Abstract
Background
This study is aimed at exploring the correlation of sirtuin 2 (SIRT2) with clinical characteristics as well as overall survival (OS) in breast cancer patients.
Methods
Totally, 296 primary breast cancer patients who underwent surgical resection were retrospectively reviewed in this study, and SIRT2 expression in tumor and adjacent tissues was determined by immunohistochemistry (IHC) and scored by semiquantitative scoring (0–12). Clinicopathological features were retrieved, and OS was calculated.
Results
Both SIRT2 IHC semiquantitative score and percentage of SIRT2 high expression by IHC score > 3 were lower in tumor tissues compared with adjacent tissues. Additionally, tumor SIRT2 high expression was associated with lower T stage, decreased N stage, and reduced TNM stage. Kaplan-Meier curves displayed that tumor SIRT2 high expression predicted longer OS. Univariate Cox’s regression analysis showed that tumor SIRT2 high expression was associated with prolonged OS, while multivariate Cox’s regression analysis displayed that tumor SIRT2 high expression was not an independent predictive factor for OS, which implied that tumor SIRT2 might predict OS indirectly through the interaction of tumor features (such as TNM stage) in breast cancer patients.
Conclusion
SIRT2 expression is lower in tumor tissues compared with adjacent tissues, and tumor SIRT2 high expression correlates with lower T stage, decreased N stage, reduced TNM stage, and longer OS in breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in females and one of the three most frequent malignancies worldwide, which causes almost 1,700,000 new cases and around 520,000 deaths during 2015 [1, 2]. Despite great advances in medical technology and novel drugs have been achieved in these years, the incidence of breast cancer is raising in developing countries partly due to the changed lifestyle and initiated screening programs, and it also remains a critical cause of cancer-related death due to high rates of relapse and metastasis [2, 3]. Thus, search for novel biomarkers that could monitor disease progression and predicting prognosis may facilitate exactitude and early therapy and further improve treatment outcomes of breast cancer patients.
Sirtuins are highly conservative protein deacetylases consisting of seven isotypes, which regulate essential biological processes related to cell proliferation and metabolism [4,5,6]. Sirtuin 2 (SIRT2), one of the sirtuin family members, acts as NAD+-dependent lysine deacetylase, and it is generally known as a regulator involved in mitosis such as the regulation of chromosomal condensation and mitotic structures [7,8,9]. Several recent studies have paid attentions to the role of SIRT2 in cancers and uncover that dysregulated SIRT2 may contribute to the initiation or progression of tumors [10,11,12,13]. For example, SIRT2 expression has been found decreased in tumor tissues of gliomas, nonsmall cell lung cancer (NSCLC), and head and neck squamous cell carcinoma (HNSCC). Moreover, deficient SIRT2 expression triggers tumorigenesis and accelerates cell proliferation, which eventually leads to aggravated disease progression and worse survival profiles in several cancers (including prostate cancer and serous ovarian carcinoma) [10,11,12,13,14]. Based on the indication that SIRT2 is a potential biomarker for attenuated disease progression as well as favorable survival profiles in some cancers, we speculated that SIRT2 may also play an antitumor role in the progression and prognosis of breast cancer, whereas related investigation is seldomly reported. Hence, we conducted this study to explore the correlation of SIRT2 with clinical characteristics as well as overall survival (OS) in breast cancer patients.
Methods
Patients
This study retrospectively reviewed 296 primary breast cancer patients who underwent surgical resection at our hospital from January 2014 to December 2016. The screening criteria were as follows: (i) confirmed diagnosis of primary breast cancer by histopathological examination, (ii) underwent surgical resection, (iii) tumor tissue and adjacent tissue specimens taken from surgery were available, and (iv) data of clinicopathological features and follow-up information were complete. Patients with following conditions were excluded: (i) received neoadjuvant therapy before surgery, (ii) relapsed or metastasis disease, (iii) accompanied with other malignancies, and (iv) missed follow-up information. The Ethical Committee of our hospital approved the protocol of this study. All enrolled patients or their guardians provided written informed consents or verbal agreements with recording.
