Abstract
Background
There is contradictory evidence regarding the merits of restricted versus liberal perioperative intravenous (IV) fluid administration in bowel surgery. This study sought to audit perioperative fluid management in elective colectomy in Ireland and to analyse the impact of such on operative outcomes.
Methods
A national surgical trainee collaborative audit of perioperative fluid management was performed. Data from each site was collected prospectively over a selected 3-week period within a pre-defined 2-month block. Collected variables included demographics, type of operation/anaesthethic, volume/type of fluid administration pre-, intra- and post-operatively, 30-day morbidity and mortality. Primary outcome was fluid balance 24-h post-operatively with further analysis to identify the impact of this on 30-day morbidity. ROC curves were generated to identify the critical volume at which fluid balance was associated with 30-day morbidity.
Results
Ninety-four patients were enrolled from 17 hospitals. Mean age was 64 years. A total of 48.9% (N = 46) were managed by ERAS and 51.1% (N = 48) received bowel preparation. Almost 70% of cases (N = 63) were completed by minimally invasive techniques. Significant 30-day morbidity requiring hospital readmission was low [6.4% (n = 6)]. Median fluid balance at 24 h was + 715 ml (IQR 165–1486 ml). On multivariate analysis, high BMI (p = 0.02), indication for surgery (p = 0.02) and critical care admission (p = 0.008) were significantly predictive of 30-day morbidity. Twenty-four hour fluid balance >+ 665 ml was associated with increased risk of 30-day morbidity on univariate but not multivariate analysis, implying association but not causation.
Conclusion
Overall, perioperative fluid management was within an acceptable range with minimal impact on 30-day morbidity following elective colorectal surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgery remains the cornerstone of treating malignant colorectal neoplasia with over 2 500 new cases diagnosed in Ireland annually [1]. Of these, 74% will undergo surgery within a year of initial diagnosis [1]. Treatment of rectal cancer has been standardised in Ireland through the development of dedicated rectal cancer centres with a subsequent improvement in outcomes [2]. Colon cancer and benign disease including Crohn’s and ulcerative colitis still account for the vast majority of colorectal resections. Indeed, almost three quarters of patients with Crohn’s disease will require a surgical intervention (including colectomy) due to their inflammatory bowel disease (IBD) [3].
The introduction of enhanced recovery after surgery (ERAS) programmes to colorectal surgery has significantly improved outcomes [4, 5]. Within this, perioperative fluid use has remained contentious. A number of randomised controlled trials (RCTs) have attempted to ascertain whether the use of perioperative fluid restriction is beneficial within the context of the ERAS protocol [6,7,8,9]. Excess of fluids can result in hyperchloremia, fluid overload, and renal vasoconstriction [10]. Furthermore, certain studies have suggested that such an excess of fluid promotes bowel wall oedema, resulting in increased rates of anastomotic complications [11]. Yet, more recent reviews have suggested that fluid restriction does not confer the benefit initially proposed by the ERAS protocol [12, 13]. Indeed, a recent study of over 3000 patients in the New England Journal of Medicine suggested that too restrictive a fluid regimen is as bad as too liberal, with increased acute kidney injury and no improvement in morbidity [14]. There remains a paucity of evidence regarding the use of fluids in the perioperative period as well as its sequelae on outcomes for patients undergoing colorectal resection. The available evidence would suggest a large variation in practice between different institutions [15]. Identifying perioperative fluid practice variations and the effect of this on patient outcomes is important to guide practice.
The increasing output from surgical research collaboratives, primarily formulated and managed by trainees, within the United Kingdom in the last 10 years has provided a new wave of high-quality research [16]. Such collaboratives have provided highly useful information on common surgical problems such as gallstone disease [17], using data from across multiple institutions. This study represents a landmark within Ireland as it is the first study to be carried out by the Irish Surgical Research Collaborative and follows the example set by other such collaboratives in the United Kingdom and Internationally.
The primary aim of this study was to audit perioperative fluid management in elective colorectal surgery in Ireland, in a multi-centre, prospective cohort study and to analyse the impact of perioperative fluid practice on operative outcomes.
