Abstract
Introduction
Mutations in mitochondrial DNA (mtDNA) are the most important causes for Leber’s hereditary optic neuropathy (LHON). Of these, three primary mtDNA mutations account for more than 90% cases of this disease. However, to date, little is known regarding the relationship between mitochondrial tRNA (mt-tRNA) variants and LHON.
Aim
In this study, we aimed to investigate the association between mt-tRNA variants and LHON.
Methodology
One hundred thirty-eight LHON patients lacking three primary mutations (ND1 3460G > A, ND4 11778Gxs > A, and ND6 14484 T > C), as well as 266 controls were enrolled in this study. PCR-Sanger sequencing was performed to screen the mt-tRNA variants. Moreover, the phylogenetic analysis, pathogenicity scoring system, as well as mitochondrial functions were performed.
Results
We identified 8 possible pathogenic variants: tRNAPhe 593 T > C, tRNALeu(UUR) 3275C > T, tRNAGln 4363 T > C, tRNAMet 4435A > G, tRNAAla 5587 T > C, tRNAGlu 14693A > G, tRNAThr 15927G > A, and 15951A > G, which may change the structural and functional impact on the corresponding tRNAs, and subsequently lead to a failure in tRNA metabolism. Furthermore, significant reductions in mitochondrial ATP and MMP levels and an overproduction of ROS were observed in cybrid cells containing these mt-tRNA variants, suggesting that these variants may lead to mitochondrial dysfunction which was responsible for LHON.
Conclusion
Our study indicated that mt-tRNA variants were associated with LHON, and screening for mt-tRNA variants were recommended for early detection, diagnosis, and prevention of maternally inherited LHON.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leber’s hereditary optic neuropathy (LHON) was a typical mitochondrial disorder, which was associated with a rapid, painless, acute, or subacute bilateral visual loss in young adults [1, 2]. It has been reported that 1:8500 individuals harbored a primary LHON-causing mutation and 1:31,000 experienced visual loss as a result of LHON in the North East of England [3]. The typical features in this disorder included the primary degeneration of retinal ganglion cells (RGCs) accompanied by ascending optic atrophy [4, 5]. Mutations in mitochondrial DNA (mtDNA) had been identified to contribute to the pathogenesis of LHON though to varying degrees [6,7,8]; in particular, three primary mutations—ND1 3460G > A, ND4 11778G > A, and ND6 14484 T > C—had been reported to account for more than 90% of LHON cases [9]. Incomplete penetrances and gender bias were two features of LHON, but the underlying molecular mechanisms for the onset of these two features had not been fully understood.
Human mitochondrial tRNA (mt-tRNA) was a short, non-coding RNA, which constituted approximately 4 ~ 10% of all nuclear RNAs [10]. In fact, human mitochondrial genome encoded 13 peptides for oxidative phosphorylation (OXPHOS) system, as well as 22 tRNAs for mitochondrial translation [11]. Variations in mt-tRNAs, either in a sporadic status or maternally inherited, constituted the most common mtDNA alternations that were associated with human disorders [12]. Most recently, several LHON-associated mt-tRNA variants have been reported, such as tRNAMet 4435A > G [13], tRNAThr 15927G > A [14], and tRNAAla 5601C > T [15]. However, the pathophysiology of mt-tRNA variants in LHON was not fully understood.
To investigate the role of mitochondrial genetic defects in the pathogenesis of LHON, we recently carried out a systematic and extended mutational screening of 22 mt-tRNA genes in a cohort of 138 patients with LHON lacking three primary mutations and 266 control subjects. PCR-Sanger sequencing identified 27 nucleotide alternations in 13 mt-tRNA genes. Through the application of phylogenetic conservation analysis and tRNA structural-function prediction, 8 mt-tRNA variants were found to be pathogenic. To see the contributions of mtDNA genetic background to mt-tRNA variants, we sequenced the entire mitochondrial genomes of the probands carrying one of these pathogenic/likely pathogenic mt-tRNA variants. In addition, the trans-mitochondrial cybrid cells were used to analyze the mitochondrial function.
Materials and methods
Study population
A total of 138 genetically unrelated LHON patients lacking the known LHON-associated mtDNA mutations (ND1 3460G > A, ND4 11778G > A, and ND6 14484 T > C) participated in this study; these subjects were recruited from the Department of Ophthalmology, the First Affiliated Hospital of Nanjing Medical University. Moreover, 266 healthy subjects, including 130 males and 136 females, were obtained from the Healthy Examination Center of the First Affiliated Hospital of Nanjing Medical University. The inclusion criteria of patients for this study, included acute or subacute visual loss in both eyes simultaneously or sequentially within 1 year, clinical evidence of relatively symmetric optic neuropathies with central visual loss, and age less than 50 years at onset of visual symptoms. Patients who had hearing loss, cardiovascular or muscle diseases will be excluded. Furthermore, 266 controls were healthy individuals, without any clinical diseases; the subjects who have a family history of mitochondrial diseases will be excluded.
This study was in compliance with the Declaration of Helsinki, and informed consent, blood samples, and clinical evaluations were obtained from all participating subjects, under the protocol approved by the First Affiliated Hospital of Nanjing Medical University.
Clinical examinations
All patients and control subjects were received the following ophthalmic examinations including the visual acuity, visual field examination, visually evoked potentials, and fundus photography. The degree of visual impairment was defined according the following standard: normal, > 0.3; mild, 0.3 to 0.1; moderate, < 0.1 to 0.05; severe, < 0.05 to 0.02; and profound, < 0.02.