Data collection
Clinicopathological features of patients including age, pathological grade, T stage, N stage, TNM stage, estrogen receptors (ER) status, progesterone receptors (PR) status, human epidermal growth factor (HER-2) status, and molecular subtype were extracted from medical records. Moreover, the information of adjuvant therapies was also extracted.
Immunohistochemistry
Specimens of tumor and adjacent tissue (referred to the normal tissue with a distance of 2–5 cm far from the tumor tissue, and adjacent tissue and tumor tissue in our study were separate samples from the same patients) were acquired from the Specimen Storage Room of our hospital, which were resected from primary breast cancer lesions and were formalin-fixed and paraffin-embedded. Tissue samples were sliced from paraffin blocks (5-μm sections), deparaffinated in xylene, and hydrated in a methanol gradient (100%, 95%, 70%, and 50%). Unspecific peroxidase activity was blocked with 3% H2O2 and 90% methanol. Heat-mediated antigen retrieval was performed with Tris/EDTA buffer pH 9.0. After blocked with normal goat serum, sections were incubated overnight with Rabbit Anti-SIRT2 antibody (1:100 dilutions, Abcam, MA, USA); then the sections were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:200 dilutions, Abcam, MA, USA). 3,3′-Diaminobenzidine (DAB) with 5% H2O2 was used as chromogenic agent, and hematoxylin was applied for counterstaining. Slides were finally viewed under the Nikon microscope (Nikon Instruments, NY, USA).
SIRT2 assessment by IHC scoring
The quantification of SIRT2 expression in tumor and adjacent tissue was performed using a semiquantitative scoring method according to the previously reported methodology [15]. Briefly, each immunohistochemistry (IHC) slide was assessed for the intensity of the staining and the density of positively stained cells. The staining intensity was scored using the following scale: 0 = negative, 1 = weak, 2 = moderate, and 3 = strong; the density of positively stained cells was scored using the following scale: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The final IHC score was calculated by multiplying staining intensity and staining density. SIRT2 high and low expressions were divided by the cutoff value of 3: high expression (IHC score > 3) and low expression (IHC score ≤ 3).
OS evaluation
Follow-up records of enrolled patients were collected and reviewed. The last follow-up date was June 30, 2018, and the median follow-up duration was 36.0 months ranging from 2.0 to 54.0 months, with an interquartile range of 15.0 months. OS was calculated from the date of surgery to the date of death or last follow-up.
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, USA) and GraphPad Prism 7.02 (GraphPad Software, La Jolla, USA) were used for data analysis and graph plotting. Continuous variable was displayed as mean ± standard deviation (SD). Categorized variable was expressed as count (percentage). Differences between two groups were detected by Student’s t test, chi-square test, or Wilcoxon rank-sum test. OS curves were constructed with the Kaplan-Meier method and compared with the log-rank test. The influence of variables on OS was examined by the univariable and multivariable Cox’s proportional hazards regression model analyses. A P value < 0.05 was considered statistically significant.
Results
Study flow
Six hundred fifteen breast cancer patients who underwent surgical resection were screened in this study. Whereas 287 patients were excluded, including 105 patients with no available tumor tissue and adjacent tissue, 85 patients received neoadjuvant therapy, 64 patients with no complete clinical data and follow-up information, 17 patients with relapsed disease, and 16 patients with other malignancies (Fig. 1). In the remaining 328 patients, 32 patients who were unable to contact or obtain informed consents were excluded. Finally, 296 patients were reviewed in the study.
Characteristics of breast cancer patients
Mean age of 296 breast cancer patients was 53.3 ± 13.8 years (Table 1). For pathological grade, the numbers of patients with pathological grades G1, G2, and G3 were 68 (23.0%), 205 (69.2%), and 23 (7.8%), respectively. Besides, the numbers of patients with TNM stages I, II, and III were 71 (24.0%), 160 (54.0%), and 65 (22.0), respectively. Additionally, there were 175 (59.1%), 153 (51.7%), and 96 (32.4%) patients presented with ER positive, PR positive, and HER-2 positive, respectively. Regarding the molecular subtype, numbers of patients with ERBB2+, basal-like, luminal A, and luminal B were 53 (17.9%), 63 (21.3%), 126 (42.6%), and 54 (18.2%), respectively. As to the adjuvant therapies, there were 16 (5.4%) patients did not receive adjuvant therapies, and 280 (94.6%) patients received adjuvant therapies, among which, 169 (57.1%) patients received endocrine therapy, 181 (61.1%) patients received chemotherapy, and 71 (24.0%) patients received anti-HER2 therapy.
