study showed that both methods yielded a significant improvement in the NRS and BCTQ scores at all follow-ups compared to baseline. At 3 months of the treatment, the NRS pain scores and BCTQ scores were similar between the groups. In addition, patient satisfaction levels at the third month of follow-up were similar in both treatment methods. The mean time to pain relief was significantly shorter in the PRF group.
Steroid injection to the median nerve has been proven to be effective in CTS in the short term [4, 8, 9, 18,19,20]. In a double-blind, randomized-controlled study, Hsu et al. compared the effect of US-guided steroid injections (10 mg or 40 mg triamcinolone acetonide) in mild to moderate CTS patients [18]. They observed a significant and similar improvement in the pain and BCTQ scores at 12 weeks in both groups. In another double-blind, randomized-controlled study, Karimzadeh et al. compared the efficacy and safety of two local corticosteroid injections (triamcinolone versus methylprednisolone) at two different doses (20 versus 40 mg) in mild to moderate CTS patients [19]. The authors reported a significant and similar improvement in the pain scores at three months in both groups. Moghtaderi et al. applied 4 mg dexamethasone injection to the median nerve of 20 pregnant women with carpal tunnel syndrome [21]. They reported significant improvement in pain and electrophysiological findings at 3-week follow-up. In the literature, it is observed that injection of different steroids at different doses is effective in CTS [18, 19]. Similarly, in our study, the NRS pain and BCTQ scores were significantly decreased at 3 months in the steroid injection group, consistent with the literature.
Although the exact mechanism of steroid injection in CTS has not been fully elucidated yet, it theoretically exerts its potent anti-inflammatory effects by decreasing inflammation and edema in the nerves and tendons, improving the spatial relationship between the carpal tunnel and median nerve and tendons, and reducing the intracarpal pressure [7, 9, 22]. In addition, steroids combined with local anesthetics contribute to the pain relief through its anti-inflammatory effects [23]. In the current study, the mean time to pain relief was significantly longer in the steroid injection group than the PRF group that can be attributed to the late onset of the anti-inflammatory effects of the steroid injection.
Recurrent exacerbations of symptoms are commonly encountered in CTS patients and treatment usually requires multiple injections. Corticosteroids can temporarily relieve painful symptoms in these patients; however, there is a concern regarding the possible side effects of these agents [18]. The most common side effects of corticosteroid injections include skin depigmentation and atrophy, disturbance in menstruation, flushing, transient hyperglycemia, and chronic granulomatous inflammatory reactions [18, 24]. Due to the possible side effects of steroids, new treatment modalities are needed in patients with CTS [13].
Several studies have demonstrated that PRF is an effective, safe, non-destructive, and reproducible alternative with long-term outcomes in painful conditions [6]. However, data regarding the PRF application to the median nerve in CTS are limited. In a case report, PRF to the wrist was applied to the cubital fossa level, not the carpal tunnel level in a patient with CTS who had persistent complaints and severe pain despite two surgical interventions and a revision surgery [25]. Three cycles of PRF to ventral, dorsal, and medial aspects of the median nerve were performed under the guidance of US over 90 s for each dose. A 70% reduction in pain was achieved during a 12-week follow-up period. In a randomized-controlled study, Chen et al. compared the pain and BCTQ scores in CTS patients receiving a single-dose US-guided PRF (120 s) in addition to night splint (intervention group) and night splint alone (control group) [6]. The authors reported a statistically significant decrease in the pain score and BCTQ scores in all time points in the PRF group compared to the control group. In our study, the PRF was similarly applied to the ventral aspect of the median nerve for 120 s. Although there is a number of studies showing the effectiveness of PRF in painful conditions, the exact mechanism of PRF has not been fully understood yet [26]. Some authors have advocated that PRF exerts its effect through neuromodulation by activating descending adrenergic and serotonergic pathways and inhibiting the stimulation of nociceptive C fibers [27]. In addition, L5 dorsal root ganglion PRF treatment applied to rats with sciatic nerve injury has been shown to inhibit various proinflammatory cytokines (such as TNF-α and Interleukin-6) [28].