DNA preparation, PCR amplification of mt-tRNA genes, and sequencing
The DNA was extracted from 2 mL peripheral blood using a QIAamp DNA Blood Minikit (Qiagen Chins Co., Ltd, China). The DNA concentrations > 1.0 ng/µl were employed for the next experiments.
For screening the mt-tRNA variants, subjects’ DNA (138 LHON patients and 266 controls) fragments spanning 22 mt-tRNA genes were amplified by PCR using the primers as described in Table 1. The PCR reagents (Takara Bio, Inc.) were as follows: 200 µM dNTPs, 2 µl 10 × PCR buffer (10 × 0.2 µl Taq DNA polymerase and 15 mmol/l Mg2+). Subsequently, the PCR products were purified and sequenced using the ABI PRISM™ 3700 machine (Applied Biosystems; Thermo Fisher Scientific, Inc.), as previously described [16]. The sequence data was handled by the DNASTAR program (DNASTAR Inc., Madison, USA). Mt-tRNA variants were scored relative to the revised Cambridge Reference Sequence (rCRS, GenBank Accessible No. NC_012920.1) [17].
Phylogenetic analysis
We carried out a phylogenetic conservation analysis for the identified mt-tRNA variants, as described previously [18]. The conservation index (CI) was then calculated by comparing the human nucleotide variants with the other 14 vertebrates. These species were as follows: Mus musculus, Rattus norvegicus, Cebus albifrons, Pongo pygmaeus, Bos taurus, Sus scrofa, Phoca vitulina, Kogia breviceps, Gorilla gorilla, Orycteropus afer, Zaglossus bruijni, Ornithorhynchus anatinus, Dromiciops gliroides, and Microtus kikuchii.
Assessment of the pathogenicity
To further assess the pathogenicity of mt-tRNA variants, we used the following criteria: first, the variant itself occurred < 1% in control subjects; second, the CI ≥ 75%, as proposed by Ruiz-Pesini and Wallace [19]; third, the variant may have functional impact on mt-tRNA genes; finally, the pathogenicity scoring system which was originally proposed by Yarham et al. [20], the variants were classified as “definitely pathogenic” with a score of ≥ 11 points, “possibly pathogenic” with a score of 7–10 points and a “neutral polymorphism” with a score of ≤ 6 points. Patients carrying potential pathogenic mt-tRNA variants that met these criteria were selected for further molecular and biochemical analysis.
Mutational screening for the complete mitochondrial genomes in probands carrying putative pathogenic mt-tRNA variants
To see the contributions of mitochondrial genetic background to LHON expression, we screened the entire mtDNA genes in 23 probands carrying one of the putative pathogenic LHON-associated tRNA variants. Briefly, 24 overlapping primers were used to amplify the whole mtDNA genes, as described previously [16]. The sequence results were compared with the rCRS (GenBank Accession No. NC_012920.1) [17], as described above.
Determining the mitochondrial haplogroups
The mtDNA sequences of the 23 subjects carrying the putative pathogenic mt-tRNA variants were assigned to the Asian mitochondrial haplogroups proposed by Kong et al. [21].
Cell lines
Lymphoblastoid cell lines were immortalized by transformation with the EB virus, as described previously [22]. Cell lines derived from the LHON patients carrying tRNAPhe 593 T > C, tRNALeu(UUR) 3275C > T, tRNAGln 4363 T > C, tRNAMet 4435A > G, tRNAAla 5587 T > C, tRNAGlu 14693A > G, tRNAThr 15927G > A, and 15951A > G variants, as well as 10 healthy subjects without these variants were grown in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS).
Analysis of ATP production
The mitochondrial ATP levels of LHON patients with pathogenic mt-tRNA variants and control subjects were analyzed by using the Cell Titer-Glo® Luminescent Cell Viability Assay kit (Promega), according to the protocol provided by the manufacturer [23]. Briefly, the assay buffer and substrate were equilibrated to room temperature, and the buffer was transferred to and gently mixed with the substrate to obtain a homogeneous solution. After a 30-min equilibration of the cell plate to room temperature, 100 µl of the assay reagent was added into each well with 20,000 cells and the content was mixed for 2 min on an orbital shaker to induce cell lysis. After 10-min incubation in room temperature, the luminescence was read on a microplate reader (Syneregy H1, Bio-Tek).
ROS analysis
The ROS level of the LHON patients carrying these mt-tRNA variants and controls were analyzed by fluorometry, as described in a previous study [24]. Cells were incubated with the fluorescent probe (5 × 10−6 mol/L) 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) for 30 min. The ROS production was assessed by fluorimetry using a Synergy Mx plate reader (BioTek Instruments, Winooski, VT). Subsequently, it was assessed using a fluorescence microscope (IX81, Olympus, Hamburg, Germany) coupled with the static cytometry software “ScanR” version 2.03.2 (Olympus).
Determining the MMP
Loss of mitochondrial membrane potential (MMP) was implicated to be involved in apoptosis [25]. For evaluating MMP, cells were plated onto 96-well cell culture plate overnight in growth medium. JC-10 dye-loading solution was added for 30 min at 37 °C, 5% CO2. Alternatively, plated cells were preincubated with 10 µM of the protonophore uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP) for 30 min at 37 °C, 5% CO2 prior to staining with JC-10 dye. The fluorescent intensities for both J-aggregates and monomeric forms of JC-10 were measured at Ex/Em = 490/530 and 490/590 nm with a microplate reader, as described in a previous study [23].
