Abstract
Background
Failure to restore femoral offset in metal on polyethylene total hip arthroplasty (THA) causes polyethylene wear and aseptic loosening. To our knowledge, no study to date has investigated the relationship between femoral offset and wear in metal-on-metal (MOM) THA.
Aims
In this study, we investigated the relationship between femoral offset and wear by measuring circulating metal ion levels in MOM THA.
Methods
In this retrospective study, we identified patients who had undergone MOM THA with the ASR XL system (DePuy International Ltd., Leeds, UK). Femoral offset was measured using anteroposterior radiographs, and circulating metal ion levels (cobalt and chromium) were recorded.
Results
In total, 95 patients were included (68 males and 27 females). The mean age at the time of surgery was 64.9. Mean time from surgery to blood sampling was 15.4 months. No statistically significant relationship was found between femoral offset and cobalt (p = 0.313) or chromium (p = 0.401) ions.
Conclusion
It is known that failure to restore femoral offset during THA can lead to high rates of wear in metal-on-polyethylene articulations. In our study, no statistically significant relationship was found between femoral offset and serum cobalt or chromium ions. This study adds to the information available to surgeons regarding factors that increase wear in metal-on-metal total hip arthroplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal-on-metal (MOM) bearings in total hip arthroplasty (THA) were proposed to have several advantages over conventional metal-on-polyethylene bearings. These advantages, including lower rates of wear and improved stability [1,2,3], led to increased use of metal-on-metal bearings in THA [4]. The articular surface replacement XL system (Depuy International Ltd., Leeds, United Kingdom) is a metal-on-metal hip arthroplasty system which was designed as a hip resurfacing system (ASR hip resurfacing system) or as the ASR XL total hip arthroplasty system using a femoral component articulating with the same acetabular component as used in the resurfacing implant.
However, joint registries have reported higher than expected failure rates with metal-on-metal arthroplasty [5] and concerns remain regarding the adverse effects of high circulating metal ions in patients with MOM bearings. These adverse effects include pseudotumor formation, metal hypersensitivity, and osteolysis [6,7,8,9]. In August 2010, DePuy voluntarily recalled the ASR hip resurfacing and XL stemmed implant system due to high failure rates.
Circulating levels of metal ions are used as a marker for the degree of in vivo wear in metal-on-metal bearing arthroplasty [10, 11]. This has lead to attempts to determine the technical and mechanical factors that affect the levels of circulating metal ions. A variety of factors, including cup inclination angle, degree of anteversion, edge loading, and impingement can affect the wear rate in MOM articulations, and thus may cause an increase in circulating metal ion levels [11,12,13,14,15]. To our knowledge, no study has yet investigated the effect of femoral offset on metal ion levels in MOM bearing THA.
Femoral offset is defined as the perpendicular distance between the long axis of the femur and the center of rotation of the femoral head. The restoration of normal femoral offset has several advantages in total hip arthroplasty. These include improved abductor strength, enhanced stability, greater range of motion, and reduced rates of aseptic loosening and polyethylene wear [16,17,18,19,20,21,22,23]. We hypothesize that increasing the femoral offset may lead to increased wear in metal-on-metal total hip arthroplasty and thus cause increased levels of circulating metal ions. In our study, we investigated the relationship between femoral offset and circulating metal ion levels.
Materials and methods
This retrospective study was performed in a single tertiary care institution. For this type of study, formal consent is not required. All patients were identified from our institution’s database following DePuy’s recall of the ASR resurfacing and XL stemmed implant in August 2010. All patients underwent MOM THA between December 2005 and the product recall. At follow-up, patients were evaluated biochemically and radiologically with peripheral blood samples for analysis of serum cobalt and chromium ion levels and standard anteroposterior radiographs of the pelvis. Serum metal ions were utilized as a surrogate marker for wear at the bearing surface. All blood samples were analyzed in an independent laboratory, using phlebotomy protocols recommended by the laboratory.
In total, 128 patients were identified initially. Patients who had undergone resurfacing arthroplasty were not included in the study. Thirty-three patients were excluded as they had a THA on the contralateral side. Consequently, 95 patients were analyzed. There were 68 males and 27 females in the cohort. The mean age at the time of surgery was 64.9 ± 9.4. The mean time in months between surgery and measurement of metal ions was 37 ± 15.4 (range 11–66).