Methods
As a cross-sectional prospective cohort study, the STROBE (Strengthening of the Reporting of Observational Studies in Epidemiology) consensus statement was adhered to [18].
Collaborative study design
This study was the first collaborative study performed by the Irish Surgical Research Collaborative (ISRC). The ISRC is a trainee-led surgical research collaborative established by Surgical Trainees in the Republic of Ireland with the aim of undertaking high-quality collaborative multicentre research projects. All surgical trainees in all hospitals in Ireland were invited to participate in this study through registered Royal College of Surgeons in Ireland (RCSI) mailing lists to ensure inclusivity. Lead trainees who agreed to participate in each centre established a local network including a Consultant Surgeon (local Principal Investigator), Consultant Anaesthetist and other participating surgical trainees. Ethical approval was sought prospectively from each regional clinical ethics committee prior to commencing data collection. Authorship was assigned in keeping with the Association of Surgeons in Training (ASiT)/National Research Collaborative (NRC) authorship model.
Study timeline and patient selection
Participating centres could select a 3-week period for data collection within a predefined 2-month study window as outlined in the study protocol. All patients over the age of 18 years undergoing elective colonic or rectal resection in each centre were eligible for screening for inclusion. Both benign and malignant indications for surgery were included. Patients undergoing emergency operations were excluded. Patients were identified through elective theatre operating lists and informed consent obtained prospectively.
Data collection
A full study data collection sheet is included as Appendix 1. Basic demographic information was recorded and a combination of pre-, peri- and post-operative data tabulated. Data was collected in a standardised database across all centres. Each local PI coded data prior to central submission and all patient identifiers were removed. Patient identifiers were maintained onsite only in a secure local server to facilitate accurate patient identification for recording 30-day follow-up outcomes. Pre-operative data was collected from patients’ medical notes. Operative data was recorded by a trainee present in/assisting in procedures and post-operative data collection continued for the 30 days post-operatively.
The primary endpoint was an analysis of fluid balance at 24 h post-operatively by subtraction of IV fluid input and output and the impact of fluid balance at 24 h on 30-day morbidity. Fluid balance at 24 h post-operatively was defined from the time at which the operation ended to 24 h post-operatively. Secondary endpoints included change in patient’s baseline weight (where available); demographics; method of operation; use of ERAS protocols and bowel preparation; use of GDFT; hospital length of stay (LOS); all cause 30-day morbidity; all cause 30-day mortality. Further analysis sought to examine the impact of ERAS and bowel preparation practices on 30-day morbidity.
Outcome analysis
The statistical data from this study is reported in accordance with the guidelines set by the STROBE (Strengthening of the Reporting of Observational Studies in Epidemiology) consensus statement [13]. Data was divided into clinically relevant categories. Categorical variables were analysed using Fisher exact or Chi-square test where appropriate and continuous variables were analysed using Student’s t test. Statistical significance was observed at p < 0.05. All variables achieving p < 0.25 on univariate analysis were entered into a multivariate analysis to assess impact of predictive variables on outcomes. An ordinal regression model was used to produce odds ratios (OR), i.e., the odds of an adverse event which in this case was the odds of developing morbidity by day 30 post-operatively. A receiver operating curve (ROC) was generated to calculate Youden’s Index (J), the critical point at which 24-h fluid balance predicts 30-day morbidity. Statistical analysis was performed using SPSS, version 21.
Pilot study
Prior to study rollout, a 2-week pilot study was undertaken to assess feasibility of study design and to validate data collection techniques. Following the pilot study, a number of changes were made to data collection variables due to practical considerations.
Results
Demographics and clinical parameters
A total of 94 patients were recruited across 17 hospitals in the Republic of Ireland. Demographics are outlined in Table 1. Fifty-three percent (n = 9) was model 3 hospitals and 47% (n = 8) model 4. Sixty-one percent (n = 57) of patients were male and 39% (n = 37) were female. Mean age was 64 years (range: 23–89 years). Most patients were ASA grade III or less, with the majority assessed as ASA II [63.8% (n = 60)]. Over three-quarters of patients underwent surgery for malignancy [78.7% (n = 74)], 13.8% (n = 13) for benign causes and 7.4% (n = 7) for dysplasia.