Expression of SIRT2 in tumor tissues and adjacent tissues
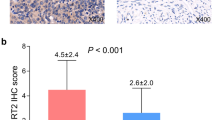
Examples of SIRT2 expressions in tumor and adjacent tissues are exhibited in Fig. 2. SIRT2 IHC score was lower in tumor tissues compared with adjacent tissues (3.0 ± 1.9 vs. 4.5 ± 2.7) (P < 0.001) (Fig. 3a), and the percentage of SIRT2 high expression by IHC score > 3 was decreased in tumor tissues compared with adjacent tissues (24.7% vs. 51.7%) (P < 0.001) (Fig. 3b), suggesting that SIRT2 expression was lower in tumor tissues compared with adjacent tissues.
Comparison of SIRT2 expression between tumor tissues and adjacent tissues. SIRT2 IHC score in tumor tissues and adjacent tissues (a). SIRT2 high expression by IHC score > 3 in tumor tissues and adjacent tissues (b). Comparison between groups was determined by the paired t test or McNemar test. SIRT2, sirtuin 2; IHC, immunohistochemistry. P < 0.05 was considered significant
Correlation of tumor SIRT2 expression with clinical characteristics in breast cancer patients
Patients were classified as SIRT2 high expression group (n = 73) and SIRT2 low expression group (n = 223) according to the tumor SIRT2 expression by IHC score (cutoff value = 3). Tumor SIRT2 high expression was associated with lower T stage (P = 0.001), decreased N stage (P = 0.004), and reduced TNM stage (P < 0.001) in breast cancer patients (Table 2), whereas no correlation of tumor SIRT2 expression with age (P = 0.174), pathological grade (P = 0.921), ER (P = 0.614), PR (P = 0.732), HER-2 (P = 0.178), and molecular subtype (P = 0.677) was found in breast cancer patients.
Correlation of tumor SIRT2 expression with OS in breast cancer patients
To investigate the correlation of tumor SIRT2 expression with OS in breast cancer patients, K-M curves were performed and the log-rank test was utilized, which revealed that tumor SIRT2 high expression was correlated with better OS in breast cancer patients (P = 0.024) (Fig. 4).
OS in SIRT2 high expression patients and SIRT2 low expression patients. K-M curves displayed OS in SIRT2 high expression patients and SIRT2 low expression patients. Comparison between groups was determined by the log-rank test. OS, overall survival; SIRT2, sirtuin 2; K-M curves, Kaplan-Meier curves. P < 0.05 was considered significant
Analysis of factors affecting OS in breast cancer patients
Univariate Cox’s regression analysis displayed that tumor SIRT2 high expression was associated with prolonged OS (P = 0.033), while higher pathological grade (P = 0.006), higher T stage (P < 0.001), higher N stage (P < 0.001), and higher TNM stage (P < 0.001) were associated with shorter OS in breast cancer patients (Table 3). Besides, chemotherapy was associated with decreased OS in breast cancer patients (P = 0.015). Moreover, multivariate Cox’s regression analysis showed that tumor SIRT2 high expression was not an independent factor for predicting OS in breast cancer patients, whereas higher pathological grade (P = 0.002) and higher T stage (P = 0.018) were independent predictive factors for worse OS. These results implied that tumor SIRT2 might predict OS indirectly through affecting T stage, N stage, and TNM stage in breast cancer patients.
Discussion
Our results indicated that (1) SIRT2 expression was lower in tumor tissues compared with adjacent tissues, and tumor SIRT2 high expression was associated with lower T stage, decreased N stage, and reduced TNM stage in breast cancer patients; (2) tumor SIRT2 expression positively correlated with OS in breast cancer patients.