Statistical analysis
The SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. Student t test was used to calculate the P values; moreover, the Fisher’s exact test was carried out to evaluate the difference in mt-tRNA variants between LHON and controls; the P < 0.05 was regarded as having statistical significance.
Results
Clinical information for the LHON patients and controls
The study samples lacking the known LHON-related three primary mutations (ND1 3460G > A, ND4 11778G > A, and ND6 14484 T > C) consisted of 88 males and 50 females. All participants were Han Chinese subjects from separate families who came to the Department of Ophthalmology, the First Affiliated Hospital of Nanjing Medical University. Clinical examination revealed that 55 subjects exhibited profound visual loss, 32 patients had severe visual impairment, and 51 patients suffered from moderate visual impairment, whereas the 266 controls had normal vision. The age at onset of LHON ranged from 3 to 39 years, with an average of 17 years. However, these LHON patients did not harbor other clinical abnormalities, such as hearing loss, diabetes, cancer, or other mitochondrial diseases.
Mutational screening for LHON-associated mt-tRNA variants
To analyze the frequencies of LHON-related mt-tRNA variants, the DNA fragments spanning 22 mt-tRNA genes from 138 LHON patients and 266 control subjects were PCR amplified and analyzed by Sanger sequencing. Compared with the rCRS [17], we identified 27 mt-tRNA variants, as shown in Table 2. All of these genetic variants were well-known mutational hot spots, and none of these variants could be classified as “novel” [26]. These variants were as follows: tRNAPhe 593 T > C and 628C > T, tRNAVal 1647 T > C and 1655A > G, tRNALeu(UUR) 3275C > T and 3290 T > C, tRNAGln 4363 T > C, 4386 T > C and 4395 T > C, tRNAMet 4435A > G and 4454 T > C, tRNACys 5802 T > C and 5821G > A, tRNASer(UCN) 7492C > T and 7498C > T, tRNALys 8343A > G, tRNAArg 10454 T > C, tRNAGlu 14693A > G, tRNAThr 15889 T > C, 15896A > G, 15927G > A, 15930G > A, 15941 T > C and 15951A > G, tRNAPro 16000C > T. Moreover, we noticed that there were 7 variants only presented in controls but absent in LHON patients: tRNAVal 1647 T > C, tRNAGln 4386 T > C, tRNACys 5821G > A, tRNASer(UCN) 7498C > T, tRNAThr 15889 T > C, 15896A > G, and 15930G > A, suggesting that they were neutral polymorphisms.
To evaluate the potential pathogenicity of mt-tRNA variants, we used the following criteria: (a) the variant presented in < 1% of controls, (b) the CI ≥ 75%, (c) potential structural and functional alterations, and (d) the pathogenicity scoring system [20]. Since most mt-tRNA from all domains of life had a highly conserved cloverleaf structure, consisting of acceptor arm, D-arm, anticodon stem, variable region, and TψC loop [27]. As shown in Fig. 1 and Table 2, 5 variants were localized at acceptor arm, 5 variants occurred at D-arm, 5 variants were located at anticodon stem, 1 variant occurred at variable region, and 10 variants occurred at TψC loop. Moreover, the 4363 T > C variant disrupted the T-A base-pairing, by contrast, the 4395 T > C variant created a novel C-G base-pairing in tRNAGln, while the 5802 T > C and 5821G > C variants abolished the A-T and C-G base-pairings in tRNACys, respectively. In addition, the 15889 T > C, 15927G > A, and 15951A > G variants disrupted the T-A, C-G, and T-A base-pairings in tRNAThr, respectively. Furthermore, phylogenetic conservation analysis was performed by comparing the human tRNA nucleotide variants with those in 14 other vertebrates. As shown in Fig. 2 and Table 2, the CIs among these variants ranged from 11.5% (tRNAPro 16000C > T) to 100% (tRNALeu(UUR) 3275C > T, tRNAMet 4435A > G, and tRNAThr 15896A > G). Furthermore, the Fisher’s exact test was used to evaluate the difference in mt-tRNA variants between LHON patients and controls, the variants with P < 0.05 were tRNAPhe 593 T > C, tRNALeu(UUR) 3275C > T, tRNAMet 4435A > G, tRNAAla 5587 T > C, tRNAGlu 14693A > G, tRNAThr 15927G > A, and 15951A > G, while the P values of other variants > 0.05.
Clinical features of 23 probands carrying one of the putative pathogenic mt-tRNA variants
As shown in Table 3, 23 probands with LHON carried the pathogenic/likely pathogenic tRNA variants, accounting for 16.67% of cases in our cohort. The age at onset of LHON in these subjects ranged from 7 to 36 years. Moreover, comprehensive medical histories showed that none of these subjects’ relatives suffered from LHON. Notably, these subjects carrying the putative pathogenic mt-tRNA variants did not manifestate diabetes, deafness, or cardiovascular diseases. There were variable degrees of vision loss among these probands, 3 patients suffered from profound vision loss, 2 subjects had severe vision loss, 7 individuals had moderate vision loss, and 11 patients had mild vision loss.