Summary of operative outcomes
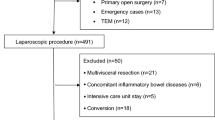
Figure 1 summarises the procedures performed with breakdown by operative technique (open, laparoscopic (lap), lap-assisted, converted, robotic). Anterior resection [41.5% (n = 39)] and right hemi colectomy [27.7% (n = 26)] were the most commonly performed procedures. Most procedures were performed laparoscopic or lap-assisted [69.1% (n = 65)], with one quarter performed open [25.5% (n = 24)] and five cases robotic (5.3%). A 10% conversion rate was observed for minimally invasive procedures (n = 7). Thirty-six covering stomas were formed (42%) along with nine definitive (n = 7 APR and n = 2 completion proctectomy). Sixty-nine percent of covering stomas were performed for anterior resections (n = 25). Median operative time was 210 min (IQR 144–289 min). Less than 50% of patients were managed using standardised enhanced recovery protocols [48.9% (n = 46)]. Fifty percent (n = 47) of patients received bowel preparation pre-operatively 48.9% (n = 23) as part of ERAS pathway.
Perioperative fluid balance
Median fluid balance at 24 h post-op was 714.5 ml (IQR = 165–1486) as outlined in Table 2. GDFT was described in only 17% (n = 16) of cases and no cases utilised oesophageal Doppler monitoring. The median 24-h fluid input was 2593 ml (IQR 2205–3178 ml) and median 24-h output was 1710 ml (IQR 1271–2473 ml). No significant difference was observed in median fluid balance at 24 h post-op between those managed by ERAS protocols (+ 832 v 857, p = 0.91), those who received bowel prep (+ 802 v + 886, p = 0.71) and those managed using GDFT (+ 455 v + 937, p = 0.10). Pre- and post-operative weight was available for 47 patients as a measure of perioperative fluid balance. There was no median change in weight from pre- to post-operative day 1.
Operative complications and 30-day outcomes
Six intra-operative complications occurred (6.4%): two bowel injuries, one bladder injury, one significant haemorrhage and two others (not defined). At 30-day follow-up, 32 (34%) morbidities were reported as outlined in Table 3. A 6.4% (n = 6) SSI rate was reported, 2.1% (n = 2) anastomotic leak rate and 5.3% (n = 5) intra-abdominal collections. Six patients (6.4%) developed a post-operative ileus with median time to return of gut function as 2 days (IQR = 1–3 days). Median hospital length of stay (LOS) was 9 days (IQR 6–13). No significant difference in LOS was observed in those managed by ERAS (median 8 days; IQR 6–13) versus traditional pathways [median 9 days; IQR 7–12 (p = 0.159)]. During 30-day follow-up, 11.7% (n = 11) represented to ED, 6.4% were re-admitted and there were no deaths.
Impact of perioperative fluid balance, ERAS and bowel preparation on 30-day morbidity
On univariate analysis BMI, indication for surgery and operation performed, requirement for critical care admission and 24-h fluid balance were all significantly associated with 30-day morbidity. When a multivariate analysis was further performed only BMI [OR 5.65 (95% CI 0.018–0.192) p = 0.02], indication for surgery [OR 3.39 (95% CI − 0.089–2.871) p = 0.02] and critical care admission [OR 7.045 (95% CI 0.767–5.095) p = 0.008] maintained significance. A receiver-operating curve was generated to identify the critical point at which fluid balance volume is significantly associated with 30-day morbidity (Fig. 2a). With an area under the curve (AUC) of 0.625, the critical cut-off was identified at 665 ml (sensitivity 62.9%, specificity 60%) [Youden’s index (J) = 0.229]. Therefore, in this cohort of elective colectomy patients, when fluid balance at 24 h exceeded + 665 ml, risk of developing morbidity within the first 30 days post-operatively significantly increased [OR 2.05 [− 0.01–0.10] 0.152]. Figure 2b further shows that the risk of 30-day morbidity is three times higher than the risk at the critical cut-off point when fluid balance exceeds 1 l [OR 7.14 (95% CI 2.86–17.8) p < 0.001] and risk almost doubles again > 1.5 l [OR 14.76 (95% CI 3.87–16.32) p < 0.001] Table 4.