Sirtuin 2 (SIRT2), which belongs to the sirtuin family, is identified to act with multiple genes or pathways including p53, peroxisome proliferator-activated receptor-γ (PPAR-γ), forkhead box O (FOXO), and nuclear factor kappa B (NF-κB), and it participates in the various biological processes such as cellular stress response, metabolism, mitosis, and apoptosis through deacetylating different function proteins [10, 16,17,18]. In recent years, the role of SIRT2 as regulator in maintaining genome integrity as well as a tumor suppressor has been disclosed in some previous studies [9, 10, 12, 19]. For example, SIRT2 inhibits cell proliferation in vitro via targeting JMJD2A in NSCLC cells and represses tumor growth in vivo [10]. Also, an interesting study discloses that SIRT-2-deficient mouse embryonic fibroblasts gradually gain a rapid proliferation rate and become malignantly transformed after immortalization; moreover, in vivo experiments show that the SIRT2-deficient mice develop tumors, implying that absence of SIRT2 triggers tumorigenesis [9]. Another study uncovers that decreased SIRT2 fails to suppress cyclin-dependent kinase 4 (CDK4) expression, which eventually results in accelerated cell proliferation and cell invasion in serous ovarian carcinoma [12]. Additionally, a study displays that SIRT2 sensitizes breast cancer cells to intracellular DNA damage and leads to enhanced cell apoptosis that induced by oxidative stress [19]. These studies reveal that SIRT2 plays a critical role in maintaining genetic stability and suppressing tumor formation in these cancers.
In clinical studies, SIRT2 expression has been shown to be decreased in tumor tissues of several cancers (such as NSCLC, gliomas, and HNSCC) [10, 11, 13]. As for the correlation of SIRT2 expression with clinical characteristics in carcinomas, a study shows that tumor SIRT2 expression is negatively associated with TNM stage in HNSCC patients [11]. And another study displays that tumor SIRT2 high expression is associated with decreased clinical stage in prostate cancer patients [14]. These previous studies indicate that tumor SIRT2 expression is negatively correlated with disease stage in some cancers. Considering that SIRT2 might act as a biomarker for attenuated disease progression in these mentioned cancers, we hypothesized that it might also act as a suppressor in the breast cancer, whereas related data in breast cancer is still indefinite. Our study enrolled 296 breast cancer patients and measured the SIRT2 expression in tumor tissues as well as adjacent tissues. We found that SIRT2 expression was reduced in tumor tissues compared with adjacent tissues. Furthermore, we further investigated the correlation of SIRT2 expression with clinical characteristics in these patients, and we observed that tumor SIRT2 high expression was associated with lower T stage, decreased N stage, and reduced TNM stage in breast cancer patients. The possible reasons for these results were that (1) SIRT2 might inhibit cell proliferation or enhance cell apoptosis via targeting JMJD2A or p53, thereby suppressed tumor growth and resulted in lower T stage as well as TNM stage in breast cancer patients [20]; (2) SIRT2 might repress cell invasion and migration via deacetylating CDK4; therefore, SIRT2 reduced tumor invasion and metastasis, which associated with lower N stage and TNM stage in breast cancer patients [12]; (3) SIRT2 was reported to bind to chromatin in the nucleus, and the overexpressed SIRT2 delayed mitotic exit; thereby, SIRT2 was a mitotic checkpoint protein that prevented chromatin condensation in response to mitotic stress, and for cancer cells, SIRT2 was regarded as an important tumor suppressor through preventing tumor cell division, and this mechanism might also explain our results [21]; (4) elevated levels of SIRT2 could sensitize breast cancer cells to intracellular DNA damage and cell apoptosis induced by oxidative stress; thus, SIRT2 high expression indicated more SIRT2-induced tumor suppressor activity in breast cancer, which led to the attenuated tumor growth and decreased TNM stage in breast cancer patients [8].