Analysis of entire mitochondrial genomes of 23 LHON patients carrying one of pathogenic/likely pathogenic variants
The affected subjects carrying one of pathogenic/likely pathogenic tRNA variants were further examined to ascertain if there were any other functional mtDNA variants. As shown in Table 3, 6 variants were identified to be coexisted with these potential pathogenic tRNA variants, but we failed to detect any LHON patients carrying more than one mt-tRNA variant. Among them, the ND1 4216 T > C (p.Y304H) variant coexisted with tRNAPhe 593 T > C variant. In fact, the 4216 T > C variant changed the conserved thymine to cytosine at position 304, occurred at ND1 gene which encoded a key member of Complex I of electron transport chain (ETC), which was regarded as a risk factor for maternally inherited diabetes according to a recent study [28]. In addition, ND1 3394 T > C and 3308 T > C coexisted with tRNALeu(UUR) 3275C > T and tRNAGlu 14693A > G variants, respectively. Interestingly, the 3394 T > C (p.Y30H) was localized at extremely conserved nucleotide of ND1 gene and was involved in the pathogenesis and progression of LHON [29]. While the well-known 3308 T > C (p.M1T) variant resulted in the replacement of the first amino acid, translation-initiating methionine with a threonine in ND1 [30]. Furthermore, the 3308 T > C variant located in two nucleotides adjacent to the 3′ end of tRNALeu(UUR). Thus, this variant caused an alteration on the processing of the H-strand polycistronic RNA precursors or the destabilization of ND1 mRNA [31]. Similarly, the 12338 T > C (p.M1T) variant resulted in the replacement of the first amino acid, translation-initiating methionine with a threonine in ND5 polypeptide; thus, the truncated ND5 mRNA was expected to be shortened by two amino acids [32]. Moreover, 12338 T > C variant located in two nucleotides adjacent to the 3′ end of tRNALeu(CUN) [32]. Therefore, the 12338 T > C variant altered the respiratory function, as well as the processing of RNA precursors, thereby leading to a reduction in tRNALeu(CUN) level [33]. Furthermore, the 11696G > A (p.V313I) variant was identified in patient harboring the tRNAGln 4363 T > C variant. The G-to-A transition at position 11,696 (11696G > A) in ND4, caused by the substitution of an isoleucine for valine at amino acid position 313 [34]. In fact, the 11696G > A variant had been associated with LHON in a large Dutch family [35], and acted as a risk factor for increasing the penetrance and expressivity of deafness-associated 12S rRNA 1555A > G mutation in a Chinese family [36], while the 14502 T > C (p.I58V) caused the substitution of a highly conserved isoleucine for valine at position 58 in ND6 [37]. Previous studies had suggested that the 14502 T > C variant may play a synergistic role with the primary mutations (14484 T > C and 11778G > A) [38]. According to their distinct sets of polymorphisms, the mtDNA of these probands belonged to Eastern Asian haplogroups D4j, F2, D4h, F3, G2a, B4c1b, M12, F1a, B5, M7b, M10, D4, D4c1, G1a1, F2a, D4b2b, B5b1, F3b, Z4a, G3a2, M71, and D4b2b2, respectively (Table 3) [21].
The pathogenicity scoring system
We next utilized the pathogenicity scoring system [20] to evaluate the status of tRNAPhe 593 T > C, tRNALeu(UUR) 3275C > T, tRNAGln 4363 T > C, tRNAMet 4435A > G, tRNAAla 5587 T > C, tRNAGlu 14693A > G, tRNAThr 15927G > A, and 15951A > G variants, which showed the statistical significance between LHON group and controls. As shown in Table 4, we noticed that the total scores of 15927G > A and 15951A > G were 12 and 9 points, and belonging to “definitely pathogenic” and “possibly pathogenic” at this stage. Similarly, the total scores of 593 T > C, 3275C > T, 4435A > G, 5587 T > C, and 14693A > G variants were 11, 11, 12, 13, and 10 points, respectively.
Reduced in mitochondrial ATP production
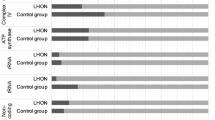
Because mitochondria generated ATP via OXPHOS, defects in ATP synthesis were found to be an important cause for mitochondrial dysfunction [39]. For this purpose, we constructed the cybrid cells containing the LHON-associated putative pathogenic variants (tRNAPhe 593 T > C, tRNALeu(UUR) 3275C > T, tRNAGln 4363 T > C, tRNAMet 4435A > G, tRNAAla 5587 T > C, tRNAGlu 14693A > G, tRNAThr 15927G > A, and 15951A > G), as well as 10 healthy subjects lacking these variants, according to the study as described in elsewhere [22]. As shown in Fig. 3A, we found that LHON patients carrying these variants had a lower level of ATP when compared with the controls (P = 0.0031).
ROS increased
The levels of ROS from LHON patients carrying the pathogenic mt-tRNA variants and controls were determined by using the fluorometry. As shown in Fig. 3B, approximately ~ 50% in the increasing ROS production when compared with the controls (P = 0.0020).
MMP decreased
The MMP generated by proton pumps was an essential component in the process of energy storage during OXPHOS [40]. To see whether mt-tRNA pathogenic variants affected mitochondrial functions, we analyzed MMP in cybrids cells with and without mt-tRNA pathogenic variants. As shown in Fig. 3C, we noticed that MMP decreased significantly in mutant cell lines when compared with the controls (P = 0.0042).
Discussion
Dysfunction of mitochondrial activities was frequently associated with LHON [41]. Mt-tRNA point mutations typically caused a loss of mt-tRNA stability leading to defective mitochondrial translation and a combined respiratory chain deficiency. Today, approximately 200 pathogenic variants had been mapped to mt-tRNA genes (https://www.mitomap.org/MITOMAP) [42], emphasizing the importance of mt-tRNAs for mitochondrial function.