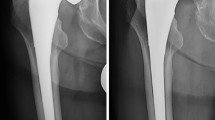
All radiographs were stored in the picture archiving and communication system (PACS), and femoral offset of both the operated hip and the contralateral hip was recorded using PACS tools. A protocol is used within the radiology department for anteroposterior radiographs of the pelvis following arthroplasty to ensure conformity between images and this protocol was adhered to during the study period. Offset was measured as the distance between the center of rotation of the hip and femoral anatomical axis, as this measurement has been shown to remain constant regardless of femoral position in abduction or adduction [24]. Patients who had bilateral total hip arthroplasty were excluded, regardless of the bearing surface of the contralateral hip, due to fears that corrosion at modular junctions in the contralateral hip arthroplasty may cause elevated circulating metal ions [25, 26]. Pre-operative radiographs were also reviewed to ensure there were no discrepancies in offset between the hips prior to surgery, thus allowing the contralateral hip offset to be used as a comparison for the operated side in the post-operative period. Patients who were deceased or lost to follow-up before radiological and biochemical evaluation could be completed were also excluded.
Once all measurements had been recorded, patients were grouped into one of three categories: decreased offset (decrease ≥ 5 mm when compared with contralateral hip), increased offset (increase ≥ 5 mm compared with the contralateral hip), and normal offset (< 5 mm difference between operated hip and contralateral hip). Similar categorization of offset into “increased,” “normal,” and “decreased” has previously been described in studies of femoral offset following THA [27, 28].
In a document from their Expert Advisory Group published in 2010, the United Kingdom Medicines and Healthcare Products Regulatory Agency (MHRA) recommended 7 ppb as the limit above which metal ion levels should be a concern [29]. This is equivalent to 119 nmol/L cobalt and 134 nmol/L chromium. Although alternative metal ion levels have been suggested [30], in this study, we used the levels recommended by the MRHA, as these are the levels that we apply in our clinical practice.
Statistical analysis was performed using IBM Corp. SPSS version 24 (Armonk, NY). Patients’ metal ion levels, patient demographics, and prosthesis details were compared. Variables with skewed distribution (cobalt and chromium concentrations) were compared using the Spearman’s correlation coefficient. The other numerical variables were normally distributed and compared using independent t tests. Categorical variables were compared using the chi-square test. Changes in blood cobalt and chromium levels over time for all patients and subgroups of offset were compared using linear mixed-effects models with random intercept. In all analysis, a p value of less than 0.05 was taken to be statistically significant.
Results
In total, 95 patients were included. All patients had undergone metal-on-metal total hip arthroplasty with the ASR XL total hip arthroplasty system between December 2005 and August 2010. There were 68 males and 27 females. The mean age at the time of surgery was 64.9 ± 9.4 with a minimum age of 43 and maximum age of 82. The mean time in months between surgery and measurement of metal ions was 37 ± 15.4 (range 11–66).
One assessor measured the femoral offset in all cases to ensure consistency. Offset was compared to the contralateral limb to determine if offset was restored. As described above, patients were divided into one of three categories: decreased offset, normal offset, and increased offset. Thirty-three patients were classified as low offset (34% of the cohort). Forty-six patients (48%) had normal offset and 16 patients (16.8% of the cohort) were classified as high offset. Table 1 divides patients with elevated ion levels into categories based on offset.
Firstly, cobalt ion levels were analyzed. Twenty-six patients had high cobalt ion levels (> 119 nmol/L). Spearman’s rank coefficient was utilized as the data was found to be non-parametric using the Kolmogorov-Smirnov test. There was no statistically significant difference in the relationship between femoral offset and cobalt ion levels (r = 0.187, p = 0.313). Next, the three discrete femoral offset groups were evaluated to see if any relationship existed in relation to cobalt ion levels. The chi-square test was carried out. There was no association between femoral offset groups and cobalt ion levels x2 = 1.803, p 0.406.