Interventional procedures to the median nerve can be performed using a blind technique or under the guidance of US in CTS. Although these procedures are safe and well-tolerated, tendon rupture, arterial occlusion, and median nerve injury can be rarely seen in injections through the blind technique [12, 29]. In recent years, US-guided steroid injections have been increasingly used owing to high accuracy and low median nerve injury rates [6]. Additionally, US allows an accurate visualization of the delivery of the injection agent with less or no discomfort for the patient [11]. Recent studies have demonstrated that steroid injections to the median nerve under the US guidance yield a higher rate of effectiveness [10, 11, 30]. In our clinical practice, steroid injections to the median nerve and PRF are performed under the guidance of US in the treatment of CTS. In our study, none of the patients experienced any serious complications. This can be attributed to the accurate visualization of the median nerve and adjacent structures through US. In the present study, three patients in the steroid injection group had fullness at the injection site which can be due to the transient compression on the median nerve via the injection agent.
There are some limitations of this study. The most important limitation is the retrospective design. Other limitations are small sample size and short-term follow-up. Nevertheless, this study is the first to compare the US-guided steroid injection and PRF in CTS patients and is valuable, as it provides additional information to the body of knowledge on this topic in the literature.
In conclusion, both US-guided steroid injection to the median nerve and PRF are effective and safe methods in the short-term in the treatment of CTS. Considering the side effects of steroid injection, PRF can be an important alternative. However, further prospective, randomized-controlled studies with long-term follow-up are needed to gain a better understanding of the effects of PRF in the long-term.
References
Ly-Pen D, Andreu JL, Millán I et al (2020 )Long-term outcome of local steroid injections versus surgery in carpal tunnel syndrome: observational extension of a randomized clinical trial Hand (NY) 1558944720944263 https://doi.org/10.1177/1558944720944263
Wang JC, Liao KK, Lin KP et al (2017) Efficacy of combined ultrasound-guided steroid injection and splinting in patients with carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil 98(5):947–956
Aroori S, Spence RA (2008) Carpal tunnel syndrome. Ulster Med J 77(1):6–17
Babaei-Ghazani A, Nikbakht N, Forogh B et al (2018) Comparison between effectiveness of ultrasound-guided corticosteroid injection above versus below the median nerve in mild to moderate carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil 97(6):407–413. https://doi.org/10.1097/PHM.0000000000000877
Karadas O, Tok F, Ulas UH et al (2011) The effectiveness of triamcinolone acetonide vs. procaine hydrochloride injection in the management of carpal tunnel syndrome: a double-blind randomized clinical trial. Am J Phys Med Rehabil 90(4):287–292. https://doi.org/10.1097/PHM.0b013e31820639ec
Chen LC, Ho CW, Sun CH et al (2015) Ultrasound-guided pulsed radiofrequency for carpal tunnel syndrome: a single-blinded randomized controlled study. PLoS ONE 10(6):e0129918. https://doi.org/10.1371/journal.pone.0129918
Padua L, Coraci D, Erra C et al (2016) Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol 15(12):1273–1284. https://doi.org/10.1016/S1474-4422(16)30231-9
Roh YH, Hwangbo K, Gong HS et al (2019) Comparison of ultrasound-guided versus landmark-based corticosteroid injection for carpal tunnel syndrome: a prospective randomized trial. J Hand Surg Am 44(4):304–310. https://doi.org/10.1016/j.jhsa.2019.02.007
Armstrong T, Devor W, Borschel L et al (2004) Intracarpal steroid injection is safe and effective for short-term management of carpal tunnel syndrome. Muscle Nerve 29(1):82–88
Eslamian F, Eftekharsadat B, Babaei-Ghazani A et al (2017) A randomized prospective comparison of ultrasound-guided and landmark-guided steroid injections for carpal tunnel syndrome. J Clin Neurophysiol 34(2):107–113. https://doi.org/10.1097/WNP.0000000000000342