In the current study, we analyzed the frequencies of mt-tRNA variants in 138 genetically unrelated LHON patients lacking three primary mtDNA mutations and 266 control subjects by using PCR-Sanger sequencing. Mutational screening for the entire mt-tRNA genes identified 27 nucleotide alternations in 13 mt-tRNA genes. Among these, 8 mt-tRNA variants exhibited the statistical significance between LHON and controls. In fact, the 593 T > C variant occurred at the D-arm of tRNAPhe (conventional position 17), variation at that position was important for the structure and function of tRNA. Functional analysis showed that approximately ~ 46% decreases in the steady-state level of tRNAPhe in the cell lines harboring this variant [43]. In addition, the 593 T > C variant was implicated to play a synergistic effect on LHON-associated ND4 11778G > A mutation [44], while the homoplasmic 3275C > T variant was localized at the variable region of tRNALeu(UUR), which had been reported to be associated with LHON and polycystic ovary syndrome (PCOS) [45, 46]. Moreover, the 4363 T > C variant occurred at the anticodon stem of tRNAGln, which was highly conserved across various species (conventional position 38). Nucleotide at that position was often chemically modified during tRNAGln processing and function [27]. Thus, the 4363 T > C variant may reduce the steady-state level of tRNAGln and cause mitochondrial dysfunction [47]. In addition, the well-known 4435A > G variant affected a highly conserved adenosine at position 37, 3′ adjacent to the tRNAMet anticodon, which was important for the fidelity of codon recognition and stabilization. Functional analysis revealed that the 4435A > G variant introduced an m1G37 modification of tRNAMet, altering its structure and function [48,49,50].
While the T to C transition at position 5587 was localized at the end of tRNAAla and may alter the tertiary structure of this tRNA, the 5587 T > C variant had been reported to be associated with deafness and LHON [51, 52]. Furthermore, the homoplasmic 14693A > G variant occurred at the first base (conventional position 54) of the TψC loop of tRNAGlu, nucleotide at position 54 was often modified, and contributed to the structural and stabilization of functional tRNAs [27]; thus, the 14693A > G variant may lead to mitochondrial dysfunction which was responsible for LHON [53]. Recent experimental studies revealed that the 14693A > G variant was associated with hearing loss and may increase the penetrance and expressivity of deafness-associated 12S rRNA mutations [54, 55]. Moreover, 2 variants in tRNAThr were found to be associated with LHON: 15927G > A and 15951A > G. The 15927G > A variant abolished the C-G base-pairing in the anticodon stem of tRNAThr and caused approximately 60% reductions in the steady-state level of tRNAThr [14]. Furthermore, this variant decreased the activities of mitochondrial complex I and III, marked diminished mitochondrial ATP level [14]. Interestingly, the 15927G > A had been regarded as pathogenic variant associate with hearing loss [56] and coronary heart disease [57], while the 15951A > G variant was located at the acceptor arm of tRNAThr, which was important for tRNA identity and pre-tRNA processing [58]. In fact, the significant reduction of the steady-state levels in tRNAThr was observed in cells carrying the 15951A > G variant [59]. Therefore, these mt-tRNA variants may lead to the failure in tRNA metabolism and impair the mitochondrial translation and respiration.
It has been suggested that mitochondrial genetic background (haplogroups) may contribute to the phenotypic expression of LHON. For instance, the haplogroup J specific ND1 4216 T > C and ND5 13708G > A variants may increase the penetrance and expressivity of LHON-related primary mutations in European countries [60, 61]. Furthermore, mtDNA haplogroups M7b1′2 and M8a had been implicated in the clinical expression of the LHON-associated ND4 11778G > A mutation in Han Chinese population [62]. More recently, mitochondrial haplogroup D4j specific variant 11696G > A was found to increase the penetrance and expressivity of the LHON-associated 11778G > A mutation in several Han Chinese pedigrees [63]. To see the contributions of rare mutations to the clinical expression of LHON-related mt-tRNA variants, we sequenced the whole mitochondrial genomes of 23 probands carrying one of the pathogenic/likely pathogenic mt-tRNA variants. Consequently, 6 probands carrying functional mtDNA variants were identified. These mtDNA variants included the following: ND1 3308 T > C, 3394 T > C and 4216 T > C, ND4 11696G > A, ND5 12338 T > C and ND6 14502 T > C. We noticed that these variants occurred at extremely conserved nucleotides of mtDNA and may affect the respiratory chain function, which aggravated mitochondrial dysfunction caused by putative pathogenic mt-tRNA variants (Table 3).
To whether mt-tRNA variants caused mitochondrial dysfunction, we generated the cybrid cell lines containing these pathogenic/likely pathogenic variants. As shown in Fig. 3, we found that LHON patients harboring these variants had lower levels of ATP and MMP when compared with the controls; by contrast, the ROS level increased significantly (P < 0.05 for all). In fact, the shortage in ATP generation in LHON patients was most probably a result of the decreased in the proton electrochemical potential gradient of impaired mitochondria [64]. Moreover, reduction of MMP can contribute to abnormal mitochondrial function and was an important parameter for indicating the early cell death [65]. The impairment of MMP will in turn increase the ROS generation; on the other hand, overproduction of ROS may lead to serious consequence such as increasing the oxidative stress in cells, damaging DNA, RNA, and lipids and contributing to programmed cell death [66]. Therefore, these mt-tRNA variants were involved in the pathogenesis of LHON.