Next, chromium ions were analyzed. Eleven patients had high chromium ion levels (> 134 nmol/L). Spearman’s rank coefficient was utilized as the data was again found to be non-parametric, using the Kolmogorov-Smirnov test. There was no statistically significant difference in the relationship between femoral offset and chromium ion levels (r = − 0.180, p = 0.401). The chi-square test was conducted to evaluate any correlation between the discrete femoral offset groups and chromium ion levels. There was no statistically significant correlation between femoral offset groups and chromium ion levels, x2 = 5.22, p = 0.073.
Discussion
Total hip arthroplasty has been used for many years, and is believed to have originated in Germany in 1891. Philip Wiles is credited with performing the first metal-on-metal THA when he implanted steel prostheses in six patients in 1938. English surgeon George McKee was one of the first surgeons to use metal-on-metal prosthesis regularly when he began using a modified Thompson stem with a cobalt-chrome acetabular cup [31]. However, these implants were associated with accelerated wear, early loosening, and poor overall survivorship [32], and with the advent of Sir John Charnley’s low friction metal-on-polyethylene arthroplasty, their use significantly declined. It was reintroduced over the last two decades because of low volumetric wear rates in comparison with metal-on-polyethylene bearings, which has the potential to reduce wear particle-induced osteolysis and aseptic loosening [2]. It also had the advantage of greater implant stability with lower rates of dislocation [3]. Resurfacing arthroplasty using MOM bearings was also seen as an attractive option, as it has the advantage of bone stock conservation and relatively straightforward revision to total hip arthroplasty [33].
Unfortunately, national joint registries have reported failure rates in hip arthroplasty with MOM bearings 2–3-fold higher than with other established bearings [5]. Furthermore, adverse periprosthetic soft tissue reactions have emerged which are locally destructive to muscle and bone and can cause implant failure [34]. This local soft tissue destruction is thought to be caused by immune responses to metal wear particles, with activation of lymphocytes and macrophages leading to inflammation, necrosis, and granuloma formation [6, 35]. Although volumetric wear with MOM articulations is lower than MOP articulations, the particles produced are smaller and more numerous [36]. As the role of these particles in adverse soft tissue reactions became apparent, multiple studies attempted to characterize factors which influence wear, using circulating metal ions as a marker for the degree of wear [37, 38].
Studies in MOM resurfacing arthroplasty have shown that a variety of factors, such as cup inclination angles greater than 55 degrees, anteversion less than 10 degrees or greater than 20 degrees, edge loading, impingement, female sex, and smaller femoral head sizes can affect the wear rate, and thus may cause an increase in circulating metal ion levels [11,12,13,14]. It is not clear, however, to what extend these factors affect wear in MOM total hip arthroplasty. Bayley et al. evaluated 258 hips with mean 4-year follow-up treated with MOM THA with head sizes 42 to 60 mm. In this study, elevated metal ion levels were associated with smaller head size, bilateral MOM THA, and female sex [15]. Engh et al. examined 105 patients 5 years following MOM THA and concluded that 36-mm MOM bearings underperformed with respect to metal ion levels when compared to 28-mm bearings [39]. Clearly, the relationship between component size, as well as position, and metal ion levels is complex.
For MOM bearings, the 7 ppb limit for cobalt and chromium ions recommended by the Medicines and Healthcare products Regulatory Agency (MHRA) in a document from their Expert Advisory Group [29] has been shown by Hart et al. [30] to have a sensitivity of 52% and a specificity of 89% for detecting failing prostheses. In this same study, the optimal cut-off level was noted to be 4.97 ppb, which had a sensitivity of 63% and a specificity of 86%, although there is no single upper limit of normal for ions that is agreed upon the world over. In our analysis, we used the limit recommended by the MHRA, as this is the limit we apply in our clinical practice.
We feel that the exclusion of all patients with a THA on the contralateral side is one of strengths of this study. As evidence emerges that corrosion at modular interfaces, in particular the trunnion, can lead to release of metal debris [25, 26], studies examining the factors leading to wear in MOM bearings that ignore potential sources of ion release elsewhere may not be as accurate as previously thought. To our knowledge, this is the first study that seeks to investigate the relationship between offset and serum metal ion levels in MOM THA.