Table 5 summarises the impact of ERAS protocol and pre-operative bowel preparation use on 30-day morbidity. Thirty-day morbidity was observed in 39% (n = 18) of patients managed with ERAS protocols compared to 29% (n = 14) of patients not managed by strict ERAS protocols. This was not statistically significant (p = 0.308). No difference was observed in patients treated with pre-operative bowel preparation with an identical 30-day morbidity rate observed in each group [34% (n = 16)].
Discussion
This study is the first to nationally examine peri-operative fluid use as well as its impact on surgical outcomes in patients undergoing elective colorectal surgery in Ireland. A number of studies have previously examined variation in fluid use within colorectal surgery, but only at an institutional level [19, 20]. Our study demonstrates that across 17 hospitals in the Republic of Ireland, there remains a significant variation in the volume of fluid being administered perioperatively in patients undergoing elective colorectal resections, but that this did not significantly affect morbidity. There was a high rate of minimally invasive surgery reported (70%), which may have improved fluid balance overall.
Our study demonstrated that the overall median fluid balance in the 24-h period after surgery was + 714.5 ml; yet, the interquartile range of 165–1486 ml shows a significant difference in fluid administration between cases. Only 18% of patients had a negative fluid balance, making the numbers too small to determine whether this impacted morbidity. Previous evidence has demonstrated that morbidity is lowest when a “zero balance” regarding fluid status [21] is achieved. Although this study showed higher complications in those with a positive fluid balance, this was not significant on multivariate analysis and caution must be exercised in interpretation. A recent NEJM publication highlighted the dangers of an overly restrictive fluid policy [14], and as mentioned, our study did not have sufficient numbers to interpret the impact of a negative fluid balance. On a national level, surgical and anaesthetic bodies need to continue to work together to continually update and improve guidelines to improve consistency in perioperative care.
A cornerstone of fluid use within the ERAS protocol involves goal-directed fluid therapy (GDFT) to guide both anaesthetists and surgeons. Previous randomised controlled trials have attempted to assess the efficacy of GDFT and in particular the use of intra-operative oesophageal doppler monitoring as a guide to fluid status and subsequent fluid administration [22,23,24]. These trials demonstrated a significant reduction in post-operative morbidity in patients treated in such a manner. Our study shows that few institutions (17%) in Ireland routinely use GDFT for guiding fluid use and none of the hospitals involved reported using intraoperative oesophageal dopplers to monitor fluid status. This is in keeping with a survey of anaesthetists in the United Kingdom that examined the prevalence of GDFT as part of an ERAS programme [25], with only 33% of those surveyed stating they used GDFT within the setting of colorectal surgery, although NICE guidelines advocate the use of GDFT [26]. Our study also demonstrates that fluid balance was lower in the GDFT group compared to the non-GDFT group, but this was not statistically significant (median: 455 ml vs 927 ml; p = 0.10). This may also be due to a type 2 error due to the small sample size, or there may not be a difference between the two groups.
There are multiple other findings of interest within this study. The laparoscopic approach was the most common operative technique utilised, consistent with previous evidence demonstrating an advantage to this approach [27, 28]. Five cases were performed robotically indicating the increasing role of such an approach in Ireland. This figure is likely to increase and this group of patients will become a new focus for investigation when considering perioperative fluid use in the future. Less than half of patients were treated within an enhanced recovery protocol even though there is now a strong body of evidence to support the use of such a protocol, even if there remains controversy regarding some aspects [4, 5]. However, the 30-day morbidity rate was not significantly different between those managed by ERAS and those managed by a more traditional approach with similar findings observed with pre-operative bowel preparation use. This may be due to the adoption of ERAS principles by units that do not adhere formally to an ERAS protocol.