Based on these observations, we proposed that the possible molecular mechanism underlying the roles of mt-tRNA mutations in the pathogenesis of LHON may be as follows: first, the mutation itself disrupted the secondary structure of mt-tRNA, thus causing a failure in tRNA metabolism, such as CCA addition, posttranscriptional modification, or aminoacylation [67, 68]. Failures in mt-tRNA metabolism caused by these mutations would impair mitochondrial protein synthesis and respiration. As a result, abnormal mitochondrial respiration caused oxidative stress and uncoupling of oxidative pathways for ATP synthesis [69], which caused the RGC dysfunction and apoptosis [5], thus contributing to the LHON progression.
Several limitations existed in the present study; first, this study only included Chinese individuals and the results needed to be validated in other ethnic groups. Second, the statistical power may be limited due to the small sample size. Third, further investigations such as examining tRNA steady-state level, mitochondrial protein expression were required to verify the conclusions.
In summary, our data provided the evidence that mt-tRNA variants may be associated with LHON. Mt-tRNAPhe 593 T > C, tRNALeu(UUR) 3275C > T, tRNAGln 4363 T > C, tRNAMet 4435A > G, tRNAAla 5587 T > C, tRNAGlu 14693A > G, tRNAThr 15927G > A, and 15951A > G variants should be added as risk factors for LHON. Thus, our findings may provide novel insights into the understanding of the pathophysiology and valuable information for the management and prevention of LHON.
Abbreviations
- mtDNA:
-
Mitochondrial DNA
- LHON:
-
Leber’s hereditary optic neuropathy
- mt-tRNA:
-
Mitochondrial tRNA
- RGCs:
-
Retinal ganglion cells
- OXPHOS:
-
Oxidative phosphorylation
- rCRS:
-
Revised Cambridge Reference Sequence
- CI:
-
Conservation index
- FBS:
-
Fetal bovine serum
- DCFH-DA:
-
2’,7’-Dichlorodihydrofluorescein diacetate
- MMP:
-
Mitochondrial membrane potential
- CCCP:
-
Carbonyl cyanide 3-chlorophenylhydrazone
- ETC:
-
Electron transport chain
- PCOS:
-
Polycystic ovary syndrome
References
Yu-Wai-Man P, Griffiths PG, Hudson G et al (2009) Inherited mitochondrial optic neuropathies. J Med Genet 46(3):145–158
Rasool N, Lessell S, Cestari DM (2016) Leber hereditary optic neuropathy: Bringing the lab to the clinic. Semin Ophthalmol 31(1–2):107–116
Yu-Wai-Man P, Griffiths PG, Brown DT et al (2003) The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet 72(2):333–339
Newman NJ (2005) Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol 140(3):517–523
Carelli V, La Morgia C, Valentino ML et al (2009) Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta 1787(5):518–528
Brown MD, Trounce IA, Jun AS et al (2000) Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber’s hereditary optic neuropathy mitochondrial DNA mutation. J Biol Chem 275(51):39831–39836
Mashima Y, Yamada K, Wakakura M et al (1998) Spectrum of pathogenic mitochondrial DNA mutations and clinical features in Japanese families with Leber’s hereditary optic neuropathy. Curr Eye Res 17(4):403–408
Wallace DC, Singh G, Lott MT et al (1988) Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242(4884):1427–1430
Jia X, Li S, Xiao X et al (2006) Molecular epidemiology of mtDNA mutations in 903 Chinese families suspected with Leber hereditary optic neuropathy. J Hum Genet 51(10):851–856
Kirchner S, Ignatova Z (2015) Emerging roles of tRNA in adaptive translation, signaling dynamics and disease. Nat Rev Genet 16(2):98–112
Wallace DC (2013) A mitochondrial bioenergetic etiology of disease. J Clin Invest 123(4):1405–1412
Elson JL, Swalwell H, Blakely EL et al (2009) Pathogenic mitochondrial tRNA mutations–which mutations are inherited and why? Hum Mutat 30(11):E984-992
Qu J, Li R, Zhou X et al (2006) The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation. Invest Ophthalmol Vis Sci 47(2):475–483
Zhang J, Ji Y, Liu X et al (2018) Leber’s hereditary optic neuropathy caused by a mutation in mitochondrial tRNAThr in eight Chinese pedigrees. Mitochondrion 42:84–91
Ding Y, Ye YF, Li MY et al (2020) Mitochondrial tRNAAla 5601C>T variant may affect the clinical expr16ession of the LHON-related ND4 11778G>A mutation in a family. Mol Med Rep 21(1):201–208
Wang HW, Jia X, Ji Y et al (2008) Strikingly different penetrance of LHON in two Chinese families with primary mutation G11778A is independent of mtDNA haplogroup background and secondary mutation G13708A. Mutat Res 643(1–2):48–53
Andrews RM, Kubacka I, Chinnery PF et al (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23(2):147
Qu J, Wang Y, Tong Y et al (2010) Leber’s hereditary optic neuropathy affects only female matrilineal relatives in two Chinese families. Invest Ophthalmol Vis Sci 51(10):4906–4912
Ruiz-Pesini E, Wallace DC (2006) Evidence for adaptive selection acting on the tRNA and rRNA genes of human mitochondrial DNA. Hum Mutat 27(11):1072–1081