Our study has limitations. These include its retrospective design and that due to the nature of the implant recall, there was a wide variation in the time from initial surgery to the measurement of circulating ions. With regard to the use of metal ions as a marker for wear, we are aware that in vivo measurement of metal ion levels do not always reflect the local ion load around the implant, and does not discriminate between ion release from the bearing surface and ions released from modular junctions in the THA. Furthermore, the measurement of femoral offset from radiographs is not as accurate as computed tomography and is sensitive to femoral rotation, although every attempt was made to ensure consistency with regard to this, with a standard protocol used for radiographs as well as the method used to measure offset which remains constant regardless of the position of the hip in adduction or abduction.
In our study, we did not find any statistically significant relationship between femoral offset in MOM THA and serum metal ion levels. As described above, a number of other factors have been shown to increase circulating ion levels in MOM bearings. We feel that our study provides useful information for the surgeon reviewing patients with these MOM bearings, particularly when attempting to isolate patients at higher risk of failure and need for revision in the future.
References
Sieber HP, Rieker CB, Köttig P (1999) Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg (Br) 81:46–50
Cuckler JM (2005) The rationale for metal-on-metal total hip arthroplasty. Clin Orthop Relat Res 441:132–136
Komistek RD, Dennis DA, Ochoa JA, Haas BD, Hammill C (2002) In vivo comparison of hip separation after metal-on-metal or metal-on-polyethylene total hip arthroplasty. J Bone Joint Surg Am 84–A:1836–1841
Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ (2009) The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am 91:1614–1620. https://doi.org/10.2106/JBJS.H.01220
de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC (2011) Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am 93:2287–2293. https://doi.org/10.2106/JBJS.J.01727
Pandit H, Whitwell D, Gibbons CLM, Athanasou N, Gill HS, Murray DW (2008) Pseudotumours associated with metal-on- metal hip resurfacings. J Bone Joint Surg 90:847–851. https://doi.org/10.1302/0301-620X.90B7
Campbell P, Shimmin A, Walter L, Solomon M (2008) Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplast 23:1080–1085. https://doi.org/10.1016/j.arth.2007.09.024
Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS (2011) Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am 93:2164–2171. https://doi.org/10.2106/JBJS.J.01884
Kwon Y-M, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW (2016) “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty. J Arthroplast 26:511–518. https://doi.org/10.1016/j.arth.2010.05.030
De Smet K, De Haan R, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C et al (2008) Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am 90(Suppl 4):202–208. https://doi.org/10.2106/JBJS.H.00672
Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AVF (2008) The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg (Br) 90:1143–1151. https://doi.org/10.1302/0301-620X.90B9
Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A et al (2011) The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings. Bone Joint J 93–B:315–320
De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K (2008) Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. Bone Joint J 90–B:1291–1297
Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P et al (2009) Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and birmingham hip resurfacing arthroplasties. J Bone Joint Surg (Br) 9191:1287–1295. https://doi.org/10.1302/0301-620X.91B10
Bayley N, Khan H, Grosso P, Hupel T, Stevens D, Snider M, Schemitsch E, Kuzyk P (2014) What are the predictors and prevalence of pseudotumor and elevated metal ions after large-diameter metal-on-metal THA? Clin Orthop Relat Res 473:477–484. https://doi.org/10.1007/s11999-014-3824-2
Asayama I, Chamnongkich S, Simpson KJ, Kinsey TL, Mahoney OM (2016) Reconstructed hip joint position and abductor muscle strength after total hip arthroplasty. J Arthroplast 20:414–420. https://doi.org/10.1016/j.arth.2004.01.016
Devane PA, Robinson EJ, Bourne RB, Rorabeck CH, Nayak NN, Horne JG (1997) Measurement of polyethylene wear in acetabular components inserted with and without cement. A randomized trial. J Bone Joint Surg Am 79:682–689