This project is the first trainee-run collaborative study carried out in the Republic of Ireland. Performed across 17 hospitals, data was collated by trainees under the supervision of surgical consultants. Such a study has allowed us to attempt to add to the literature regarding the issue of fluid use in elective colorectal surgery at a national level, which has not been done before, demonstrating the benefit of such a collaborative. This follows from large collaborative studies in the United Kingdom, such as the CholeS study [16], which provided invaluable information regarding gallbladder surgery. Although the number of cases in this study is smaller than previously published collaborative studies, we believe it provides useful information regarding fluid use in elective colorectal surgery going forward. We also believe that this study will act as an important platform for developing future collaborative studies to develop internationally with the Irish Surgical Research Collaborative.
Conclusion
Practice variation is observed in the administration of intravenous fluid in patients undergoing elective colorectal resection across hospitals in Ireland but no significant impact on 30-day morbidity is observed on multivariate analysis. Variation also exists in patient management by ERAS and use of pre-operative mechanical bowel preparation.
Change history
03 July 2019
The above article originally published with Christina Fleming and Irish Surgical Research Collaborative listed in the authorship line.
References
https://www.ncri.ie/sites/ncri/files/factsheets/colorectal.pdf
Burke JP, Coffey JC, Boyle E, Keane F, McNamara DA (2013) Early outcomes for rectal cancer surgery in the republic of Ireland following a national centralization program. Ann Surg Oncol 20(11):3414–3421
Jones DW, Finlayson SR (2010) Trends in surgery for Crohn's disease in the era of infliximab. Ann Surg 252:307–312
Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, Gouma DJ, Bemelman WA, Laparoscopy and/or Fast Track Multimodal Management Versus Standard Care (LAFA) Study Group, Enhanced Recovery after Surgery (ERAS) Group (2006) Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 93(7):800–809
The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection 2015 Results from an international registry. Ann Surg.
Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I (2005) Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 103(1):25–32
Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP (2002) Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 359(9320):1812–1818
Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K et al (2003) Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 238(5):641–648
Kalyan JP, Rosbergen M, Pal N, Sargen K, Fletcher SJ, Nunn DL et al (2013) Randomized clinical trial of fluid and salt restriction compared with a controlled liberal regimen in elective gastrointestinal surgery. Br J Surg 100(13):1746–1747
Bedi SS, Hetz R, Thomas C, Smith P, Olsen AB, Williams S, Xue H, Aroom K, Uray K, Hamilton J, Mays RW, Cox CS Jr (2013) Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem Cells Transl Med 2:953–960
Boesen AK, Maeda Y, Rorbaek Madsen M. Perioperative fluid infusion and its influence on anastomotic leakage after rectal cancer surgery: implications for prevention strategies
Bundgaard-Nielsen M, Secher NH, Kehlet H (2009) 'Liberal' vs. 'restrictive' perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand 53:843–851
Boland MR, Noorani A, Varty K, Coffey JC, Agha R, Walsh SR (2013) Perioperative fluid restriction in major abdominal surgery: systematic review and meta-analysis of randomized, clinical trials. World J Surg 37(6):1193–1202
Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, Christophi C, Leslie K, McGuinness S, Parke R, Serpell J, Chan MTV, Painter T, McCluskey S, Minto G, Wallace S (2018) Restrictive versus Liberal fluid therapy for major abdominal surgery. N Engl J Med 378:2263–2274. https://doi.org/10.1056/NEJMoa1801601. [Epub ahead of print]
Chong PC, Greco EF, Stothart D, Maziak DE, Sundaresan S, Shamji FM et al (2009) Substantial variation of both opinions and practice regarding perioperative fluid resuscitation. Can J Surg 52:207–214
G D, Bartlett DC, Futaba K, Whisker L, Pinkney TD, West Midlands Research Collaborative (2014) (WMRC), Birmingham, UK. BMC med Educ. How to set up and manage a trainee-led research collaborative. BMC Med Educ 14:94
CholeS study group, west midlands research Collaborative (2016) Population-based cohort study of outcomes following cholecystectomy for benign gallbladder diseases. Br J Surg 103(12):1704–1715
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 370:1453–1457
Lee SY, Kang SB, Kim DW, Oh HK, Ihn MH (2015) Risk factors and preventive measures for acute urinary retention after rectal cancer surgery. World J Surg 39(1):275–282
Boesen AK, Maeda Y, Rorbaek Madsen M (2013) Perioperative fluid infusion and its influence on anastomotic leakage after rectal cancer surgery: implications for prevention strategies. Color Dis 15(9):e522–e527
Lobo DN, Macafee DA, Allison SP (2006) How perioperative fluid balance influences postoperative outcomes. Best Pract Res Clin Anaesthesiol 20(3):439–455
Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C (2002) Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 57:845–849
Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF (2005) Barclay GR et al. intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 95:634–642
Noblett SE, Snowden CP, Shenton BK, Horgan AF (2006) Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 93:1069–1076
Srinivasa S, Kahokehr A, Soop M, Taylor M, Hill AG (2013) Goal-directed fluid therapy- a survey of anaesthetists in the UK, USA, Australia and New Zealand. BMC anaesthesiology 13:5
Group TCOoSTS (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Kennedy GD, Heise C, Rajamanickam V, Harms B, Foley EF (2009) Laparoscopy decreases postoperative complication rates after abdominal colectomy: results from the national surgical quality improvement program. Ann Surg 249:596–601
Author information
Authors and Affiliations
Consortia
Ethics declarations
Ethical approval was sought prospectively from each regional clinical ethics committee prior to commencing data collection. Authorship was assigned in keeping with the Association of Surgeons in Training (ASiT)/National Research Collaborative (NRC) authorship model.