Karaahmet OZ, Gurcay E, Kara M et al (2017) Comparing the effectiveness of ultrasound-guided versus blind steroid injection in the treatment of severe carpal tunnel syndrome. Turk J Med Sci 47(6):1785–1790. https://doi.org/10.3906/sag-1704-97
Makhlouf T, Emil NS, Sibbitt WL et al (2014) Outcomes and cost-effectiveness of carpal tunnel injections using sonographic needle guidance. Clin Rheumatol 33(6):849–858. https://doi.org/10.1007/s10067-013-2438-5
Chang MC (2018) Efficacy of pulsed radiofrequency stimulation in patients with peripheral neuropathic pain: a narrative review. Pain Physician 21(3):E225-e234
Chua NH, Vissers KC, Sluijter ME (2011) Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir 153(4):763–771. https://doi.org/10.1007/s00701-010-0881-5
So H, Chung VCH, Cheng JCK et al (2018) Local steroid injection versus wrist splinting for carpal tunnel syndrome: A randomized clinical trial. Int J Rheum Dis 21(1):102–107
Sezgin M, Incel NA, Serhan S et al (2006) Assessment of symptom severity and functional status in patients with carpal tunnel syndrome: reliability and functionality of the Turkish version of the Boston Questionnaire. Disabil Rehabil 28(20):1281–1285
Kim DH, Jang JE, Park BK (2013) Anatomical basis of ulnar approach in carpal tunnel injection. Pain Physician 16(3):E191-198
Hsu PC, Liao KK, Lin KP et al (2020) Comparison of corticosteroid injection dosages in mild to moderate idiopathic carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil 101(11):1857–1864. https://doi.org/10.1016/j.apmr.2020.06.018
Karimzadeh A, Bagheri S, Raeissadat SA et al (2019) The comparison of the effectiveness between different doses of local methylprednisolone injection versus triamcinolone in Carpal Tunnel Syndrome: a double-blind clinical trial. J Pain Res 12:579–584
Chesterton LS, Blagojevic-Bucknall M, Burton C et al (2018) The clinical and cost-effectiveness of corticosteroid injection versus night splints for carpal tunnel syndrome (INSTINCTS trial): an open-label, parallel group, randomised controlled trial. Lancet 392(10156):1423–1433
Moghtaderi AR, Moghtaderi N, Loghmani A (2011) Evaluating the effectiveness of local dexamethasone injection in pregnant women with carpal tunnel syndrome. J Res Med Sci 16(5):687–690
Buntragulpoontawee M, Chang KV, Vitoonpong T et al (2020) The effectiveness and safety of commonly used injectates for ultrasound-guided hydrodissection treatment of peripheral nerve entrapment syndromes: a systematic review. Front Pharmacol 11:621150. https://doi.org/10.3389/fphar.2020.621150
Salman Roghani R, Holisaz MT, Tarkashvand M et al (2018) Different doses of steroid injection in elderly patients with carpal tunnel syndrome: a triple-blind, randomized, controlled trial. Clin Interv Aging 13:117–124
Brinks A, Koes BW, Volkers AC et al (2010) Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord 11:206
Haider N, Mekasha D, Chiravuri S et al (2007) Pulsed radiofrequency of the median nerve under ultrasound guidance. Pain Physician 10(6):765–770
Mercadal B, Vicente R, Ivorra A (2020) Pulsed radiofrequency for chronic pain: In vitro evidence of an electroporation mediated calcium uptake. Bioelectrochemistry 136:107624.
Hagiwara S, Iwasaka H, Takeshima N et al (2009) Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain 13(3):249–252
Vallejo R, Tilley DM, Williams J et al (2013) Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician 16(5):E601-613
McConnell JR, Bush DC (1990) Intraneural steroid injection as a complication in the management of carpal tunnel syndrome. A report of three cases. Clin Orthop Relat Res 250:181–184
Ustun N, Tok F, Yagiz AE et al (2013) Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: A single-blind randomized prospective study. Am J Phys Med Rehabil 92(11):999–1004. https://doi.org/10.1097/PHM.0b013e31829b4d72
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alp Eren Çelenlioğlu, Hanzade Aybüke Ünal Artık, and Gülen Güler. The manuscript was written by Alp Eren Çelenlioğlu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Celenlioglu, A.E., Unal-Artık, H.A. & Guler, G. Comparison of ultrasound-guided pulsed radiofrequency versus steroid injection in the treatment of carpal tunnel syndrome. Ir J Med Sci 191, 2751–2757 (2022). https://doi.org/10.1007/s11845-022-02923-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-02923-0