The predictive value of SIRT2 in cancers has also been explored in some previous clinical trials [14, 22,23,24]. For instance, tumor SIRT2 high expression is reported to be correlated with better OS and recurrence-free survival (RFS) in NSCLC patients [22, 23]. Also, a study shows that tumor SIRT2 expression is positively associated with OS in chronic lymphocytic leukemia patients [24]. Another study discloses that SIRT2-deleted prostate patients have a trend of shorter RFS compared with SIRT2 diploid prostate patients [14]. These studies imply that tumor SIRT2 high expression predicts better survival profiles in some cancer patients, whereas evidence about the predictive value of SIRT2 in breast cancer patients is limited. In our study, we observed that tumor SIRT2 high expression was associated with prolonged OS in breast cancer patients, which might due to (1) SIRT2 inhibited cell proliferation and promoted cell apoptosis through regulating some genes or kinases (such as JMJD2A, p53, and CDK4); therefore, SIRT2 led to alleviated disease progression and further resulted in better OS [10, 12]; (2) SIRT2 might amplify lethal effects of agents through inducing nuclear accumulation of FOXO3A or inactivating Prdx-1; thus, SIRT2 contributed to the increase in treatment efficiency, which led to prolonged OS [8]. Furthermore, we found that SIRT2 high expression was associated with longer OS, but it was not an independent predictive factor for OS in breast cancer patients. These data implied that SIRT2 might predict OS through affecting T stage, N stage, and TNM stage in breast cancer patients.
Some limitations still existed in our study: (1) this was a retrospective study, and assessment of SIRT2 expression was restricted to formalin-fixed and paraffin-embedded tissues; (2) this was a single-center study, and it might lack wide representativeness; (3) detailed mechanisms of SIRT2 in breast cancer are still unclear.
Some limitations still existed in our study: (1) this was a retrospective study, and assessment of SIRT2 expression was restricted to formalin-fixed and paraffin-embedded tissues; (2) this was a single-center study, and it might lack wide representativeness; (3) detailed mechanisms of SIRT2 in breast cancer were still unclear.
In conclusion, SIRT2 expression is lower in tumor tissues compared with adjacent tissues, and tumor SIRT2 high expression correlates with lower T stage, decreased N stage, reduced TNM stage, and longer OS in breast cancer patients.
References
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomark Prev 25(1):16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578
Harbeck N, Gnant M (2017) Breast cancer. Lancet 389(10074):1134–1150. https://doi.org/10.1016/S0140-6736(16)31891-8
Scully OJ, Bay BH, Yip G, Yu Y (2012) Breast cancer metastasis. Cancer Genomics Proteomics 9(5):311–320
Min JS, Kim JC, Kim JA, Kang I, Ahn JK (2018) SIRT2 reduces actin polymerization and cell migration through deacetylation and degradation of HSP90. Biochim Biophys Acta, Mol Cell Res 1865(9):1230–1238. https://doi.org/10.1016/j.bbamcr.2018.06.005
Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273(2):793–798. https://doi.org/10.1006/bbrc.2000.3000
Verdin E (2015) NAD(+) in aging, metabolism, and neurodegeneration. Science 350(6265):1208–1213. https://doi.org/10.1126/science.aac4854
Hiratsuka M, Inoue T, Toda T, Kimura N, Shirayoshi Y, Kamitani H, Watanabe T, Ohama E, Tahimic CGT, Kurimasa A, Oshimura M (2003) Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene. Biochem Biophys Res Commun 309(3):558–566
Fiskus W, Coothankandaswamy V, Chen J, Ma H, Ha K, Saenz DT, Krieger SS, Mill CP, Sun B, Huang P, Mumm JS, Melnick AM, Bhalla KN (2016) SIRT2 deacetylates and inhibits the peroxidase activity of peroxiredoxin-1 to sensitize breast cancer cells to oxidant stress-inducing agents. Cancer Res 76(18):5467–5478. https://doi.org/10.1158/0008-5472.CAN-16-0126
Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, Ji J, Wang XW, Park SH, Cha YI, Gius D, Deng CX (2011) SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20(4):487–499. https://doi.org/10.1016/j.ccr.2011.09.004