Yarham JW, Al-Dosary M, Blakely EL et al (2011) A comparative analysis approach to determining the pathogenicity of mitochondrial tRNA mutations. Hum Mutat 32(11):1319–1325
Kong QP, Bandelt HJ, Sun C et al (2006) Updating the East Asian mtDNA phylogeny: a prerequisite for the identification of pathogenic mutations. Hum Mol Genet 15(13):2076–2086
Wang M, Liu H, Zheng J et al (2016) A deafness- and diabetes-associated tRNA mutation causes deficient pseudouridinylation at position 55 in tRNAGlu and mitochondrial dysfunction. J Biol Chem 291(40):21029–21041
Ding Y, Xia BH, Zhang CJ et al (2018) Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene 642:299–306
Victor VM, Rovira-Llopis S, Bañuls C et al (2016) Insulin resistance in PCOS patients enhances oxidative stress and leukocyte adhesion: role of myeloperoxidase. PLoS ONE 11(3):e0151960
Adrie C, Bachelet M, Vayssier-Taussat M et al (2001) Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med 164(3):389–395
Bandelt HJ, Salas A, Taylor RW et al (2009) Exaggerated status of “novel” and “pathogenic” mtDNA sequence variants due to inadequate database searches. Hum Mutat 30(2):191–196
Suzuki T, Nagao A, Suzuki T (2011) Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet 45:299–329
Jiang Z, Teng L, Zhang S et al (2021) Mitochondrial ND1 T4216C and ND2 C5178A mutations are associated with maternally transmitted diabetes mellitus. Mitochondrial DNA A DNA Mapp Seq Anal 32(2):59–65
Liang M, Guan M, Zhao F et al (2009) Leber’s hereditary optic neuropathy is associated with mitochondrial ND1 T3394C mutation. Biochem Biophys Res Commun 383(3):286–292
Campos Y, Martín MA, Rubio JC et al (1997) Bilateral striatal necrosis and MELAS associated with a new T3308C mutation in the mitochondrial ND1 gene. Biochem Biophys Res Commun 238(2):323–325
Liu Y, Li Z, Yang L et al (2008) The mitochondrial ND1 T3308C mutation in a Chinese family with the secondary hypertension. Biochem Biophys Res Commun 368(1):18–22
Liu XL, Zhou X, Zhou J et al (2011) Leber’s hereditary optic neuropathy is associated with the T12338C mutation in mitochondrial ND5 gene in six Han Chinese families. Ophthalmology 118(5):978–985
Chen B, Sun D, Yang L et al (2008) Mitochondrial ND5 T12338C, tRNA(Cys) T5802C, and tRNA(Thr) G15927A variants may have a modifying role in the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in three Han Chinese pedigrees. Am J Med Genet A 146A(10):1248–1258
Qu J, Li R, Zhou X et al (2007) Cosegregation of the ND4 G11696A mutation with the LHON-associated ND4 G11778A mutation in a four generation Chinese family. Mitochondrion 7(1–2):140–146
De Vries DD, Went LN, Bruyn GW et al (1996) Genetic and biochemical impairment of mitochondrial complex I activity in a family with Leber hereditary optic neuropathy and hereditary spastic dystonia. Am J Hum Genet 58(4):703–711
Liao Z, Zhao J, Zhu Y et al (2007) The ND4 G11696A mutation may influence the phenotypic manifestation of the deafness-associated 12S rRNA A1555G mutation in a four-generation Chinese family. Biochem Biophys Res Commun 362(3):670–676
Zhao F, Guan M, Zhou X et al (2009) Leber’s hereditary optic neuropathy is associated with mitochondrial ND6 T14502C mutation. Biochem Biophys Res Commun 389(3):466–472
Zhang S, Wang L, Hao Y et al (2008) T14484C and T14502C in the mitochondrial ND6 gene are associated with Leber’s hereditary optic neuropathy in a Chinese family. Mitochondrion 8(3):205–210
Rybalka E, Timpani CA, Cooke MB et al (2014) Defects in mitochondrial ATP synthesis in dystrophin-deficient mdx skeletal muscles may be caused by complex I insufficiency. PLoS ONE 9(12):e115763
Zorova LD, Popkov VA, Plotnikov EY et al (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59
Datta S, Baudouin C, Brignole-Baudouin F et al (2017) The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest Ophthalmol Vis Sci 58(4):2406–2412
Brandon MC, Lott MT, Nguyen KC et al (2005) MITOMAP: a human mitochondrial genome database—2004 update. Nucleic Acids Res 33:D611-613
Chen X, Nie Z, Wang F et al (2017) Late onset nonsyndromic hearing loss in a Dongxiang Chinese family is associated with the 593T>C variant in the mitochondrial tRNAPhe gene. Mitochondrion 35:111–118
Zhang AM, Bandelt HJ, Jia X et al (2011) Is mitochondrial tRNA(phe) variant m.593T>C a synergistically pathogenic mutation in Chinese LHON families with m.11778G>A? PLoS One 6(10): e26511
Garcia-Lozano JR, Aguilera I, Bautista J et al (2000) A new mitochondrial DNA mutation in the tRNA leucine 1 gene (C3275A) in a patient with Leber’s Hereditary Optic Neuropathy. Hum Mutat 15(1):120–121
Ding Y, Xia BH, Zhang CJ et al (2017) Mutations in mitochondrial tRNA genes may be related to insulin resistance in women with polycystic ovary syndrome. Am J Transl Res 9(6):2984–2996
Wang L, Dong Z, Lin W et al (2017) Molecular characterization of a pedigree carrying the hypertension-associated mitochondrial tRNAGln T4363C mutation. Mol Med Rep 16(5):6029–6033