Bourne RB, Rorabeck CH (2002) Soft tissue balancing: the hip. J Arthroplast 17:17–22. https://doi.org/10.1054/arth.2002.33263
McGrory BJ, Morrey BF, Cahalan TD, An KN, Cabanela ME (1995) Effect of femoral offset on range of motion and abductor muscle strength after total hip arthroplasty. Bone Joint J 77–B:865–869
Fackler CD, Poss R (1980) Dislocation in total hip arthroplasties. Clin Orthop Relat Res 151:169–178
Devane PA, Horne JG (1999) Assessment of polyethylene wear in total hip replacement. Clin Orthop Relat Res 369:59–72
Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB (2009) Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res 467:2895–2900. https://doi.org/10.1007/s11999-009-0845-3
Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH (2001) Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res 388:125–134
Bonin N, Jacquot L, Boulard L, Reynaud P, Saffarini M, Lustig S (2016) How to best measure femoral length and lateralisation after total hip arthroplasty on antero-posterior pelvic radiographs. Int Orthop 40:1–7. https://doi.org/10.1007/s00264-016-3145-z
Craig P, Bancroft G, Burton A, Collier S, Shaylor P, Sinha A (2014) Raised levels of metal ions in the blood in patients who have undergone uncemented metal-on-polyethylene Trident-Accolade total hip replacement. Bone Joint J 96–B:43–47. https://doi.org/10.1302/0301-620X.96B1.30923
White PB, Meftah M, Ranawat AS, Ranawat CS (2016) A comparison of blood metal ions in total hip arthroplasty using metal and ceramic heads. J Arthroplast 31:16–20. https://doi.org/10.1016/j.arth.2016.03.024
Cassidy KA, Noticewala MS, Macaulay W, Lee JH, Geller JA (2012) Effect of femoral offset on pain and function after total hip arthroplasty. J Arthroplast 27:1863–1869. https://doi.org/10.1016/j.arth.2012.05.001
Liebs TR, Nasser L, Herzberg W, Rüther W, Hassenpflug J (2014) The influence of femoral offset on health-related quality of life after total hip replacement. Bone Joint J 96–B:36–42
Mri M (2012) Ref : No authors listed. Medicines and Healthcare products Regulatory Agency (MHRA). Medical device alert: all metal-on-metal (MOM) hip replacements, 2010 MDA/2010/033. http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON079157:6–7
Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B et al (2011) Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg (Br) 93–B:1308–1313. https://doi.org/10.1302/0301-620X.93B10.26249
Knight SR, Aujla R, Biswas SP (2011) Total Hip Arthroplasty - over 100 years of operative history. Orthop Rev (Pavia) 3:2–4
Dobbs H (1980) Survivorship of total hip replacements. J Bone Joint Surg (Br) 62-B(2):168–173
Vendittoli P-A, Lavigne M, Girard J, Roy AG (2006) A randomised study comparing resection of acetabular bone at resurfacing and total hip replacement. Bone Joint J 88–B:997–1002
Drummond J, Tran P, Fary C (2015) Metal-on-metal hip arthroplasty: a review of adverse reactions and patient management. J Funct Biomater 6:486–499. https://doi.org/10.3390/jfb6030486
Hallab N, Merritt K, Jacobs JJ (2001) Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am 83:428–436
MacDonald SJ (2004) Metal-on-metal total hip arthroplasty: the concerns. Clin Orthop Relat Res 429:86–93
Emmanuel AR, Bergin KM, Kelly GE, McCoy GF, Wozniak AP, Quinlan JF (2014) The effect of acetabular inclination on metal ion levels following metal-on-metal hip arthroplasty. J Arthroplast 29:186–191. https://doi.org/10.1016/j.arth.2013.04.022
Langton DJ, Joyce TJ, Jameson SS, Lord J, Van Orsouw M, Holland JP et al (2011) Adverse reaction to metal debris following hip resurfacing. Bone Joint J 93–B:164–171
Engh CA, MacDonald SJ, Sritulanondha S, Korczak A, Naudie D, Engh C (2014) Metal ion levels after metal-on-metal total hip arthroplasty: a five-year, prospective randomized trial. J Bone Joint Surg Am 96:448–455. https://doi.org/10.2106/JBJS.M.00164
Acknowledgements
The authors would like to thank Paula Blake, orthopedic nurse at Our Lady’s Hospital, who worked tirelessly in assisting us with data collection and patient identification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Prior to undertaking this study, the local institutional review board was consulted. As it is a retrospective study, the decision was made that formal consent was not required.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pomeroy, E., Macnamara, F., Murphy, E. et al. Femoral offset found not to affect metal ion levels in metal-on-metal total hip arthroplasty. Ir J Med Sci 188, 149–153 (2019). https://doi.org/10.1007/s11845-018-1808-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-018-1808-z