Xu W, Jiang K, Shen M, Qian Y, Peng Y (2015) SIRT2 suppresses non-small cell lung cancer growth by targeting JMJD2A. Biol Chem 396(8):929–936. https://doi.org/10.1515/hsz-2014-0284
Lai CC, Lin PM, Lin SF, Hsu CH, Lin HC, Hu ML, Hsu CM, Yang MY (2013) Altered expression of SIRT gene family in head and neck squamous cell carcinoma. Tumour Biol 34(3):1847–1854. https://doi.org/10.1007/s13277-013-0726-y
Du Y, Wu J, Zhang H et al (2017) Reduced expression of SIRT2 in serous ovarian carcinoma promotes cell proliferation through disinhibition of CDK4 expression. Mol Med Rep 15(4):1638–1646. https://doi.org/10.3892/mmr.2017.6183
Li Y, Dai D, Lu Q et al (2013) Sirt2 suppresses glioma cell growth through targeting NF-kappaB-miR-21 axis. Biochem Biophys Res Commun 441(3):661–667. https://doi.org/10.1016/j.bbrc.2013.10.077
Damodaran S, Damaschke N, Gawdzik J, Yang B, Shi C, Allen GO, Huang W, Denu J, Jarrard D (2017) Dysregulation of Sirtuin 2 (SIRT2) and histone H3K18 acetylation pathways associates with adverse prostate cancer outcomes. BMC Cancer 17(1):874. https://doi.org/10.1186/s12885-017-3853-9
Ye SL, Li XY, Zhao K, Feng T (2017) High expression of CD8 predicts favorable prognosis in patients with lung adenocarcinoma: a cohort study. Medicine (Baltimore) 96(15):e6472. https://doi.org/10.1097/MD.0000000000006472
Arora A, Dey CS (2014) SIRT2 negatively regulates insulin resistance in C2C12 skeletal muscle cells. Biochim Biophys Acta 1842(9):1372–1378. https://doi.org/10.1016/j.bbadis.2014.04.027
Suematsu T, Li Y, Kojima H, Nakajima K, Oshimura M, Inoue T (2014) Deacetylation of the mitotic checkpoint protein BubR1 at lysine 250 by SIRT2 and subsequent effects on BubR1 degradation during the prometaphase/anaphase transition. Biochem Biophys Res Commun 453(3):588–594. https://doi.org/10.1016/j.bbrc.2014.09.128
Gal J, Bang Y, Choi HJ (2012) SIRT2 interferes with autophagy-mediated degradation of protein aggregates in neuronal cells under proteasome inhibition. Neurochem Int 61(7):992–1000. https://doi.org/10.1016/j.neuint.2012.07.010
Nguyen P, Lee S, Lorang-Leins D, Trepel J, Smart DK (2014) SIRT2 interacts with beta-catenin to inhibit Wnt signaling output in response to radiation-induced stress. Mol Cancer Res 12(9):1244–1253. https://doi.org/10.1158/1541-7786.MCR-14-0223-T
Temel M, Koc MN, Ulutas S et al (2016) The expression levels of the sirtuins in patients with BCC. Tumour Biol 37(5):6429–6435. https://doi.org/10.1007/s13277-015-4522-8
McGlynn LM, Zino S, MacDonald AI et al (2014) SIRT2: tumour suppressor or tumour promoter in operable breast cancer? Eur J Cancer 50(2):290–301. https://doi.org/10.1016/j.ejca.2013.10.005
Li Z, Huang J, Yuan H, Chen Z, Luo Q, Lu S (2016) SIRT2 inhibits non-small cell lung cancer cell growth through impairing Skp2-mediated p27 degradation. Oncotarget 7(14):18927–18939. https://doi.org/10.18632/oncotarget.7816
Gong J, Wang H, Lou W, Wang G, Tao H, Wen H, Liu Y, Xie Q (2018) Associations of sirtuins with clinicopathological parameters and prognosis in non-small cell lung cancer. Cancer Manag Res 10:3341–3356. https://doi.org/10.2147/CMAR.S166946
Van Damme M, Crompot E, Meuleman N et al (2012) HDAC isoenzyme expression is deregulated in chronic lymphocytic leukemia B-cells and has a complex prognostic significance. Epigenetics 7(12):1403–1412. https://doi.org/10.4161/epi.22674
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Ethical Committee of our hospital approved the protocol of this study. All enrolled patients or their guardians provided written informed consents or verbal agreements with recording.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, P., Zhou, M. & Yang, Y. Upregulated tumor sirtuin 2 expression correlates with reduced TNM stage and better overall survival in surgical breast cancer patients. Ir J Med Sci 189, 83–89 (2020). https://doi.org/10.1007/s11845-019-02071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-019-02071-y