Conflict of interest
Nil
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The above article originally published with Christina Fleming and Irish Surgical Research Collaborative listed in the authorship line. Christina Fleming’s name was included in error by the Publisher, and should have instead been included in the Collaborators section.
Appendix
Appendix
Pre-operative | |
|---|---|
MRN (for data collection only) | |
Age (years) | |
Gender | |
ASA | |
Indication | |
Admission date | |
Enhanced recovery (Y/N) | |
Pre-op weight (kg) | |
BMI | |
Diabetic (Y/N) | |
Baseline creatinine | |
Baseline haemoglobin | |
Baseline diuretic Type Dose | |
IV fluid preop Type Volume | |
Pre-op bowel prep (Y/N) Type | |
Intra-operative | |
Date of surgery | |
Time of induction | |
Time of skin closure | |
Lap/converted/open/robotic | |
Operation | |
Operation duration (min) | |
General anaesthesia (Y/N) | |
Spinal anaesthesia (Y/N) | |
Epidural (Y/N) | |
Cannula Size Number | |
Urinary catheter | |
Goal-directed fluid therapy (Y/N) Type | |
Oesophageal Doppler use (Y/N) | |
IV fluids Type Volume | |
Blood products Type Volume | |
Intra-op urinary output | |
Intra-op blood loss (ml) | |
Intra-op complications (Y/N) Type | |
Inotropes (Y/N) | |
Post-op day 1 | |
POD1 fluid balance (ml) | |
Total post op fluid input (ml) | |
Total post op fluid output (ml) | |
Weight (kg) | |
IV fluid post op to 24 h Type Volume | |
Blood products post op to 24 h Type Volume | |
Enteral fluid input post-op to 24 h Tpye Volume | |
Urinary output POD-24 h (ml) | |
GI output POD-24 h (ml) | |
Stoma (Y/N) Volume POD-24 h | |
Creatinine day 1 | |
Post-op diuretic Type Dose | |
Post-op day 30 | |
HDU (Y/N)–planned (Y/N) | |
ICU (Y/N)–planned (Y/N) | |
Total LOS (days) | |
High output stoma (Y/N) | |
Renal compromise from HOS (Y/N) | |
Return to theatre (Y/N) | |
Time to return to oral fluids (POD) | |
Time to return to oral diet (POD) | |
Time to passing flatus (POD) | |
Time to BO (POD) | |
30-day morbidity (Y/N) | |
Specific morbidity | |
Clavien-Dindo grade | |
Complication description | |
All cause 30-day ED attendance | |
All cause 30-day re-admission | |
All cause 30-day mortality | |
Cause of death |
Rights and permissions
About this article
Cite this article
Irish Surgical Research Collaborative. PERioperative Fluid Management in Elective ColecTomy (PERFECT)—a national prospective cohort study. Ir J Med Sci 188, 1363–1371 (2019). https://doi.org/10.1007/s11845-019-02003-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-019-02003-w