Zhou M, Xue L, Chen Y et al (2018) A hypertension-associated mitochondrial DNA mutation introduces an m1G37 modification into tRNAMet, altering its structure and function. J Biol Chem 293(4):1425–1438
Ding Y, Xia BH, Zhuo GC et al (2019) Premature ovarian insufficiency may be associated with the mutations in mitochondrial tRNA genes. Endocr J 66(1):81–88
Lu Z, Chen H, Meng Y et al (2011) The tRNAMet 4435A>G mutation in the mitochondrial haplogroup G2a1 is responsible for maternally inherited hypertension in a Chinese pedigree. Eur J Hum Genet 19(11):1181–1186
Tang X, Li R, Zheng J et al (2010) Maternally inherited hearing loss is associated with the novel mitochondrial tRNA Ser (UCN) 7505T>C mutation in a Han Chinese family. Mol Genet Metab 100(1):57–64
Ji Y, Qiao L, Liang X et al (2017) Leber’s hereditary optic neuropathy is potentially associated with a novel m.5587T>C mutation in two pedigrees. Mol Med Rep 16(6): 8997–9004
Tong Y, Mao Y, Zhou X et al (2007) The mitochondrial tRNA(Glu) A14693G mutation may influence the phenotypic manifestation of ND1 G3460A mutation in a Chinese family with Leber’s hereditary optic neuropathy. Biochem Biophys Res Commun 357(2):524–530
Young WY, Zhao L, Qian Y et al (2006) Variants in mitochondrial tRNAGlu, tRNAArg, and tRNAThr may influence the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in three Han Chinese families with hearing loss. Am J Med Genet A 140(20):2188–2197
Ding Y, Li Y, You J et al (2009) Mitochondrial tRNA(Glu) A14693G variant may modulate the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in a Han Chinese family. J Genet Genomics 36(4):241–250
Ding Y, Teng YS, Zhuo GC et al (2019) The mitochondrial tRNAHis G12192A mutation may modulate the clinical expression of deafness-associated tRNAThr G15927A mutation in a Chinese pedigree. Curr Mol Med 19(2):136–146
Qin Y, Xue L, Jiang P et al (2014) Mitochondrial tRNA variants in Chinese subjects with coronary heart disease. J Am Heart Assoc 3(1):e000437
Helm M, Brulé H, Friede D et al (2000) Search for characteristic structural features of mammalian mitochondrial tRNAs. RNA 6(10):1356–1379
Li R, Qu J, Zhou X et al (2006) The mitochondrial tRNA(Thr) A15951G mutation may influence the phenotypic expression of the LHON-associated ND4 G11778A mutation in a Chinese family. Gene 376(1):79–86
Ghelli A, Porcelli AM, Zanna C et al (2009) The background of mitochondrial DNA haplogroup J increases the sensitivity of Leber’s hereditary optic neuropathy cells to 2,5-hexanedione toxicity. PLoS ONE 4(11):e7922
Brown MD, Starikovskaya E, Derbeneva O et al (2002) The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup. J Hum Genet 110(2):130–138
Ji Y, Zhang AM, Jia X et al (2008) Mitochondrial DNA haplogroups M7b1'2 and M8a affect clinical expression of leber hereditary optic neuropathy in Chinese families with the m.11778G‑>a mutation. Am J Hum Genet 83(6): 760–768
Xie S, Zhang J, Sun J et al (2017) Mitochondrial haplogroup D4j specific variant m.11696G > a (MT-ND4) may increase the penetrance and expressivity of the LHON-associated m.11778G > a mutation in Chinese pedigrees. Mitochondrial DNA A DNA Mapp Seq Anal 28(3):434–441
James AM, Sheard PW, Wei YH et al (1999) Decreased ATP synthesis is phenotypically expressed during increased energy demand in fibroblasts containing mitochondrial tRNA mutations. Eur J Biochem 259(1–2):462–469
Gong S, Peng Y, Jiang P et al (2014) A deafness-associated tRNAHis mutation alters the mitochondrial function, ROS production and membrane potential. Nucleic Acids Res 42(12):8039–8048
Böttger EC, Schacht J (2013) The mitochondrion: a perpetrator of acquired hearing loss. Hear Res 303:12–19
Ding Y, Zhuo G, Guo Q et al (2021) Leber’s hereditary optic neuropathy: the roles of mitochondrial transfer RNA variants. PeerJ 9:e10651
Lin Y, Xu X, Wang W et al (2021) A mitochondrial myopathy-associated tRNA(Ser(UCN)) 7453G>A mutation alters tRNA metabolism and mitochondrial function. Mitochondrion 57:1–8
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407
Author information
Authors and Affiliations
Contributions
Zhilan Yuan and Jie Shuai designed the study. Jie Shuai and Jian Shi performed the mutational screening of mt-tRNA genes in all subjects involved in this study. Ya Liang and Fangfang Ji performed the mitochondrial functional analysis. Luo Gu analyzed the data. Zhilan Yuan wrote the paper. All authors have read and approved final draft.
Corresponding author
Ethics declarations
Ethical approval
This work is approved by the Ethics Committee of First Affiliated Hospital of Nanjing Medical University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shuai, J., Shi, J., Liang, Y. et al. Mutational analysis of mitochondrial tRNA genes in 138 patients with Leber’s hereditary optic neuropathy. Ir J Med Sci 191, 865–876 (2022). https://doi.org/10.1007/s11845-021-02656-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02656-6